Abstract
The purpose of this study was to elucidate the role of alpha-tocopherol succinate (TS)- and AMD3100-mobilized progenitors in mitigating combined injury associated with acute radiation exposure in combination with secondary physical wounding. CD2F1 mice were exposed to high doses of cobalt-60 gamma-radiation and then transfused intravenously with 5 million peripheral blood mononuclear cells (PBMCs) from TS- and AMD3100-injected mice after irradiation. Within 1 h after irradiation, mice were exposed to secondary wounding. Mice were observed for 30 d after irradiation and cytokine analysis was conducted by multiplex Luminex assay at various time-points after irradiation and wounding. Our results initially demonstrated that transfusion of TS-mobilized progenitors from normal mice enhanced survival of acutely irradiated mice exposed 24 h prior to transfusion to supralethal doses (11.5–12.5 Gy) of 60Co gamma-radiation. Subsequently, comparable transfusions of TS-mobilized progenitors were shown to significantly mitigate severe combined injuries in acutely irradiated mice. TS administered 24 h before irradiation was able to protect mice against combined injury as well. Cytokine results demonstrated that wounding modulates irradiation-induced cytokines. This study further supports the conclusion that the infusion of TS-mobilized progenitor-containing PBMCs acts as a bridging therapy in radiation-combined-injury mice. We suggest that this novel bridging therapeutic approach involving the infusion of TS-mobilized hematopoietic progenitors following acute radiation exposure or combined injury might be applicable to humans.
Keywords: cytokines, gamma-radiation, hematopoietic progenitor cells, mice, transfusion
INTRODUCTION
There has been increasing awareness that terrorists might attack using sophisticated weapons of mass destruction, including nuclear/radiological devices. Such attacks could involve the placement of a concealed radiation source, a simple radiological device, the use of a radiological dispersal device (also known as dirty bomb), detonation of an improvised nuclear device or a sophisticated nuclear weapon (nuclear bomb), or an attack on a nuclear power plant or nuclear waste facility. The most destructive scenario would be the detonation of a nuclear weapon, which in an urban setting might cause enormous loss of lives from blast and heat, produce an intense burst of radiation and a large amount of radioactive fallout, and lead to thousands of injured survivors [1–3]. The actual numbers of casualties will depend on several factors, including, but not limited to: device design, time of day and weather conditions at detonation, and its precise location. Care of the injured may require application of some sort of radiation countermeasure, which is defined as an agent that prevents, mitigates or treats radiation injury. Despite the extensive research on the effects of radiation on normal tissue, there is an extremely limited number of effective radiation countermeasures currently available either to prevent, to mitigate, or to treat such radiation injuries, especially in the context of unwanted (terrorist-associated) or unintended (radiation accidents) exposures. The US Food and Drug Administration (FDA) has approved several therapies such as potassium iodine (KI), calcium and zinc salts of diethyl-triamine-penta-acetate (DTPA), and Prussian blue for decorporation or blocking of internalized isotopes. The latter two agents are available in strategic national stockpile. Amifostine has been approved for preventing radiation injury in the salivary glands of head and neck cancer patients receiving radiotherapy to reduce xerostomia. However, no safe and effective radiation countermeasure has yet been approved by the FDA for acute radiation syndrome. To improve preparedness for such attacks, the US Government has established programs to promote the development and licensure of medical countermeasures to treat victims of radiological or nuclear terrorism. ‘Radiation combined injury’ is a term used to describe conditions where radiation injury is coupled with other insults such as wounds, burns, infections, or blunt trauma. Radiation combined injury would be expected after a radiological or nuclear attack [4]. Radiation exposure combined with other insults was observed at Hiroshima and Nagasaki, Japan, among nuclear detonation victims. Approximately 60% of these victims received only radiation exposure while the remaining 40% received other injuries (wounds and/or thermal burns) in addition to radiation exposure [5, 6]. Approximately 10% of radiation-exposed victims of the Chernobyl reactor meltdown in the former USSR received thermal burns [7]. There are several reports demonstrating that burns and wounds increase mortality after exposure with non-lethal doses of radiation in mice [8, 9], rats [10, 11], guinea pigs [12], canines [13], and swine [14]. In mice, radiation exposure combined with wounds decreases body weight, increases gut bacterial translocation to various organs, and increases mortality compared with wounds or radiation exposure alone [9, 15]. The combination of radiation with trauma, burns or both confers a worse prognosis than the same dose of radiation alone [16]. Combined injury results in immunosuppression, acute myelosuppression, cellular damage, sepsis, and fluid imbalance resulting in multiorgan dysfunction and multiorgan failure, the most frequent cause of mortality after irradiation and combined injury [17–21]. The molecular and cellular events underlying increased mortality by combined injury are not well understood.
Tocopherol succinate (TS) is a hemisuccinate ester of α-tocopherol that has been shown to protect mice against ionizing radiation injury when administered 24 h prior to irradiation [22, 23]. We demonstrated recently that TS induces high levels of granulocyte colony-stimulating factor (G-CSF) in a dose-dependent manner [24, 25]. In addition, the protective efficacy of TS against hematopoietic and gastrointestinal (GI) syndromes can be abrogated by use of an exogenous neutralizing antibody to G-CSF [24]. We also demonstrated that infusion of whole blood or peripheral blood mononuclear cells (PBMCs) from TS-injected mice improved their chances of extended survival after exposure to escalating doses of acute radiation that cause not only potentially fatal hematopoietic injuries but also severe, potentially fatal GI injuries [26, 27]. In addition, we have demonstrated that infusion of PBMCs from TS- and AMD3100-injected mice significantly inhibited apoptosis, increased cell proliferation in the analyzed tissues of recipient mice, and inhibited gut bacterial translocation to various organs compared with mice receiving cells from vehicle-mobilized cells [28]. All of the tocol species tend to enjoy a wide margin of safety. No doubt, TS also has a very low level of toxicity associated with it. AMD3100 in combination with G-CSF has been shown to mobilize more progenitors than G-CSF alone. AMD3100 is a reversible, pure antagonist of chemokine (C-X-C) receptor 4 (CXCR4) that competes with stromal-derived factor-1 (SDF-1) [29]. It disrupts the interactions between CXCR4 on CD34+ hematopoietic stem cells and stromal cell-derived factor on bone marrow stromal cells, essentially blocking the chemotactic actions of stromal cell-derived factor [30]. This displacement of previously anchored CD34+ hematopoietic stem cells causes their release from stromal cells, allowing their subsequent migration from the bone marrow into the peripheral blood [31, 32]. AMD3100 has been approved by the US FDA for mobilizing hematopoietic stem cells in patients with non-Hodgkins lymphoma and multiple myeloma [33].
Our objectives for this study were to investigate the radio-mitigation potential of TS-mobilized progenitors against supralethal radiation exposures and combined injury, and subsequently, to evaluate the effect of this treatment on cytokine production. Our results demonstrated that transfusions of TS-mobilized progenitors were clearly able to protect CD2F1 mice, with or without ‘combined injuries,’ against the potential lethal effects of doses of ionizing radiation as high as 12 Gy, and that the protective/mitigative effect(s) of the transfused TS-mobilized progenitors is temporally, but causally associated with the modulation of various cytokines.
MATERIALS AND METHODS
Mice
For this study, 6–8-week-old male, CD2F1 specific-pathogen-free mice were purchased from Harlan (Indianapolis, IN, USA) and housed in an air-conditioned facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International [34]. All mice were kept in rooms with a 12 h light/dark cycle. The mouse holding room was maintained at 21 ± 2°C, having 10–15 hourly cycles of fresh air and a relative humidity of 50 ± 10%. Upon arrival, the mice were held in quarantine for 10 days. A microbiological examination of representative samples ensured the absence of Pseudomonas aeruginosa. Mice were provided certified rodent rations (Harlan Teklad Rodent Diet, Harlan Teklad, WI, USA) and acidified water (HCl, pH = 2.5–2.8) ad libitum. The mice were 8–9 weeks old when experiments began. All animal procedures were performed according to a protocol approved by the Armed Forces Radiobiology Research Institute (AFRRI) Institutional Animal Care and Use Committee. Research was conducted according to the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources, National Research Council, US National Academy of Sciences [35].
Drug preparation and administration
TS (Sigma-Aldrich, St Louis, MO, USA) was administered in suspension as described earlier [23]. For a 400-mg/kg dose (10 mg for a 25 g mouse; all experimental mice at the time of testing were approximately 25 ± 2 g), 1000 mg of TS was dispersed in 8.35 ml of PEG-400 and 0.5 ml of Tween-80 for a total volume of 10 ml. The same composition of Tween-80 and PEG-400 was used for the control vehicle. AMD3100 (Sigma-Aldrich, St Louis, MO, USA) was diluted to 1.25 mg/ml with phosphate buffer saline (PBS) for a 5-mg/kg dose. Donor mice were injected with TS (400 mg/kg, 72 h before harvesting) or the vehicle subcutaneously (sc). All donor mice received AMD3100 1 h before harvesting. AMD3100 was administered sc in a volume of 0.1 ml using a 23-gauge needle.
Blood collection, separation, and transfusion of PBMCs
The efficacy of PBMC infusions after total-body irradiation was evaluated using CD2F1 mice as donors as well as recipients. Donor mice were anaesthetized with isoflurane (Abbott Laboratories, Chicago, IL, USA), and blood samples were collected 72 h after TS injection. For preparation of the PBMC transfusates, blood was drawn from the caudal vena cava of donor mice into syringes coated with citrate dextrose (BD Diagnostics, Franklin Lakes, NJ, USA), using a 23-gauge needle. The ratio of the anticoagulant was 0.188 ml citrate dextrose per 1 ml of blood, according to the recommendation of the anticoagulant supplier. Blood samples from the donor group (8–16 mice) were pooled for PBMC separation and transfusion. To mobilize progenitors from the bone marrow into peripheral blood and to improve the yield of progenitor cells within the PBMC fractions, AMD3100 (commercially known as plerixafor or Mozobil) (5 mg/kg) was injected sc 1 h prior to harvest [36]. It has been well established that administration of AMD3100 helps in mobilizing progenitors from bone marrow to peripheral circulation and improves the yield of progenitors when injected to mice 1 h prior to harvest [37–39]. To isolate PBMCs, blood was diluted 1:1 with PBS and layered on histopaque-1083 (Sigma-Aldrich, St Louis, MO, USA), centrifuged for 30 min at 400 g, and mononuclear cells were separated as described earlier [24]. Cells were washed three times (PBS with 1% fetal bovine serum) and the final cell concentration for transfusion was adjusted to 50 million cells/ml in a Hanks balanced salt solution (HBSS, Sigma-Aldrich, St Louis, MO, USA), with 1% fetal bovine serum. Recipient mice were transfused with 5 million cells in 0.1 ml 24 h after irradiation via the retro-orbital sinus using a 28-gauge needle. A total of 5 million PBMCs were obtained from approximately 600 µl of whole blood. For mobilized progenitor cell analysis, blood samples were collected into potassium-EDTA blood collection tubes (lavender screw cap, 1.6 mg EDTA/ml blood; Sarstedt, Germany) and mixed in a rotary shaker. Each million PBMCs collected from TS-injected mice contained approximately 1800 Lin− sca-1+ and 2600 Lin− c-Kit+ cells. For the vehicle group these values were approximately 1100 (Lin− sca-1+) and 1250 (Lin− c-Kit+ cells) [28]. These numbers of sca-1+ and c-kit+ cells were present in blood samples collected 1 h after AMD3100 injection.
Irradiation
Mice were placed in ventilated Plexiglas boxes compartmentalized to accommodate eight mice per box and exposed to bilateral irradiation in the AFRRI 60Co facility at a dose rate of 0.6 Gy/min [25]. After irradiation, mice were returned to their cages and monitored. Sham-irradiated mice were treated in the same manner as irradiated animals except that the facility's 60Co rods were not raised from their pool of shielding water. Radiation dosimetry was based primarily on the alanine/EPR (electron paramagnetic resonance) system [40, 41], currently accepted as one of the most accurate methods and used for intercomparison between national metrology institutions. The calibration curves (spectrometer e-Scan, Burker Biospin, Inc., Madison, WI, USA) used in dose measurements at the AFRRI are based on standard alanine calibration sets purchased from The United States National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA. The alanine dosimeters obtained from NIST had been calibrated in terms of absorbed dose to water using the US national standard radiation sources. At AFRRI, identical alanine dosimeters were irradiated in mice phantoms (Plexiglas 2.5 cm diameter, 7.6 cm length) for a predefined period of time. Measurement of their EPR signals using the calibration curve constructed with alanine dosimeters from NIST provided dose rates to water in the cores of mice. A small correction was subsequently applied for the difference in mass energy absorption coefficients between water and soft tissue.
Wounding
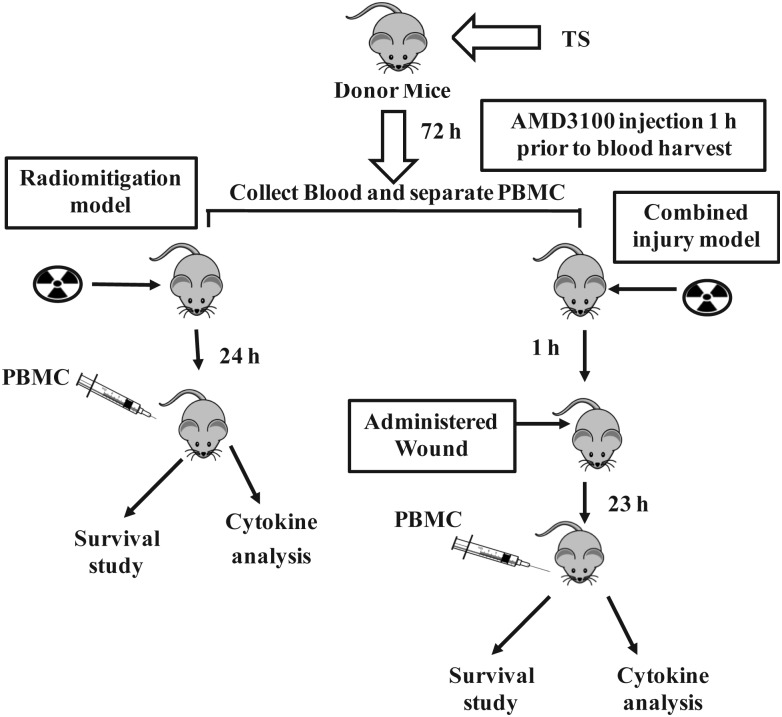
Mice were anesthetized with isoflurane (Abbott Laboratories, Chicago, IL, USA) prior to wounding within 1 h after radiation exposure. Fur was shaved in the wounding area, and a nonlethal surface area wound was administered 20 mm from the occipital bone and between the scapulae using a stainless steel arch punch (9/16", CS Osborne & Company, Harrison, NJ, USA) on a Teflon covered board cleaned with 70% alcohol before each use. The panniculus carnosus muscle and overlying skin were removed as a result of wounding with the steel arch punch. Immediately after wounding, mice were administered 0.5 ml saline (Hospira Inc., Lake Forest, IL, USA) intraperitoneally. Five minutes before and 24 h after wounding, 100 µg/kg Buprenex® (buprenorphine, Reckitt Benckiser Healthcare (UK) Ltd, Hull, England) was administered sc in 0.1 ml volume. Mice receiving no wounds were manipulated identically except that wounding was not administered (fur was shaved as for the wound group). Mice were transfused with 5 million PBMCs from TS- and AMD3100-injected mice 24 h after irradiation, and monitored for survival for 30 days post-irradiation. Diagrammatic representation of the experiment is shown in Fig. 1.
Fig. 1.
Diagrammatic representation of experimental model for evaluating TS mobilized progenitors as a radio-mitigator and countermeasure against combined injury (radiation plus wound). Donor mice received TS and AMD3100 72 h and 1 h, respectively, before blood collection. PBMCs were transfused into irradiated mice 24 h after irradiation. Combined injury mice received wound 1 h after irradiation.
Analysis of cytokines by multiplex Luminex in serum samples
Mice were anaesthetized with isoflurane, and blood samples were collected from the caudal vena cava, transferred to CapiJect serum separator tubes (3T-MG; Terumo Medical Corp., Elkton, MD, USA), allowed to clot for 30 min and centrifuged at 1000 rpm for 10 min. Serum was collected and stored at − 70°C until used for cytokine analysis by multiplex Luminex.
The Luminex protocol is a ‘sandwich-type’ immunoassay system that allows for the simultaneous detection of different cytokines in the Luminex-200 (Luminex Corp., Austin, TX, USA) dual-laser flow analyzer. Mouse serum samples were analyzed for interleukin-1β (IL-1β), IL-6, IL-10, IL-12(p70), G-CSF, granulocyte macrophage colony-stimulating factor (GM-CSF), keratinocyte-derived chemokine (KC) and tumor necrosis factor-α (TNF-α), as described earlier [42, 43]. Cytokine analysis kits were custom ordered (M200003JZX, Bio-Rad Inc., Hercules, CA) and included all necessary reagents for analysis. In brief, cytokine antibody-conjugated beads were added to each well of a flat-bottom 96-well plate (Bio-Rad Inc.). Serum samples were diluted 1:4, and samples were analyzed as per the manufacturer's instructions with slight modifications as described elsewhere [34, 44].
ELISA for estimation of FLT3 ligand in serum samples
FLT3 (fms-related tyrosine kinase 3) ligand measurement was carried out as described earlier [45]. Serum samples were collected and stored at –70°C until tested. Briefly, ELISA for FLT3 ligand was carried out as solid phase enzyme immunoassays with DuoSets (R&D systems, Inc., Minneapolis, MN, USA) based on the antibody sandwich principle. Absorbance was measured in a SpectraMax M5 Microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA) at 450 nm and was proportional to the concentration of cytokine present in samples. Standard curves were obtained by plotting known concentrations of respective cytokines versus absorbance. Results are presented as concentrations in pg/ml in serum.
Statistical analysis
Mean values with standard errors (when applicable) or percentages were reported. For comparing survival between groups at the end of 30 d, Fisher's exact test was used, with a Bonferroni adjustment to account for the pairwise comparisons that were made between groups. Analysis of variance (ANOVA) was used to detect whether there were significant differences between groups. If significant, a Tukey's post hoc test was used to determine significant differences between particular groups. ANOVA was used to detect whether there was a significant difference over a period of time for a particular group. If significant, a Scheffé post hoc test was used to locate the significance. To compare two different treatment groups, an independent t-test was used. The significance level was set at 5% for each test and all statistical tests were two-sided. Statistical software, SPSS, was used for statistical analyses.
RESULTS
Radio-mitigative potential of TS-mobilized progenitors against supralethal doses of irradiation
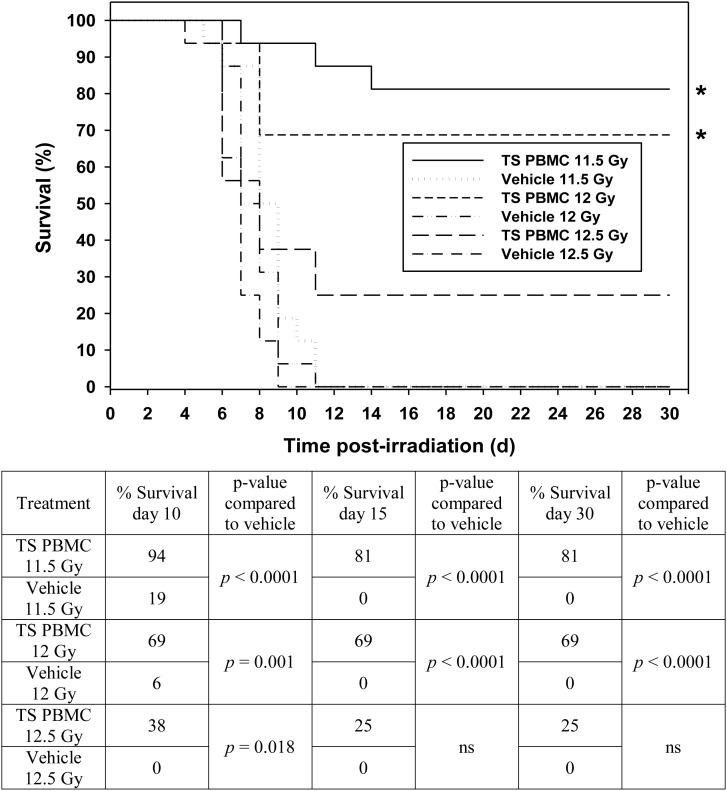
We were interested to investigate whether TS-mobilized progenitors protect mice against supralethal doses of radiation when administered 24 h after radiation exposure. TS was administered to donor mice 72 h prior to irradiation of recipient mice. AMD3100 was administered to all donor mice 1 h prior to blood collection for PBMC separation. The recipient mice were exposed to 11.5, 12.0, 12.5, 13.0 and 14.0 Gy 60Co γ-radiation doses, and then, 24 h after irradiation mice were transfused with 5 million PBMCs from TS-injected mice (details provided under ‘Materials and Methods’), and subsequently monitored for survival for 30 days post-irradiation. CD2F1 mice are relatively radio-resistant [46, 47] when compared with BALB/c or C3H animals, as indicated by the absence of the commonly noted early wave of deaths (at ∼6 d) after irradiation with radiation doses as high as 15 Gy. Data shown in Fig. 2 demonstrates significant protection by TS-mobilized progenitors from exposure to 11.5, 12.0 and 12.5 Gy on Day 10 compared with the vehicle control (P < 0.05). On Days 15 and 30, TS-mobilized progenitors significantly protected mice against 11.5 and 12.0 Gy radiation exposures (P < 0.001). On Day 30, TS-mobilized progenitors protected 81 and 69% of mice receiving 11.5 and 12.0 Gy radiation, respectively. Although the responses of test and control groups were not statistically different on Days 15 and 30 with 12.5 Gy, there was a significant positive trend. None of the vehicle groups had survivors, either on Day 15 or Day 30 after irradiation. On Day 10, the vehicle groups for 11.5 and 12.0 Gy had 19 and 6% survivors, respectively. TS-mobilized progenitors did not protect mice against 13.0 and 14.0 Gy 60Co γ-radiation (data presented in a supplemental figure).
Fig. 2.
Efficacy of TS-mobilized progenitors as a radio-mitigator against different doses of 60Co γ-radiation in mice. Five million PBMCs from TS-injected mice were transfused to each mouse 24 h after irradiation with 11.5 (n = 16), 12.0 (n = 16), and 12.5 (n = 16) Gy. Survival was monitored for 30 d. The table shows the statistical significance of the differences between vehicle control and TS-mobilized, progenitor-administered groups at each radiation dose on Days 10, 15 and 30 (ns denotes not significant).
Efficacy of TS-mobilized progenitors against radiation injury combined with wounding
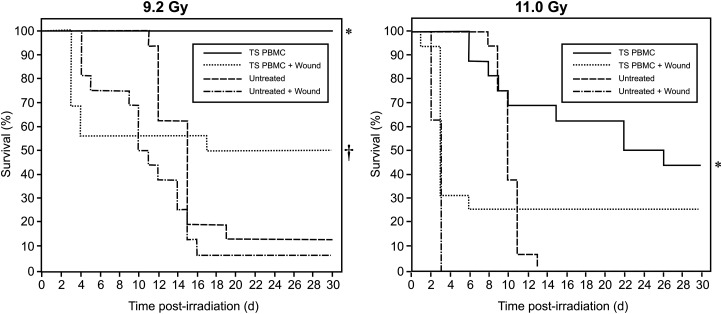
Blood was collected from TS-administered donor mice 72 h after TS injection for isolation of PBMCs. Two sets of four groups of mice (n = 16 per group) were irradiated either with 9.2 or 11 Gy. Two groups in each set were wounded 1 h following radiation exposure. A total of 5 million PBMCs obtained from donor mice were injected into each mouse of an irradiated and wounded group, and into another group treated with radiation alone. Survival was monitored for 30 d. A higher percentage of mice died in the groups receiving radiation exposure combined with wounding compared with those receiving radiation alone, and they died sooner than mice receiving only radiation exposure. In an experiment where 9.2 Gy was used, TS-mobilized PBMC treatment protected a significantly higher percentage of mice compared with respective controls (†combined injury, P < 0.05, or *irradiated only, P < 0.001) (Fig. 3, left panel). In an experiment with 11 Gy, TS-mobilized PBMC-treated mice had more survivors compared with the radiation control group (*denotes P < 0.01), but the difference between the wounded groups was not significant (Fig. 3, right panel). The body weight of the combined-injury group mice decreased compared with mice receiving only radiation exposure. In combined-injury mice, water intake increased. There was no significant difference in body weight or water intake between the TS-mobilized progenitor-administered, combined-injury group and its control.
Fig. 3.
Effect of TS-mobilized progenitors on survival of mice exposed to combined injury (irradiation and wounding). TS was administered to CD2F1 donor mice (n = 72), and blood was collected from donor mice 72 h after TS injection for isolation of PBMCS. Four groups of mice (n = 16 in each group) were irradiated either with 9.2 or 11 Gy. Two groups in each set were wounded within 1 h following radiation exposure. Five million PBMCs obtained from donor mice were injected 24 h after irradiation into each mouse of the group that had been irradiated and wounded, and also into the group treated with radiation alone. Untreated group mice did not receive cell transfusion. Survival was monitored for 30 d. In an experiment using 9.2 Gy, the TS-mobilized, PBMC-treated group had significantly higher survival compared with controls (†combined injury, P < 0.05, or *irradiated only, P < 0.001). In an experiment using 11 Gy, TS-mobilized, PBMC-treated mice had a higher number of survivors compared with its control (*P < 0.01), but the difference in the wounded groups was not significant.
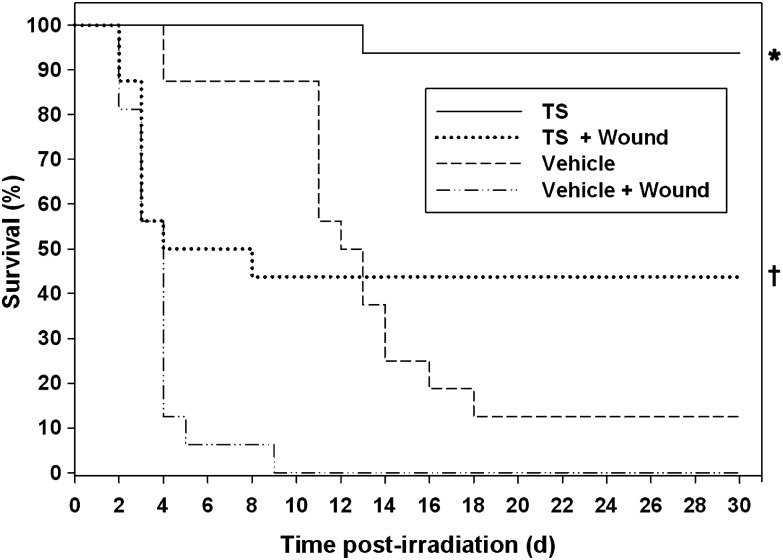
TS is a known radio-protector currently under development [23]. Based on our findings with TS-mobilized progenitors in a combined-injury model, we investigated whether TS alone can provide survival benefits in a combined-injury model. Four groups of mice (n = 16 in each group) were exposed to 9.2-Gy radiation. Out of these four groups, two were treated with TS (400 mg/kg) and two with the vehicle. Two groups (one with TS treatment and another with the vehicle) were wounded within 1 h of radiation exposure. As observed in the above-described experiment with combined injury, mortality was higher in groups receiving radiation exposure combined with wounding than in the group with radiation alone, and they died sooner than mice receiving only radiation exposure. TS treatment protected significantly higher numbers of mice compared with the vehicle control (*P < 0.001, Fig. 4). TS-treated and wounded mice had a higher number of survivors compared with the vehicle control (†P < 0.01).
Fig. 4.
Effect of TS treatment on the survival of mice with combined injury (exposed to 9.2 Gy 60Co γ-radiation and wounded). TS or vehicle was administered to mice (n = 16) 24 h before radiation exposure, and wounded groups were given a wound within 1 h following irradiation. Survival was monitored for 30 d. Mice treated with TS were protected significantly (*P < 0.001) compared with vehicle control. In combined-injury groups, TS treatment increased the number of survivors over vehicle-treated mice (†P < 0.01).
Effect of TS-mobilized progenitor administration on irradiation-induced cytokines in mice
Previous studies with other radiation countermeasures have shown a relationship between survival efficacy and an increase in levels of cytokines (G-CSF, KC and IL-6) in circulating blood [34, 44, 48]. We have proposed that these cytokines may serve as efficacy biomarkers for some radiation countermeasures. At the same time, irradiation also induces several cytokines, particularly G-CSF, and neutralization of radiation-induced G-CSF exacerbates radiation injury [49]. In addition, neutralization of G-CSF induced by radiation countermeasures abrogated radio-protective efficacy of some drugs [3, 44, 48, 50]. Thus, we wanted to determine whether the observed survival benefit afforded by TS-mobilized progenitors after total-body irradiation is associated with increased/decreased levels of cytokines in circulating blood.
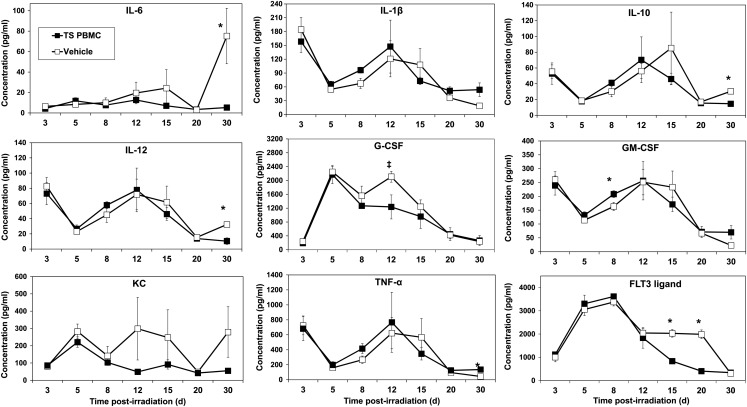
The time-course of cytokine response in circulating blood in irradiated mice in response to TS-mobilized progenitors or vehicle injection was determined by multiplex Luminex analyses. We analyzed cytokines in serum samples of two groups of mice, as shown in Fig. 5. These mice were irradiated at 8 Gy (60Co γ-radiation) and 5 million PBMCs from TS-injected mice were transfused to one group 24 h after irradiation. The second irradiated group was injected with the vehicle. Mice were exposed to a marginally lethal 8 Gy so that survivors would be present at 30 d after irradiation for cytokine analysis. Because TS-mobilized progenitor-derived cells were present in recipients, we were interested in determining cytokine levels at later time-points. Blood samples from these mice were collected 3, 5, 8, 12, 15, 20 and 30 d after irradiation and serum was separated for cytokine analysis. The results presented in Fig. 5 demonstrate that administration of TS-mobilized progenitors suppressed levels of G-CSF, IL-10, IL-6 and IL-12 at different time-points after irradiation. Although the levels of cytokines were clearly lower, albeit marginally in some cases (relative to matched controls) at selected time-points, the biological relevance of these responses is uncertain. Levels of FLT3 initially increased in both TS PMBC-treated and vehicle-treated mice. However, FLT3 ligand remained significantly elevated in vehicle-treated mice for a longer time until it finally decreased to low levels again at 30 d.
Fig. 5.
Time-course of cytokine levels in mice exposed to 8-Gy radiation and treated with TS-mobilized progenitors. CD2F1 donor mice received TS and blood was collected from donor mice for isolation of PBMCs as stated above. Two groups of mice (n = 8) were irradiated and were subsequently injected with either five million TS-mobilized PBMCs obtained from donor mice or vehicle. Blood samples for cytokine analysis were collected 3, 5, 8, 12, 15, 20 and 30 d after irradiation for serum cytokine analysis. *Denotes statistical significance of P < 0.05 between TS PBMCs and vehicle. ‡P = 0.058.
Effect of TS-mobilized progenitor administration on cytokine levels in mice receiving combined injury
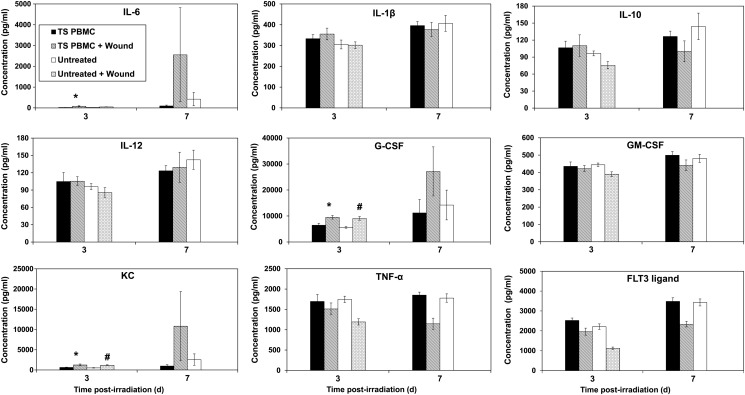
Four groups of mice were irradiated with 11 Gy. Two groups in each set were wounded 1 h following radiation exposure. A total of 5 million PBMCs obtained from donor mice were injected into groups of mice that had been either irradiated plus wounded or that had been irradiated alone. The remaining two groups were untreated controls receiving only cell transfusions. Blood samples from these mice were collected 3, 7, 10 and 15 d after irradiation, and serum was separated for cytokine analysis as described above. We could compare cytokine levels in various groups only in samples collected three and seven days after irradiation as the majority of mice died by the 10th day. Our results demonstrate that there were significantly higher levels of G-CSF, KC and IL-6 in irradiated and wounded mice receiving TS-mobilized progenitors compared with irradiated mice receiving TS PBMCs, suggesting that wounding enhanced the circulating levels of these cytokines (P < 0.05; Fig. 6). We also observed higher levels of G-CSF and KC in irradiated wounded mice (with no cell transfusion) compared with irradiated mice without wounding (P < 0.01), suggesting again that wounding stimulated production of these cytokines.
Fig. 6.
Time-course of cytokine levels in mice receiving radiation exposure and wounding, and treatment of TS-mobilized progenitors. PBMCs were collected from TS-injected mice as described above. Four groups of mice (n = 8) were irradiated with 11 Gy. Two groups were wounded 1 h following radiation exposure. Five million PBMCs from donor mice were injected into one of each group of irradiated and wounded mice, and irradiated only mice. Blood samples were collected from irradiated mice 3 and 7 d after irradiation and analyzed for serum cytokines. There were not enough survivors in the untreated wounded group 7 d post-irradiation for cytokine analysis. *Denotes statistical significance of P < 0.05 between TS PMBCs and TS PBMCs + Wounded, #P < 0.01 between untreated and untreated + wounded.
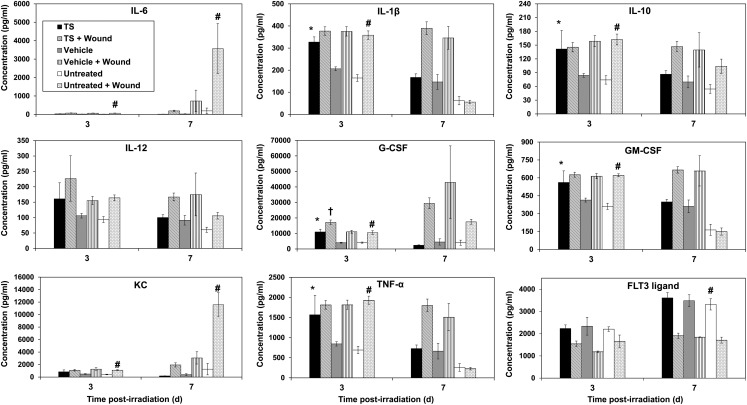
Since we observed increased survivors in combined injury mice pretreated with TS, we investigated cytokine levels in TS-treated animals at various time-points after irradiation. A total of six groups of mice were used; all receiving 9.2-Gy radiation exposure. Three groups were wounded 1 h after irradiation. Of these three groups, one was untreated, another was vehicle-treated, and the third was TS-treated (vehicle or TS treatment occurred 24 h before irradiation). The remaining three groups received similar treatments without wounding. Blood samples from these mice were collected 3, 7 and 10 d after irradiation and serum was separated for cytokine analysis, as described earlier. We could compare cytokine levels only in samples collected on Days 3 and 7 after irradiation as the majority of the mice had died by the10th day. TS-treated mice had higher levels of IL-1β, IL-10, G-CSF, GM-CSF and TNF-α compared with the vehicle group 3 d after irradiation (Fig. 7). Wounded mice pretreated with TS had higher levels of G-CSF on Day 3 after irradiation compared with wounded mice receiving vehicle treatment. Untreated wounded mice had higher levels of IL-1β, IL-10, G-CSF, KC, IL-6, GM-CSF and TNF-α compared with untreated mice without any wounding on Day 3 after irradiation. The untreated wounded group had lower levels of FLT3 ligand compared with mice without wounding on Day 7 after irradiation. These observations suggest rather profound effects of wounding on the blood levels of various cytokines.
Fig. 7.
Time-course of cytokine levels in mice receiving radiation exposure and wounding, and treatment of TS. TS or vehicle was injected to mice 24 h prior to irradiation. Mice (n = 8) were irradiated (9.2 Gy, dose rate 0.6 Gy/min), and one group of each treatment was wounded 1 h following radiation exposure. Blood samples were collected 3 and 7 d after irradiation for serum cytokine analysis. *Denotes statistical significance of P < 0.01 between TS and vehicle, †P < 0.001 between TS + wounded and vehicle + wounded, #P < 0.01 between untreated and untreated + wounded.
DISCUSSION
Difficulty obtaining sufficient hematopoietic stem cells directly from the donor has limited the clinical use of stem cell transplantation. Several attempts to stimulate the ex vivo growth of purified hematopoietic stem cells with cytokines and growth factors generally have induced only modest increases in hematopoietic stem cell numbers while decreasing their in vivo reconstituting ability. TS administration induces high levels of G-CSF in mice [24, 25, 51]. G-CSF is produced in the bone marrow, where it stimulates granulopoiesis. G-CSF has been shown to enhance the survival of irradiated mice and to minimize the effect of radiation on GI injury [52]. TS-induced G-CSF mobilizes hematopoietic progenitors from bone marrow into peripheral circulation [24]. G-CSF has also been shown to mobilize progenitors by several proposed mechanisms such as suppression of osteoblasts, disruption of the interactions between CXCR4 and chemokine ligand 12 (CXCL12, also known as SDF-1), and between very late antigen-4 (VLA-4) and vascular cell adhesion molecule 1 (VCAM 1). A number of other pathways are activated during G-CSF administration that may augment progenitor mobilization by modulating CXCR4 signaling [53]. Lai et al. have reported that a recombinant single-chain form of a naturally occurring murine hybrid cytokine of IL-7 and the beta chain of hepatocyte growth factor (rIL-7/HGFbeta), that stimulates the in vitro proliferation and/or differentiation of common lymphoid progenitors, pre-pro-B cells, and hematopoietic progenitor cells (Day 12 spleen colony-forming units) in cultures of mouse BM, efficiently induces transplantable murine hematopoietic stem cells [54]. Recently, we proposed a strategy to treat individuals who are at high risk of acute, high-dose ionizing radiation exposure (e.g. military personnel) using TS-mobilized hematopoietic progenitors [26, 27].
Our results demonstrated significant radio-mitigation by TS-mobilized progenitors following exposure to radiation doses as high as 11.5, 12.0 and 12.5 Gy, as indicated by survival on Day 10 post-exposure (Fig. 2). We reported earlier that TS-mobilized progenitors provide optimal radio-mitigation when administered 24 h after irradiation in mice. When TS-mobilized progenitors were administered 48 or 72 h after irradiation, radio-mitigation potential decreased but was still present [27]. Keeping in mind the above facts, we evaluated the efficacy of TS-mobilized progenitors against supralethal doses of radiation when cells were administered 24 h after radiation exposure. On 15 and 30 days after irradiation, TS-mobilized PBMC-treated groups had higher survival compared with their respective controls. At high radiation doses (11.5 and 12 Gy), 5 million TS-mobilized enriched PBMCs provided significant mitigation when administered 24 h after irradiation. At these supralethal radiation doses the primary cause of death is GI damage, as suggested by the early death of the control animals. Such observations suggest additional radio-mitigative effect(s) of transfused hematopoietic progenitors for repair and recovery of radiation-injured tissues and organ systems that extend beyond the primarily targeted hematopoietic system [55].
TS may be administered to select military personnel preparing for special missions having an increased risk of radiation exposures. Blood samples (either whole blood or PBMCs) of these individuals would be collected prior to a mission and stored frozen. If during the course of the mission an acute, high-dose exposure occurred, the exposed troop(s) would be removed from the field of operation and transported to a medical facility where a transfusion of their own, previously stored, blood/PBMC sample would be given in order to mitigate radiation injuries received. We consider this to be logistically feasible with minimal infrastructure because of the simplistic nature of this treatment protocol. We also studied the efficacy of gamma-tocotrienol, another component of vitamin E, in mobilizing progenitors for mitigation of radiation injury. Gamma tocotrienol-mobilized PBMCs also mitigated radiation injury in mice (unpublished observation).
Few animal models of radiation combined injury exist, and mechanisms underlying the high mortality associated with complex radiation injuries are poorly understood. Medical countermeasures currently are available for management of the non-radiation components of radiation combined injury, but it is not known whether treatment for other insults will be effective when the injury is combined with radiation exposure. Further research is needed to elucidate mechanisms behind the synergistic lethality of radiation combined injury and to identify targets for medical countermeasures. We demonstrated that as well as TS, TS-mobilized progenitors improve the numbers of survivors in mice receiving combined injury (radiation exposure and wounding). These treatments were effective when a 9.2-Gy dose of radiation (LD90/30) was used in combination with wounding. We observed no benefit when a higher dose of radiation (11 Gy) was used in combination with wounding. We have demonstrated earlier that TS mobilizes progenitors (Lin− sca-1+ and Lin− c-Kit+, Lin− sca-1+ and Lin− c-Kit+ cells) in peripheral circulation [24]. The survival sparing progenitors within TS-mobilized PBMC transfusates may include not only hematopoietic stems and early progenitors (as defined by the ckit+ lin− phenotype) but also additional, yet to be identified, progenitor subsets that have tissue reparative/renewal functions, such as innate lymphoid progenitors. It will be important to test TS-mobilized progenitors in other models of combined injury such as radiation-plus-burn, and radiation-plus-hemorrhage. Currently myeloid progenitors (mMPCs) are being developed as a promising radiation countermeasure for radiation-exposed victims [43]. Our observations suggest that mMPCs should be tested in a combined injury model.
Recently, we reported that G-CSF/IL-6 are principal mediators of the radio-protective efficacy of TS [24], 5-androstenediol [3], CBLB502 [44], and gamma-tocotrienol (unpublished observation). Our current study demonstrates that administration of TS-mobilized progenitors does not affect cytokine production to a great extent in irradiated mice (Fig. 5). Overall, the levels of cytokines were low and may not be relevant biologically [56, 57]. Our data suggest that TS-mobilized progenitor mitigation of radiation lethality is not mediated through an immediate effect on cytokine levels. Similar observations have been made earlier with mMPCs [43]. We did, however, observe a peak in some cytokines at different time-points after irradiation in the vehicle control mice compared with progenitor-treated mice. These cytokines play an important role in the inflammatory response [58–60], and their inhibition by progenitors indicates they may have anti-inflammatory activity. It needs to be investigated whether the reduction in cytokine levels after irradiation results from direct inhibition of cytokines by progenitors or from an indirect effect on hematopoietic injury in general. We have made similar observations with myeloid progenitors [43].
Our results, presented in Fig. 7, also suggest that wounding leads to induction of increased levels of IL-1β, IL-10, G-CSF, KC, IL-6, GM-CSF and TNF-α in irradiated mice. Some of these cytokines were also elevated by wounding (experimental data presented in Fig. 6). These cytokines may play a vital role in the radiation-induced injury recovery process. There are increased survivors among wounded irradiated mice when injury is administered before radiation exposure compared with injury after radiation exposure [61]. Such survival improvement in irradiated mice may be a result of increased cytokine levels induced by wounding. As stated above, we have shown the induction of various cytokines (particularly IL-6 and G-CSF) by different radiation countermeasures and their important roles in radio-protection [3, 25, 42, 44]. We have also demonstrated that irradiation-induced G-CSF plays a protective role in irradiated mice and that the use of neutralizing antibody to G-CSF exacerbates radiation injury [49]. Other investigators have also demonstrated increased levels of cytokines in wounded mice [9].
CONCLUSION
In summary, this study further supports our contention that the infusion of TS-mobilized progenitor-containing PBMCs acts as a bridging therapy. To the best of our knowledge, this is the first report demonstrating the efficacy of mobilized progenitors against radiation combined injury. This treatment may be useful for combined injury victims.
SUPPLEMENTARY DATA
Supplementary data is available at the Journal of Radiation Research online.
FUNDING
This study was supported by intramural awards RAB2CZ and RBB2GQ to V.K.S.
Supplementary Material
ACKNOWLEDGEMENTS
The opinions expressed here are those of the authors and should not be construed as official or reflecting the views of the Armed Forces Radiobiology Research Institute (AFRRI), the Uniformed Services University of Health Sciences, Bethesda, Maryland, or the Department of Defense, USA. We gratefully acknowledge Dr Cara Olsen's statistical help and the AFRRI's Veterinary Science Department and Cobalt Radiation Facility for their support during animal experimentation. The authors are thankful to Dr Mark H. Whitnall and Dr Juliann Kiang for helpful discussions.
REFERENCES
- 1.Carter AB, May MM, Perry WJ. The day after: action following a nuclear blast in a U.S. city. Washington Quarterly. 2007;30:19–32. [Google Scholar]
- 2.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 3.Grace MB, Singh VK, Rhee JG, et al. 5-AED enhances survival of irradiated mice in a G-CSF-dependent manner, stimulates innate immune cell function, reduces radiation-induced DNA damage and induces genes that modulate cell cycle progression and apoptosis. J Radiat Res. 2012;53:840–53. doi: 10.1093/jrr/rrs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin GC, McGeary M, McCutchen SR. Workshop Report. Washington, DC: The National Academies Press; 2009. Assessing medical preparedness to respond to a terrorist nuclear event. [PubMed] [Google Scholar]
- 5.Kishi HS. Effects of the “special bomb”: recollections of a neurosurgeon in Hiroshima, August 8–15, 1945. Neurosurgery. 2000;47:441–5. doi: 10.1097/00006123-200008000-00034. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 6.Iijima S. Pathology of atomic bomb casualties. Acta Pathol Jpn. 1982;32(Suppl 2):237–70. [PubMed] [Google Scholar]
- 7.Barabanova AV. Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanit Pregl. 2006;63:477–80. doi: 10.2298/vsp0605477b. [DOI] [PubMed] [Google Scholar]
- 8.Ledney GD, Elliott TB. Combined injury: factors with potential to impact radiation dose assessments. Health Phys. 2010;98:145–52. doi: 10.1097/01.HP.0000348466.09978.77. [DOI] [PubMed] [Google Scholar]
- 9.Kiang JG, Jiao W, Cary LH, et al. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2010;173:319–32. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valeriote FA, Baker DG. The combined effects of thermal trauma and X-irradiation on early mortality. Radiat Res. 1964;22:693–702. [PubMed] [Google Scholar]
- 11.Alpen EL, Sheline GE. The combined effects of thermal burns and whole body X irradiation on survival time and mortality. Ann Surg. 1954;140:113–8. doi: 10.1097/00000658-195407000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korlof B. Infection of burns. I. A bacteriological and clinical study of 99 cases. II. Animal experiments; burns and total body x-irradiation. Acta Chir Scand Suppl. 1956;209:1–144. [PubMed] [Google Scholar]
- 13.Brooks JW, Evans EI, Ham WT, Jr, et al. The influence of external body radiation on mortality from thermal burns. Ann Surg. 1952;136:533–45. doi: 10.1097/00000658-195209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxter H, Drummond JA, Stephens-Newsham LG, et al. Studies on acute total body irradiation in animals. I. Effect of streptomycin following exposure to a thermal burn and irradiation. Plast Reconstr Surg (1946) 1953;12:439–45. doi: 10.1097/00006534-195312000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Ledney GD, Elliott TB, Moore MM. Modulation of mortality by tissue trauma and sepsis in mice after radiation injury. In: Mossman KL, Mill WA, editors. The Biological Basis of Radiation Protection Practice. Baltimore: Williams and Wilkins; 1992. pp. 202–17. [Google Scholar]
- 16.DiCarlo AL, Maher C, Hick JL, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koenig KL, Goans RE, Hatchett RJ, et al. Medical treatment of radiological casualties: current concepts. Ann Emerg Med. 2005;45:643–52. doi: 10.1016/j.annemergmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Lausevic Z, Lausevic M, Trbojevic-Stankovic J, et al. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 2008;51:97–102. [PMC free article] [PubMed] [Google Scholar]
- 19.Zou Z, Sun H, Su Y, et al. Progress in research on radiation combined injury in China. Radiat Res. 2008;169:722–9. doi: 10.1667/RR1284.1. [DOI] [PubMed] [Google Scholar]
- 20.DiCarlo AL, Ramakrishnan N, Hatchett RJ. Radiation combined injury: overview of NIAID research. Health Phys. 2010;98:863–7. doi: 10.1097/HP.0b013e3181a6ee32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fliedner TM, Dorr HD, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Br J Radiol Suppl. 2005;27:1–8. [Google Scholar]
- 22.Singh VK, Singh PK, Wise SY, et al. Radioprotective properties of tocopherol succinate against ionizing radiation in mice. J Radiat Res. 2013;54:210–20. doi: 10.1093/jrr/rrs088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh PK, Wise SY, Ducey EJ, et al. Alpha-tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat Res. 2012;177:133–45. doi: 10.1667/rr2627.1. [DOI] [PubMed] [Google Scholar]
- 24.Singh VK, Brown DS, Kao TC. Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int J Radiat Biol. 2010;86:12–21. doi: 10.3109/09553000903264515. [DOI] [PubMed] [Google Scholar]
- 25.Singh PK, Wise SY, Ducey EJ, et al. Radioprotective efficacy of tocopherol succinate is mediated through granulocyte-colony stimulating factor. Cytokine. 2011;56:411–21. doi: 10.1016/j.cyto.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Singh VK, Brown DS, Kao TC, et al. Preclinical development of a bridging therapy for radiation casualties. Exp Hematol. 2010;38:61–70. doi: 10.1016/j.exphem.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Singh VK, Wise SY, Singh PK, et al. Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation-induced gastrointestinal injury in mice. Exp Hematol. 2012;40:407–17. doi: 10.1016/j.exphem.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Singh VK, Wise SY, Singh PK, et al. Alpha-tocopherol succinate-mobilized progenitors improve intestinal integrity after whole body irradiation. Int J Radiat Biol. 2013;89:334–45. doi: 10.3109/09553002.2013.762137. [DOI] [PubMed] [Google Scholar]
- 29.Uy GL, Rettig MP, Cashen AF. Plerixafor, a CXCR4 antagonist for the mobilization of hematopoietic stem cells. Expert Opin Biol Ther. 2008;8:1797–804. doi: 10.1517/14712598.8.11.1797. [DOI] [PubMed] [Google Scholar]
- 30.Gupta SK, Pillarisetti K, Thomas RA, et al. Pharmacological evidence for complex and multiple site interaction of CXCR4 with SDF-1alpha: implications for development of selective CXCR4 antagonists. Immunol Lett. 2001;78:29–34. doi: 10.1016/s0165-2478(01)00228-0. [DOI] [PubMed] [Google Scholar]
- 31.Jin F, Zhai Q, Qiu L, et al. Degradation of BM SDF-1 by MMP-9: the role in G-CSF-induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008;42:581–8. doi: 10.1038/bmt.2008.222. [DOI] [PubMed] [Google Scholar]
- 32.Hatse S, Princen K, Bridger G, et al. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 33.Damon LE. Mobilization of hematopoietic stem cells into the peripheral blood. Expert Rev Hematol. 2009;2:717–33. doi: 10.1586/ehm.09.54. [DOI] [PubMed] [Google Scholar]
- 34.Singh VK, Ducey EJ, Fatanmi OO, et al. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat Res. 2012;177:628–42. doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 35.National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 36.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flomenberg N, Devine SM, Dipersio JF, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–74. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 38.Pulliam AC, Hobson MJ, Ciccone SL, et al. AMD3100 synergizes with G-CSF to mobilize repopulating stem cells in Fanconi anemia knockout mice. Exp Hematol. 2008;36:1084–90. doi: 10.1016/j.exphem.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy VV. Accuracy considerations in EPR dosimetry. Appl Radiat Isot. 2000;52:1039–50. doi: 10.1016/s0969-8043(00)00052-x. [DOI] [PubMed] [Google Scholar]
- 41.ISO-ASTM. Standard practice for use of an alanine dosimetry. ISO/ASTM International Standard. 2004:5167. [Google Scholar]
- 42.Shakhov AN, Singh VK, Bone F, et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2) PLOS ONE. 2012;7 doi: 10.1371/journal.pone.0033044. e33044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh VK, Christensen J, Fatanmi OO, et al. Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res. 2012;177:781–91. doi: 10.1667/rr2894.1. [DOI] [PubMed] [Google Scholar]
- 44.Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther. 2012;343:497–508. doi: 10.1124/jpet.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenthal RD, Reising A, Leon E, et al. Modulation of marrow proliferation and chemosensitivity by tumor-produced cytokines from syngeneic pancreatic tumor lines. Clin Cancer Res. 2002;8:1301–9. [PubMed] [Google Scholar]
- 46.Iwakawa M, Noda S, Ohta T, et al. Different radiation susceptibility among five strains of mice detected by a skin reaction. J Radiat Res. 2003;44:7–13. doi: 10.1269/jrr.44.7. [DOI] [PubMed] [Google Scholar]
- 47.Lindsay KJ, Coates PJ, Lorimore SA, et al. The genetic basis of tissue responses to ionizing radiation. Br J Radiol. 2007;80:S2–6. doi: 10.1259/bjr/60507340. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 48.Singh VK, Ducey EJ, Brown DS, et al. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol. 2012;88:296–310. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- 49.Singh VK, Fatanmi OO, Singh PK, et al. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine. 2012;58:406–14. doi: 10.1016/j.cyto.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni S, Singh PK, Ghosh SP, et al. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62:278–85. doi: 10.1016/j.cyto.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Singh VK, Brown DS, Kao TC. Tocopherol succinate: a promising radiation countermeasure. Int Immunopharmacol. 2009;9:1423–30. doi: 10.1016/j.intimp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Kim JS, Ryoo SB, Heo K, et al. Attenuating effects of granulocyte-colony stimulating factor (G-CSF) in radiation induced intestinal injury in mice. Food Chem Toxicol. 2012;50:3174–80. doi: 10.1016/j.fct.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 53.Singh VK, Singh PK, Wise SY, et al. Mobilized progenitor cells as a bridging therapy for radiation casualties: a brief review of tocopherol succinate-based approaches. Int Immunopharmacol. 2011;11:842–47. doi: 10.1016/j.intimp.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Lai L, Zhang M, Goldschneider I. Recombinant IL-7/HGFbeta efficiently induces transplantable murine hematopoietic stem cells. J Clin Invest. 2012;122:3552–62. doi: 10.1172/JCI46055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mason KA, Withers HR, McBride WH, et al. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 1989;117:480–8. [PubMed] [Google Scholar]
- 56.Farese AM, MacVittie TJ, Roskos L, et al. Hematopoietic recovery following autologous bone marrow transplantation in a nonhuman primate: effect of variation in treatment schedule with PEG-rHuMGDF. Stem Cells. 2003;21:79–89. doi: 10.1634/stemcells.21-1-79. [DOI] [PubMed] [Google Scholar]
- 57.Johnston E, Crawford J, Blackwell S, et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol. 2000;18:2522–8. doi: 10.1200/JCO.2000.18.13.2522. [DOI] [PubMed] [Google Scholar]
- 58.Schooltink H, Rose-John S. Cytokines as therapeutic drugs. J Interferon Cytokine Res. 2002;22:505–16. doi: 10.1089/10799900252981981. [DOI] [PubMed] [Google Scholar]
- 59.Dinarello CA. A clinical perspective of IL-1 beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–17. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 60.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ledney GD, Elliott TB. Combined injury: factors with potential to impact radiation dose assessments. Health Phys. 2010;98:145–52. doi: 10.1097/01.HP.0000348466.09978.77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.