Abstract
The aim of this study was to evaluate the interfractional prostate motion of patients immobilized in the prone position using a thermoplastic shell. A total of 24 patients with prostate calcifications detectable using a kilo-voltage X-ray image-guidance system (ExacTrac X-ray system) were examined. Daily displacements of the calcification within the prostate relative to pelvic bony structures were calculated by the ExacTrac X-ray system. The average displacement and standard deviation (SD) in each of the left–right (LR), anterior–posterior (AP), and superior–inferior (SI) directions were calculated for each patient. Based on the results of interfractional prostate motion, we also calculated planning target volume (PTV) margins using the van Herk formula and examined the validity of the PTV margin of our institute (a 9-mm margin everywhere except posteriorly, where a 6-mm margin was applied). In total, 899 data measurements from 24 patients were obtained. The average prostate displacements ± SD relative to bony structures were 2.8 ± 3.3, −2.0 ± 2.0 and 0.2 ± 0.4 mm, in the SI, AP and LR directions, respectively. The required PTV margins were 9.7, 6.1 and 1.4 mm in the SI, AP and LR directions, respectively. The clinical target volumes of 21 patients (87.5%) were located within the PTV for 90% or more of all treatment sessions. Interfractional prostate motion in the prone position with a thermoplastic shell was equivalent to that reported for the supine position. The PTV margin of our institute is considered appropriate for alignment, based on bony structures.
Keywords: intensity-modulated radiotherapy, interfractional prostate motion, prone position, prostate cancer, thermoplastic shell
INTRODUCTION
Several studies have been conducted to determine whether a supine or prone fixation position is superior for external beam radiotherapy (EBRT) in patients with prostate cancer [1–11]. However, the optimal treatment position for prostate EBRT remains controversial and inconclusive because each position has its own merits and demerits. The merit of the prone position is that the irradiation dose to the rectum is reduced because the seminal vesicles are pulled away from the rectum [1, 11]. It has also been reported that the geometric relationship between the prostate and pelvic bony anatomy is more consistent in the prone position [5], a finding that is very important for centers that use bony-structure-based positioning. Conversely, a demerit of the prone position is the greater prostate motion compared with the supine position [3, 4]. Additionally, the supine position is more comfortable for patients and more convenient for therapists than the prone position [3].
We have treated patients with localized prostate cancer using intensity-modulated radiotherapy (IMRT) in the prone position fixed with a thermoplastic shell since 2000. We adopted the prone position for two reasons. First, the rectal dose is reduced because we treat mainly locally advanced prostate cancer patients in whom the seminal vesicles are included in the clinical target volume (CTV) [11]. Second, we applied bony-structure-based positioning because we had no soft-tissue-based image-guided radiotherapy (IGRT) options when we first began using IMRT for prostate cancer. To compensate for the expected large prostate motion in the prone position, we immobilized patients by using a thermoplastic shell.
To our knowledge, only three reports have examined interfractional prostate motion in the prone position when immobilized with a thermoplastic shell [3, 12, 13]. However, those reports did not document sufficient data with systematic and random errors to calculate the required planning target volume (PTV) margin because they examined only three additional computed tomography (CT) data measurements per patient or assessed the lateral portal image alone. Therefore, no report to date has determined the adequate PTV margin in the prone position while immobilized using a thermoplastic shell.
Beginning in 2007, we were able to use a dual-orthogonal kilo-voltage (kV) X-ray IGRT system (ExacTrac X-ray system; BrainLAB AG, Feldkirchen, Germany) for patient positioning. This system also allows detection of calcification within the prostate (Fig. 1). Because calcification in the prostate is a reliable marker of prostate position [14], using this IGRT system we are able to assess interfractional prostate motion in all fractions without the need for an invasive procedure, based on routinely acquired clinical data. The aim of the present study was to evaluate interfractional prostate motion in patients immobilized with a thermoplastic shell in the prone position. Additionally, we validated the adequacy of the PTV margin applied at our institute.
Fig. 1.
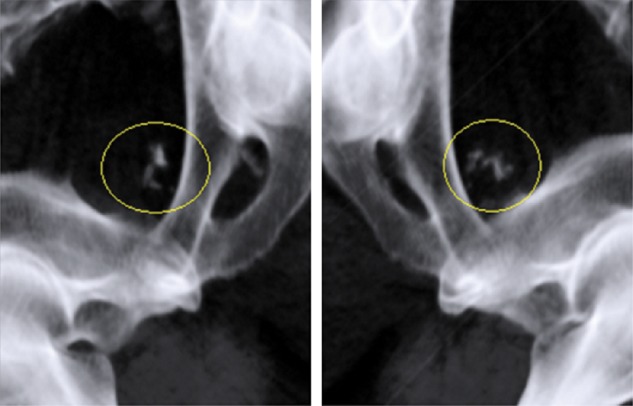
Examples of ExacTrac X-ray images. Intra-prostatic calcifications were clearly detected (yellow circle).
MATERIALS AND METHODS
Patients' characteristics
Between August 2007 and August 2010, 163 consecutive patients with localized prostate cancer (cT1c-T4N0M0) received IMRT using Novalis (BrainLAB AG) at our institute. Of these, the ExacTrac X-ray system clearly detected calcification within the prostate in 24 patients. Since all of the system's image data for localization were stored and accessible, we included these 24 patients in the present analyses. The median age of the study population was 72 years (range, 59–80 years). T-stage was 17 with T1c–T2b, 3 with T2c, and 4 with T3a. The median prostate-specific antigen (PSA) and Gleason scores were 10.55 ng/ml and 7, respectively. Patients' characteristics are summarized in Table 1. Witten informed consent was obtained from the patients to use their clinical data for research purposes and for publication.
Table 1.
Patients' characteristics and treatment parameters
| Age (years) | 59–80 (median, 72) |
| T stage (UICC 2002) | |
| T1c–T2b | 17 |
| T2c | 3 |
| T3a | 4 |
| Gleason score | |
| ≤6 | 7 |
| 7 | 8 |
| ≥8 | 9 |
| Initial PSA value (ng/ml) | 5.03–49.00 (median, 10.55) |
| NAHT period (months) (CAB) | 4–21 (median, 7) |
| RT dose (Gy) | |
| 70 | 3 |
| 74 | 12 |
| 78 | 9 |
UICC = classification of the International Union Against Cancer, NAHT = neoadjuvant hormonal therapy, CAB = combined androgen blockade, PSA = prostate-specific antigen, RT = radiotherapy.
Radiation therapy
Each patient underwent pretreatment planning CT scans (LightSpeed RT; GE Healthcare, Waukesha, WI, USA) of 2.5-mm slice thickness. All patients were instructed to void the bladder and rectum about 1–1.5 h before the CT simulation, according to their individual urinary conditions. In actual treatments, patients were also required to void the bladder and rectum at exactly the same timing as set in the CT simulation. In addition, the treatment time was fixed by each patient to maintain patients' condition. Patients were immobilized in the prone position with a thermoplastic shell (Hip Fix system; CIVCO Medical Solutions, Kalona, IA, USA) that extended from the mid-thigh to the upper third of the leg, in combination with a vacuum pillow (Vac-Lok system; CIVCO Medical Solutions) and a leg support (Fig. 2). Details of our planning protocol have been reported previously [15, 16]. Briefly, the CTV consisted of the prostate and seminal vesicles (base to the whole depending on the clinical stage), not including the lymph node area. The PTV was the CTV plus a 9-mm margin everywhere except posteriorly, where a 6-mm margin was applied. The PTV was treated with a dose of between 70 Gy in 35 fractions and 78 Gy in 39 fractions (median, 74 Gy). Patients were positioned based on their pelvic bone structures using the ExacTrac X-ray system immediately before each treatment session.
Fig. 2.
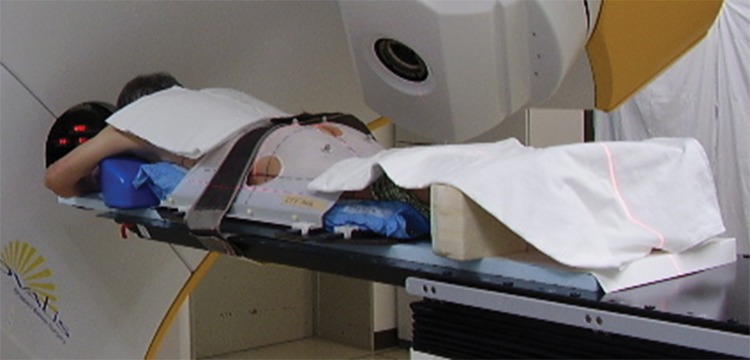
Patient fixed in the prone position with the combination of a thermoplastic shell, a vacuum pillow and a leg support.
Analysis of interfractional prostate motion
Displacement of the position of a calcification within the prostate between each daily session and the DRR image at the CT simulation was calculated based on the ExacTrac data. If more than one calcification was identified within the prostate, the largest one was used to calculate the position. The average displacement and the standard deviation (SD) in each of the left–right (LR), anterior–posterior (AP), and superior–inferior (SI) directions were calculated for each patient.
Calculation of the required PTV margin
The required PTV margins were generated using the van Herk formula (2.5Σ + 0.7σ) [17]. The Σ was calculated as the SD of the mean displacement for each individual patient. The σ was determined by computing the root mean square of the SD of an individual patient's displacements. This method is intended to guarantee that 90% of patients receive a minimum cumulative CTV dose of at least 95% of the prescribed dose.
Comparison of prostate motion with other reports and validation of the PTV margin
To validate the adequacy of the applied PTV margin, we calculated the required PTV margin using the van Herk formula to compare prostate motion with other reports, which often report Σ and σ values [8, 18–24], because we believe that the calculated PTV margin expresses the total possible variation of prostate motion simply.
In addition, we generated accumulated CTV dose reflecting interfractional prostate motion. This allowed estimation of actually delivered target dose and was generated as follows. First, isocenter-shifted plans, in which the isocenter was shifted to compensate for the corresponding positional error of the prostate by each fraction, were generated for every patient. Then, all plans of the individual patient were summed up on the Eclipse (ver. 8.6) treatment-planning system (Varian Medical Systems, Palo Alto, CA, USA), and the accumulated CTV dose was calculated. We assessed the dose–volume histogram (DVH) of this accumulated CTV dose. We also evaluated the probability of prostate motion coverage within our clinical PTV margin.
RESULTS
In total, 899 ExacTrac X-ray system data measurements from 24 patients were available for registration of bony structures and calcification. The average prostate displacements ± SD relative to the bony structure were −2.0 ± 2.0, 2.8 ± 3.3 and 0.2 ± 0.4 mm, in the AP, SI and LR directions, respectively. There was a tendency for a large shift in the SI direction, and displacement of more than 10 mm was observed only in the SI direction. The displacement in the LR direction was generally small.
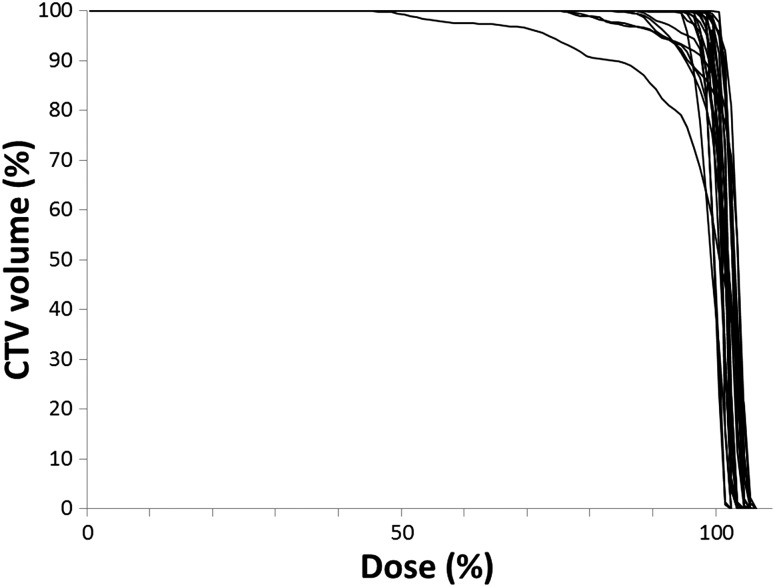
Table 2 summarizes the means, SDs, ranges, Σ, σ and PTV margins of interfractional prostate displacement in the AP, SI and LR directions. The Σ values of the AP, SI and LR directions were 2.0, 3.3 and 0.4 mm, respectively, and the σ values were 1.6, 2.2 and 0.7 mm, respectively. Both Σ and σ were also largest in the SI direction. The PTV margins calculated using the van Herk formula were 6.1, 9.7 and 1.4 mm in the AP, SI and LR directions, respectively. The prostate displacement of 21 patients (87.5%) was within the applied PTV margins (9-mm margin everywhere except posteriorly, where a 6-mm margin was applied) in 90% or more of all treatment sessions. With regard to the DVH of the accumulated CTV dose, the average mean dose ± SD and the dose at the 95% volume level of the cumulative DVH (D95) ± SD of the CTV were 100.7 ± 1.3% (range, 96.7–102.4%) and 96.2 ± 5.8% (range, 73.1–100.7%), respectively (Fig. 3).
Table 2.
Interfractional prostate displacement
| AP (mm) | SI (mm) | LR (mm) | |
|---|---|---|---|
| Mean | −2.0 | 2.8 | 0.2 |
| Minimum | −9.6 | −11.2 | −3.4 |
| Maximum | 6.0 | 15.4 | 4.7 |
| Σ | 2.0 | 3.3 | 0.4 |
| σ | 1.6 | 2.2 | 0.6 |
LR = left–right, AP = anterior–posterior, SI = superior–inferior, + = motion to the right/posterior/superior directions about isocenter, Σ = the SD of the average displacement for each individual patient, σ = the root mean square of the individual SD.
Fig. 3.
Dose–volume histograms of the accumulated CTV dose for all patients. The acceptable CTV coverage of D95 ≥ 95% was achieved in about 80% of the patients (n = 19).
DISCUSSION
Table 3 summarizes previously reported PTV margins for interfractional organ motion errors relative to a bony structure [8, 18–24]. The data include seven studies in the supine position without a thermoplastic shell, one in the supine position with a thermoplastic shell, and one prone position without a thermoplastic shell. Their calculated PTV margins were 4.7–10.5 mm (mean, 8.0 mm), 4.0–12.0 mm (mean, 8.5 mm) and 1.4–4.5 mm (mean, 3.2 mm) in the AP, SI and LR directions, respectively. Our results fall within the range of those reported previously, indicating that the prostate motion relative to the bony structure in individuals immobilized in the prone position using a thermoplastic shell is comparable to that in the supine position.
Table 3.
Comparison of required PTV margins calculated from interfractional errors relative to a bony structure using the van Herk formula
| Position | Thermoplastic shell | No. of Patients | Method | No. of data | Margin (mm) |
|||
|---|---|---|---|---|---|---|---|---|
| AP | SI | LR | ||||||
| Supine | − | Bylund et al. [19] | 24 | MVCT without M | 972 | 7.0 | 4.0 | 3.1 |
| Tanyi et al. [24] | 14 | CBCT with M | 546 | 10.2 | 8.9 | 1.6 | ||
| Nederveen et al. [21] | 23 | EPID with M | 675 | 7.5 | 11.9 | 3.1 | ||
| Osei et al. [23] | 20 | EPID with M | 642 | 6.6 | 7.4 | 4.4 | ||
| Alonso-Arrizabalaga et al. [18] | 30 | ExacTrac with M | 1330 | 10.5 | 12.0 | 4.1 | ||
| O'Daniel et al. [22] | 10 | kV CT without M | 243 | 10.4 | 8.6 | 2.8 | ||
| Stroom et al. [30] | 15 | kV CT without M | 240 | 8.3 | 8.2 | 4.0 | ||
| + | Khosa et al. [20] | 10 | EPID with M | 180 | 4.7 | 7.3 | 3.6 | |
| Prone | − | Stroom et al. [30] | 15 | kV CT without M | 240 | 8.8 | 6.6 | 3.7 |
| + | This study | 24 | ExacTrac with calcification | 899 | 6.1 | 9.7 | 1.4 | |
CT = computed tomography, MVCT = megavoltage cone beam CT, M = fiducial marker, CBCT = cone beam CT, EPID = electronic portal imaging device, AP = anterior-posterior; SI = superior-inferior, LR = left-right, ExacTrac = ExacTrac X-ray system, kV = kilo-volt.
To our knowledge, only three publications have examined prostate motion in patients immobilized with a thermoplastic shell in the prone position. Takayama et al. [12] reported prostate motion using three additional CT scans in seven patients, with or without a double-balloon rectal catheter. The mean prostate displacements ± SD were 2.8 ± 1.8, 2.7 ± 1.8 and 1.3 ± 0.7 mm in the AP, SI and LR directions, respectively. Zelefsky et al. [13] also reported interfractional prostate motion in 50 patients, as determined by using three additional CT scans. The mean prostate displacements ± SD were − 1.2 ± 2.9, −0.5 ± 3.3 and −0.6 ± 0.8 mm in the AP, SI and LR directions, respectively. Bayley et al. [3] examined 20 patients randomized with regard to treatment when immobilized in the supine or prone position, and measured prostate motion using the daily lateral film to compare bony landmarks and fiducial marker positions. The mean prostate motions ± SD in the prone position were 0.7 ± 4.0 and 0.7 ± 3.7 mm in the AP and SI directions, respectively. Although these reports did not include Σ and σ data, which enable calculation of the required PTV margin, the SD results were similar to those we report here.
Compared with other reports fixed in the supine position without any fixation devices, our study resulted in smaller displacements in the AP direction and comparable displacements in other directions (Table 3). It is reported that the prone position without any fixation devices produced greater prostate motion than the supine position [3, 4]. We believe those results were mainly due to the respiratory motion of the chest and abdomen. That is, the respiratory motion easily affects the prostate position in the prone position because chest and abdomen are touching the couch. In contrast, application of a thermoplastic shell can contribute to restricting respiratory-related movement. In addition, prostate locations in the prone position are less influenced by rectal gas than those in the supine position, because the rectal gas tends to move and be stored in part of the rectosigmoid (Fig. 4). On the other hand, rectal gas can easily push the prostate upwards in the supine position, as indicated in Fig. 5. A smaller displacement in the AP direction was also reported by Khosa et al. but using the supine position and immobilization with a thermoplastic shell [20]. As indicated before, rectal gas in the supine position without fixation devices can greatly affect the prostate position in the AP direction (Fig. 5). It is believed that the use of a thermoplastic shell in the supine position can contribute to restriction of severe rectal dilatation by gas because of the increased abdominal pressure from the thermoplastic shell. To date and to our knowledge, no study has compared prostate motion in the same position with and without a thermoplastic shell. More detailed studies are necessary in the future, but the use of a thermoplastic shell probably reduces interfractional prostate movement in the AP direction.
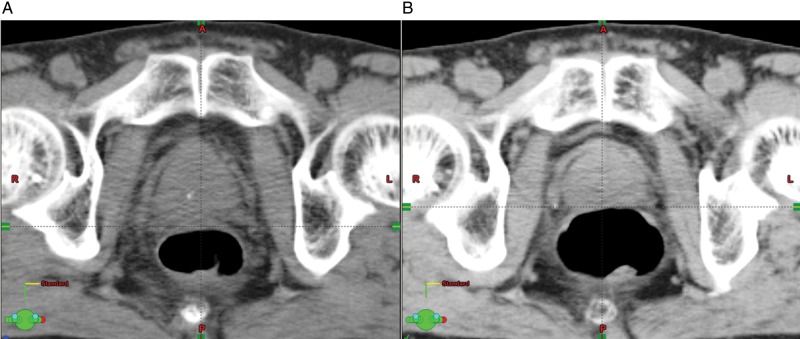
Fig. 4.
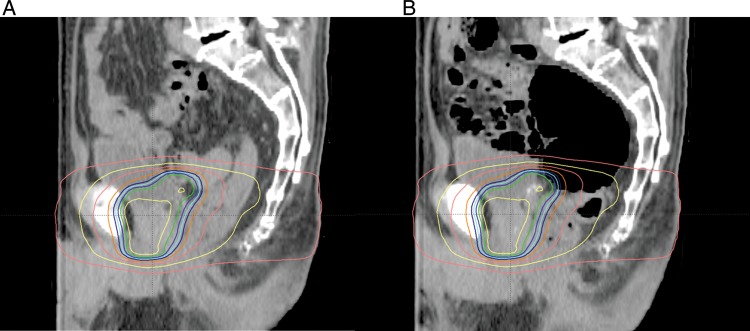
An example of the impact of rectal gas on the prostate position in the prone fixation. (A) Sagittal view of simulation CT with dose distribution curves. (B) Sagittal view of follow-up CT superimposed on dose distribution curves at planning. Although a large amount of rectal gas exists, the prostate dose was maintained because most of the gas is located in the rectosigmoid region of the rectum.
Fig. 5.
An example of impact of rectal gas on the prostate position in the supine position. (A) Axial view of simulation CT; (B) Axial view of follow-up CT. The amount of the rectal gas directly affects the position of the prostate.
Our required PTV margin using the van Herk formula (9.7, 6.1 and 1.4 mm in the SI, AP and LR directions, respectively) is similar to the PTV margin of our institute. Indeed, the CTV of 21 patients (87.5%) was within our PTV margins for 90% or more of all treatment sessions. Additionally, for the DVH of the accumulated CTV dose, dose coverage of the CTV was almost satisfied. In fact, it was reported that a 1.5-cm PTV margin had no significant impact on the PSA control rate, but had a significantly negative impact on late rectal damage, compared with a 1.0-cm margin [25]. Therefore, our PTV margin may be relevant as far as fitting to the bony structure in the prone position in a patient immobilized with a thermoplastic shell. However, to prevent delivery of an insufficient dose to the CTV in patients with relatively large prostate movements, it will be necessary to shift to the prostate-based IGRT approach. Indeed, we began to use this prostate IMRT approach in 2010.
The present study has the limitation that using only the largest calcification within the prostate cannot account for prostate rotation. However, previous study has indicated that rotation errors are small, and rotation alignment offers only 1-2 mm advantage compared with translational shift alone [26]. Another limitation is that the intrafractional prostate motion was not considered. Several reports on intrafractional prostate motion have been published to date [4, 9, 10, 27–29]. However, intrafractional prostate motion in the prone position in those immobilized with a thermoplastic shell has not yet been assessed. We are currently investigating intrafractional prostate motion, and will report the data in the near future.
Compared with other reports, our results demonstrate that the effect of immobilization with a thermoplastic shell in the prone position is comparable to that in the supine position. The superiority of the geometric relationship between the prostate and pelvic bony anatomy in the prone position compared with that in the supine position [5] was not determined in the present study. However, if identical PTV margins are necessary in both positions, the prone position may be preferable due to the reduced irradiation dose to the rectum [11]. Interfractional prostate motion in those immobilized in the prone position using a thermoplastic shell is equivalent to that in the supine position reported elsewhere. The PTV margin of our institute is generally appropriate when aligned to the bony structure, although prostate-based positioning will be required for patients with a large prostate motion.
FUNDING
This work was supported by Grants-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (24591838), Japan. The sponsor played no role in the study design, in the collection, analysis and interpretation of the data, in the writing of this manuscript, or in the decision to submit this manuscript for publication.
ACKNOWLEDGEMENTS
This work has been presented at the 24th Annual Meeting of the JASTRO.
REFERENCES
- 1.O'Neill L, Armstrong J, Buckney S, et al. A phase II trial for the optimisation of treatment position in the radiation therapy of prostate cancer. Radiother Oncol. 2008;88:61–6. doi: 10.1016/j.radonc.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Althof VG, Hoekstra CJ, te Loo HJ. Variation in prostate position relative to adjacent bony anatomy. Int J Radiat Oncol Biol Phys. 1996;34:709–15. doi: 10.1016/0360-3016(95)02162-0. [DOI] [PubMed] [Google Scholar]
- 3.Bayley AJ, Catton CN, Haycocks T, et al. A randomized trial of supine vs. prone positioning in patients undergoing escalated dose conformal radiotherapy for prostate cancer. Radiother Oncol. 2004;70:37–44. doi: 10.1016/j.radonc.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura K, Shirato H, Seppenwoolde Y, et al. Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int J Radiat Oncol Biol Phys. 2002;53:1117–23. doi: 10.1016/s0360-3016(02)02882-1. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, Lerma FA, Patel S, et al. Dosimetric effects of the prone and supine positions on image guided localized prostate cancer radiotherapy. Radiother Oncol. 2008;88:67–76. doi: 10.1016/j.radonc.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin PW, Wygoda A, Sahijdak W, et al. The effect of patient position and treatment technique in conformal treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;45:407–13. doi: 10.1016/s0360-3016(99)00207-2. [DOI] [PubMed] [Google Scholar]
- 7.Shah AP, Kupelian PA, Willoughby TR, et al. An evaluation of intrafraction motion of the prostate in the prone and supine positions using electromagnetic tracking. Radiother Oncol. 2011;99:37–43. doi: 10.1016/j.radonc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Stroom JC, Koper PC, Korevaar GA, et al. Internal organ motion in prostate cancer patients treated in prone and supine treatment position. Radiother Oncol. 1999;51:237–48. doi: 10.1016/s0167-8140(99)00061-4. [DOI] [PubMed] [Google Scholar]
- 9.Vargas C, Saito AI, Hsi WC, et al. Cine-magnetic resonance imaging assessment of intrafraction motion for prostate cancer patients supine or prone with and without a rectal balloon. Am J Clin Oncol. 2010;33:11–6. doi: 10.1097/COC.0b013e31819fdf7c. [DOI] [PubMed] [Google Scholar]
- 10.Wilder RB, Chittenden L, Mesa AV, et al. A prospective study of intrafraction prostate motion in the prone vs. supine position. Int J Radiat Oncol Biol Phys. 2010;77:165–70. doi: 10.1016/j.ijrobp.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Happersett L, Leibel SA, et al. The effect of treatment positioning on normal tissue dose in patients with prostate cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1997;37:13–9. doi: 10.1016/s0360-3016(96)00460-9. [DOI] [PubMed] [Google Scholar]
- 12.Takayama K, Mizowaki T, Negoro Y, et al. Impact of double-balloon rectal catheter use in external-beam radiotherapy for prostate cancer. Int J Clin Oncol. 2011;16:50–6. doi: 10.1007/s10147-010-0129-7. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Crean D, Mageras GS, et al. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol. 1999;50:225–34. doi: 10.1016/s0167-8140(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 14.Zeng GG, McGowan TS, Larsen TM, et al. Calcifications are potential surrogates for prostate localization in image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:963–6. doi: 10.1016/j.ijrobp.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Norihisa Y, Mizowaki T, Takayama K, et al. Detailed dosimetric evaluation of intensity-modulated radiation therapy plans created for stage C prostate cancer based on a planning protocol. Int J Clin Oncol. 2012;17:505–11. doi: 10.1007/s10147-011-0324-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhu SY, Mizowaki T, Norihisa Y, et al. Comparisons of the impact of systematic uncertainties in patient setup and prostate motion on doses to the target among different plans for definitive external-beam radiotherapy for prostate cancer. Int J Clin Oncol. 2008;13:54–61. doi: 10.1007/s10147-007-0724-4. [DOI] [PubMed] [Google Scholar]
- 17.van Herk M, Remeijer P, Rasch C, et al. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–35. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Arrizabalaga S, Brualla Gonzalez L, Rosello Ferrando JV, et al. Prostate planning treatment volume margin calculation based on the ExacTrac X-Ray 6D image-guided system: margins for various clinical implementations. Int J Radiat Oncol Biol Phys. 2007;69:936–43. doi: 10.1016/j.ijrobp.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 19.Bylund KC, Bayouth JE, Smith MC, et al. Analysis of interfraction prostate motion using megavoltage cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;72:949–56. doi: 10.1016/j.ijrobp.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Khosa R, Nangia S, Chufal KS, et al. Daily online localization using implanted fiducial markers and its impact on planning target volume for carcinoma prostate. J Cancer Res Ther. 2010;6:172–8. doi: 10.4103/0973-1482.65244. [DOI] [PubMed] [Google Scholar]
- 21.Nederveen AJ, Dehnad H, van der Heide UA, et al. Comparison of megavoltage position verification for prostate irradiation based on bony anatomy and implanted fiducials. Radiother Oncol. 2003;68:81–8. doi: 10.1016/s0167-8140(03)00129-4. [DOI] [PubMed] [Google Scholar]
- 22.O'Daniel JC, Dong L, Zhang L, et al. Dosimetric comparison of four target alignment methods for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:883–91. doi: 10.1016/j.ijrobp.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Osei EK, Jiang R, Barnett R, et al. Evaluation of daily online set-up errors and organ displacement uncertainty during conformal radiation treatment of the prostate. Br J Radiol. 2009;82:49–61. doi: 10.1259/bjr/58088207. [DOI] [PubMed] [Google Scholar]
- 24.Tanyi JA, He T, Summers PA, et al. Assessment of planning target volume margins for intensity-modulated radiotherapy of the prostate gland: role of daily inter- and intrafraction motion. Int J Radiat Oncol Biol Phys. 2010;78:1579–85. doi: 10.1016/j.ijrobp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Dearnaley DP, Hall E, Lawrence D, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer. 2005;92:488–98. doi: 10.1038/sj.bjc.6602301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nijkamp J, Pos FJ, Nuver TT, et al. Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results. Int J Radiat Oncol Biol Phys. 2008;70:75–82. doi: 10.1016/j.ijrobp.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Bittner N, Butler WM, Reed JL, et al. Electromagnetic tracking of intrafraction prostate displacement in patients externally immobilized in the prone position. Int J Radiat Oncol Biol Phys. 2010;77:490–5. doi: 10.1016/j.ijrobp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 28.Beltran C, Herman MG, Davis BJ. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int J Radiat Oncol Biol Phys. 2008;70:289–95. doi: 10.1016/j.ijrobp.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Rosewall T, Chung P, Bayley A, et al. A randomized comparison of interfraction and intrafraction prostate motion with and without abdominal compression. Radiother Oncol. 2008;88:88–94. doi: 10.1016/j.radonc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Stroom JC, Kroonwijk M, Pasma KL, et al. Detection of internal organ movement in prostate cancer patients using portal images. Med Phys. 2000;27:452–61. doi: 10.1118/1.598913. [DOI] [PubMed] [Google Scholar]