Abstract
Background:
Melanoma is one of the most aggressive cancers, and it is estimated that 76,250 men and women will be diagnosed with melanoma of the skin in the USA in 2012. Over the last few decades many drugs have been developed but only in 2011 have new drugs demonstrated an impact on survival in metastatic melanoma.
Methods:
A systematic search of literature was conducted, and studies providing data on the effectiveness of current and/or future drugs used in the treatment of metastatic melanoma were selected for review. This review discusses the advantages and limitations of these agents, evaluating past, current and future clinical trials designed to overcome such limitations.
Results:
To date, there are four drugs approved by the Food and Drug Administration for melanoma (dacarbazine, interleukin-2, ipilimumab and vemurafenib). Despite efforts to develop new drugs, few of them have demonstrated any clinical benefits. Approved in 1975, dacarbazine remains the gold standard in chemotherapy, although ipilimumab and vemurafenib have raised many hopes in the last few years. Combining dacarbazine or other chemotherapy agents with new pharmacological agents may be a new way to achieve better clinical responses in patients with metastatic melanoma.
Discussion:
Advances in the molecular knowledge of melanoma have led to major improvements in the treatment of patients with metastatic melanoma, providing new targets and insights. However, heterogeneity amongst study populations, different approaches to treatment and the different melanoma types and localisations included in the trials makes their comparison difficult. New studies focusing on drugs developed in recent decades are warranted.
Keywords: abraxane, axitinib, carboplatin, bevacizumab, bortezomib, dacarbazine, dabrafenib, etaracizumab, everolimus, imatinib, ipilimumab, lenvatinib, olimersen, malignant melanoma, new therapeutic agents, paclitaxel, review, sorafenib, sunitinib, temozolomide, temsirolimus, trametinib, tremelimumab, vemurafenib
Introduction
Malignant melanoma is a tumour that arises from melanocytes or from cells that develop from melanocytes. In 2010, approximately 68,130 new cases of melanoma were diagnosed, of which 8700 patients (approximately 12.8%) died of this disease in the United States [1]. In 2012, it is estimated that 76,250 men and women (44,250 men and 32,000 women) will be diagnosed with melanoma of the skin and 9180 men and women will die from it in the USA [2]. From 2005–2009 the median age of diagnosis was 61 years [2].
Melanomas may develop in a pre-existing lesion or in healthy-appearing skin. Lesions like common acquired nevus, dysplastic nevus, congenital nevus and cellular blue nevus can be precursors of melanoma. Approximately 5–10% of melanomas are hereditary and of these 20–40% are associated with a pathogenic mutation in the cyclin-dependent kinase inhibitor 2A p16 (CDKN2A/p16) [3, 4]. In fact, individuals at highest known risk for the development of melanoma are those who carry a CDKN2A/p16 mutation [5]. However, there are other genes implicated in the development of melanoma, such as cyclin-dependent kinase 4 (CDK4), retinoblastoma protein-1 (RB1), cyclin-dependent kinase inhibitor 2A p19 (CDKN2A/p19), phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and rat sarcoma (RAS).
Risk factors for melanoma include: (i) ultraviolet radiation – ultraviolet A (UVA) and ultraviolet B (UVB) [6]; (ii) acute, intense and intermittent sunburns (especially on areas of the body that only occasionally receive sun exposure); (iii) changing mole, dysplastic nevi in familial melanoma, more than 50 nevi (2 mm or greater in diameter), one family member with melanoma, previous history of melanoma, sporadic dysplastic nevi, congenital nevus, immunosuppression, sun sensitivity and freckling. Individuals with an inability to tan and with skin that sunburns easily, have a greater risk of developing melanoma [7].
In particular, metastasised melanoma has a poor prognosis, with a five-year survival rate of 15.1% [2]. The prognosis is worse when the tumour has disseminated to distant sites and visceral organs, with a median survival time of only 6–9 months and a 3-year survival rate of only 10–15% [8]. In a meta-analysis of 42 Phase II trials in advanced melanoma (2100 patients treated between 1975 and 2005), the median overall survival (OS) time was 6.2 months, with a 1-year survival rate of 25.5%. The median progression-free survival (PFS) rate was 1.7 months, with a 6-month PFS rate of 14.5% [9].
Metastatic melanoma is highly resistant to chemotherapy, radiation therapy, hormonal therapy and modern immunotherapeutic approaches. However, new drugs have recently been approved and there are data suggesting new efficient approaches to treat melanoma. In the last few decades OS has improved slightly, mainly due to earlier diagnosis. Nevertheless, no significant impact on survival has been made despite improvements in response rates achieved with new drugs or new combinations of drugs. This article reviews current data on the most used agents in the treatment of metastatic melanoma, as well as available data regarding the promising new drugs developed in the last few years.
Method
Search strategy
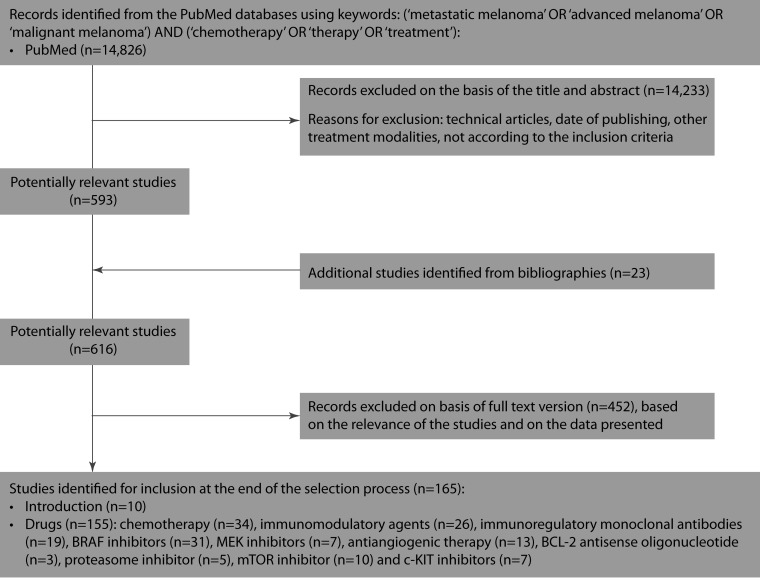
A systematic search of the literature published between January 1980 and September 2012 was conducted on drugs used in the treatment of metastatic melanoma, including approved and recently designed drugs (Figure 1). The search was performed in PubMed and followed a pre-established term list, using search terms ‘malignant melanoma’, ‘advanced melanoma’, ‘metastatic melanoma’, ‘chemotherapy’, ‘treatment’ and ‘therapy’. A language restriction to English was applied. Some references cited in the selected articles were also screened or included in the current review to identify additional potentially eligible drugs or to provide a better understanding of molecular pathways. Some unpublished studies, abstracts, posters and reviews were also included in this review, although editorials, case reports, lectures and commentaries were omitted.
Figure 1.
Selection process for the studies included in the systematic review.
Abbreviations
BCL-2, antisense oligonucleotide; BRAF, v-Raf murine sarcoma viral oncogene homolog B1; c-KIT, v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog; MEK, mitogen-activated protein kinase kinase; mTOR, mechanistic target of rapamycin
doi: 10.7573/dic.212242.f001
Inclusion criteria for studies
Abstracts of identified articles were screened and studies not meeting the following inclusion criteria were omitted: (i) development and evaluation of a drug or a combination of drugs in metastatic melanoma; (ii) the drugs were administered to individuals or groups with diagnosed metastatic melanoma; (iii) the study included data for a number of patients and tumours, tumour response and route of drug administration; (iv) studies with eligible study designs: randomised clinical trials (RCT), cohort studies, case-control studies and case series; (v) the paper was not a case study; (vi) the paper was published in English.
Study selection and data collection
The initial search identified 14,826 studies for potential review. After applying inclusion and exclusion criteria, and on the basis of the title and abstract, 14,233 studies were excluded and 593 studies were identified as potentially relevant. Twenty-three additional studies were identified from bibliographies, achieving 616 potentially relevant studies. On the basis of the full text version (based on the relevance of the studies and on the data presented), 165 studies were included in the review.
The author examined the studies identified using the search strategy, selected those for further evaluation, read the complete text and extracted the relevant data (for example, author name, year of publication, number and gender of patients, tumour response, drugs used, route of drug administration, OS time and PFS rate). If similar data were found in several studies, the most comprehensive and complete study was used.
Chemotherapy
Dacarbazine
Dacarbazine is an alkylating agent approved by the United States Food and Drug Administration (FDA) for the treatment of melanoma. Dacarbazine is one of the most effective chemotherapy agents for metastatic melanoma. Using dacarbazine as a single agent, Serrone et al. reported an approximately 20% objective response rate, with a median response duration of 5–6 months and complete response rate of 5% [10]. Middleton et al. reported a 6.4-month median survival time in a randomised Phase III study of patients treated with dacarbazine (intent-to-treat population) [11]. In a pooled analysis of 23 randomised, controlled trials, Lui et al. showed that the objective response rate for 1390 patients receiving dacarbazine monotherapy ranged between 5.3% and 28% (average 15.3%), concluding that treatment generally results in poor outcomes [12]. In patients treated with dacarbazine monotherapy, a long-term sustained complete response occurs in 1–2% of patients and fewer than 2% of patients are alive at 6 years [13]. However, Coates et al. showed that a small group of patients, with metastases to the lungs, good performance status and normal blood lactate dehydrogenase enzyme levels, can achieve long-term disease control with a good quality of life, and some of them may even achieve complete remission with a potential to cure [14].
Although it has low activity as monotherapy, dacarbazine has remained the basis for many combination chemotherapy regimens [15], including cisplatin, nitrosoureas and tubular toxins. Bottoni et al. reported complete remission of brain metastasis in three patients with Stage 4 melanoma treated with bleomycin, vincristine, lomustine and dacarbazine (BOLD). Six of the eight patients achieved clinical benefit and median survival was 12.5 months [16]. In a multicentre Phase III randomised trial using cisplatin, vinblastine and dacarbazine (CVD), Bajetta et al. observed a response rate of 21% that was increased to 33% by the addition of interleukin-2 (IL-2) and interferon-alfa (IFN-α)-2b [17]. However, a CVD regimen does not have clear survival benefits compared with best supportive care alone in patients with advanced metastatic melanoma [18]. Both BOLD and CVD regimens have induced responses by metastatic lesions in the liver, bone and brain that are commonly unresponsive to dacarbazine alone, even though they have had no impact on patient survival [10]. In a Phase II randomised study, Chiarion Sileni et al. showed that the four-drug combination dacarbazine, carmustine, cisplatin and tamoxifen (DBDT regimen) achieved an overall response rate of 26% compared with an overall response rate of 5% when using dacarbazine monotherapy [19]. The median PFS and the median survival were 4 and 9 months, respectively, for the DBDT regimen, and 2 and 7 months, respectively, for dacarbazine alone. However, this combination therapy increases toxicity with limited impact on OS [19]. Combining the DBDT regimen with low-dose IL-2 is reported to increase response rate to 32.5%, median PFS to 6.2 months and median OS to 11.3 months [20].
Intravenous (IV) dacarbazine can be administered at a dose of 2 to 4.5 mg/kg for 10 days, with doses repeated every 4 weeks, or 250 mg/m2 for 1 to 5 days, with doses repeated every 3 weeks. Common adverse effects include nausea, vomiting, leucopenia and thrombocytopenia, although other toxicities may occur.
Temozolomide
Temozolomide is an alkylating agent related to dacarbazine. It is an orally formulated prodrug of dacarbazine, with excellent oral bioavailability. Temozolomide has been shown to penetrate the brain, representing an alternative to dacarbazine against central nervous system metastases [21].
Middleton et al. reported a median survival time of 7.7 months for patients treated with temozolomide in a randomised Phase III study. The median PFS time was significantly longer in the temozolomide-treated group compared with the dacarbazine-treated group [11], although the difference between the two treatment groups was not statistically significant (HR 1.18; 95% CI: 0.92–1.52; p=0.20). Reporting another randomised Phase III trial, Kaufmann et al. showed a higher response rate for temozolomide combined with IFN-α-2b than for single-agent temozolomide (24 vs 13%). The median OS was 9.7 months for the temozolomide plus IFN-α-2b group and 8.4 months for the temozolomide group [22]. In a systematic review, the response rates for temozolomide in nine single-arm Phase I or II trials ranged from 0 to 29%, with complete responses observed in 0 to 17% of the patients. The median OS ranged from 3.2 to 13.1 months [23]. In the last few years new associations have been made, with Clark et al. reporting a 6-month PFS of 15%, a 1-year OS of 35% and a response rate of 13% in a Phase II trial combining temozolomide with thalidomide [24].
For the characteristics described above, temozolomide may be used as monotherapy or in association with whole brain radiation therapy for patients with brain melanoma metastases, increasing survival rates in these patients [25]. Additionally, for the treatment of unresectable brain metastases in malignant melanoma, temozolomide combined with radiotherapy may prolong survival compared with temozolomide without radiotherapy (9 vs 5 months, p=0.04) [25]. Boogerd et al. reported a stabilisation of brain metastasis in 11% of 52 advanced melanoma patients with small brain metastases (<2 cm) treated with temozolomide monotherapy, with a median time to neurologic progression of 7 months [26].
The choice between temozolomide and dacarbazine depends essentially on the presence or absence of brain metastases. Common adverse effects include alopecia, lymphopenia, nausea, vomiting, headache, fatigue and constipation.
Antimicrotubular agents, platinum analogues and nitrosoureas
Antimicrotubular agents such as vindesine and vinblastine (vinca alkaloids) and paclitaxel (taxanes) have been reported to have modest activity in patients with metastatic melanoma, achieving 14% and 16–17% of responses in patients, respectively [27–30].
Quagliana et al. concluded from a Phase II study that vindesine does have activity in some patients with metastatic malignant melanoma, achieving complete remissions of greater than 12 months duration in 2.5% of patients and partial remission in 17.5% of patients [27]. However, 52.5% of patients had increasing disease [27]. Moreover, a Phase II trial of vinorelbine, a semi-synthetic vinca alkaloid, showed that it has poor activity in previously treated patients with disseminated melanoma [31].
Nathan et al. reported an overall response rate of 24% in a Phase II trial with paclitaxel as a 3-hour infusion on melanoma patients [32]. In addition, Rao et al. showed that the combination of paclitaxel and carboplatin appears to have clinical activity when used as a second-line therapy after temozolomide or dacarbazine, achieving an objective partial response and clinical benefit in 26% and 45% of the patients treated, respectively [33].
A potential new drug is abraxane, an albumin-bound form of paclitaxel, already approved for breast cancer. Hersh et al. reported a median survival of 12.1 and 9.6 months, respectively, in a Phase II trial of 37 previously treated and chemotherapynaïve patients with metastatic melanoma [34]. Additionally, a Phase II trial of abraxane and carboplatin, achieved a median OS of 11.1 and 10.9 months in chemotherapy-naïve and previously treated patients, respectively [35].
Carboplatin as a single agent has induced a 19% response rate in a Phase II trial with 26 patients with metastatic malignant melanoma [36]. However, this response rate is similar to that noted for other agents, and in vitro oxaliplatin, another platinum analogue, seems to be more effective than carboplatin or cisplatin against human melanoma cell lines [37].
Nitrosoureas such as carmustine, lomustine and fotemustine induced objective responses ranging from 13 to 18% in patients with melanoma [15]. Fotemustine is probably the most active nitrosourea, with response rates between 20 and 25% [38–40]. Fotemustine appears to be a good candidate in the treatment of brain metastases because it is able to cross the blood–brain barrier [41, 42].
Immunomodulatory agents
Interferon alfa (IFN-α)-2b
IFN-α-2b is approved by the FDA for adjuvant therapy of resected high-risk melanoma, with OS and recurrence-free survival rates at 5 years of 44% and 32%, respectively [43]. On the other hand, no significant improvement in PFS is reported for low and intermediate-dose IFN-α [43, 44]. In a systematic review of randomised controlled trials, Lens et al. reported median OS rates from 3.2 to 6% in patients treated with IFN-α [45]. High-dose IFN-α may improve relapse-free and OS rates [46, 47] but it is associated with toxicities such as fever, chills, fatigue, autoimmune events and reduced quality of life. Toxicity can be reduced with the use of pegylated IFN-α that is suggested to have similar efficacy in metastatic disease [48].
IFN-α can be used alone or in regimens with IL-2 and/or chemotherapy, with more efficacy than single-agent chemotherapy. When combined with dacarbazine, it also has effects in tumour vasculature, regulating pericytes to inhibit tumour growth [49].
Interleukin-2 (IL-2)
IL-2 is a lymphokine that stimulates T-cell proliferation and function. It was approved by the FDA in 1998 for the treatment of metastatic melanoma. High dose IL-2 seems to benefit some patients with metastatic melanoma, with complete and partial responses rates of 6% and 10%, respectively [50, 51]. More effective rates are associated with greater toxicities so many trials have been conducted to identify new ways to administer IL-2. Unfortunately, IL-2 regimens involving low-doses or subcutaneous administration produce lower response rates than regimens involving high-dose and IV administration [52]. Smith et al. reported that in patients with subcutaneous or cutaneous disease only, IL-2 with melanoma vaccine gp100:209-217(210M) [gp100] was associated with higher response rates than IL-2 alone [53].
Chemotherapy in combination with IL-2 has been tested in several trials. Regimens including carmustine, cisplatin, dacarbazine, tamoxifen or vinblastine, with or without IL-2 or IL-2 associated with IFN-α achieved a response rate ranging from 23 to 32.5%, with an OS rate of between 4.6 and 11.3 months [20, 54–57]. Quan et al. recently reported that famotidine may decrease IL-2 toxicity as it enhances lymphokine-activated killer cell activity, whilst maintaining activity against melanoma [58].
Adoptive T-cell therapy (ACT) and cytolytic T-lymphocyte (CTL) response
Immunotherapeutic strategies using ACT appear to mount a powerful antitumour CTL response, provoking cell lysis by activation of the apoptotic machinery [59]. ACT can use either naturally-occurring or gene-engineered T cells to mediate tumour regression in patients with metastatic cancer [60–62]. Dudley et al. reported that host lympho-depletion followed by ACT and IL-2 results in objective response rates of 50 to 70% in patients with metastatic melanoma refractory to standard therapies [63]. ACT is a promising method of treating metastatic melanoma, achieving response rates between 49 and 72%, with median complete response rates from 18 to 75 months [64–66]. ACT is not possible in all patients and tumour cells often escape apoptotic pathways, enabling their survival despite CTL attack [67]. However, in testing bortezomib to sensitise melanoma cells towards adoptive CTL attack, Seeger et al. reported that bortezomib enhanced the susceptibility of melanoma cells toward redirected CTL attack and that tumour cell lysis was not due to the direct cytotoxic effects of bortezomib [68].
Immunoregulatory monoclonal antibodies
Ipilimumab
Ipilimumab is a fully human monoclonal antibody (IgG1) that has been recently approved by the FDA for the treatment of metastatic melanoma. Melanoma is one of the most immunogenic tumours and ipilimumab blocks CTL-associated antigen 4 (CTLA-4), an immune checkpoint molecule that downregulates pathways of T-cell activation, and promotes antitumour immunity [69, 70]. In a Phase III study, Hodi et al. tested ipilimumab plus gp100, ipilimumab alone or gp100 alone in patients with metastatic melanoma, achieving a longer OS in patients receiving ipilimumab plus gp100 or ipilimumab alone (10.0 and 10.1 months, respectively, vs 6.4 months for gp100 alone) [71]. Ipilimumab also has an effect on brain metastases, with 24% of the patients in cohort A and 10% of the patients in cohort B achieving disease control (assessed with modified World Health Organization [WHO] criteria) [72]. Additionally, ipilimumab can be combined with dacarbazine, improving OS when compared with dacarbazine alone (11.2 vs 9.1 months) [73]. New trials are combining ipilimumab with radiotherapy, such as the RADVAX study, a stratified Phase I/II dose escalation trial of radiotherapy followed by ipilimumab (ClinicalTrials.gov Identifier: NCT01497808; this study is currently recruiting patients). However, one must be aware of the potential severe autoimmune side-effects of ipilimumab, as it leads to immune-related adverse events, including rashes, diarrhoea, hepatitis, pancreatitis and neuropathies [74].
Tremelimumab
Tremelimumab is a fully human monoclonal antibody (IgG2) directed against the CTLA-4 receptor. Tremelimumab can be safely administered to humans as a single IV dose up to 15 mg/kg [75]. Camacho et al. reported median survival rates of 10.3 and 11 months in a Phase I/II trial, using 10 mg/kg monthly and 15 mg/kg quarterly dose regimens, respectively [76]. Evaluating tremelimumab in comparison with standard dacarbazine or temozolomide chemotherapy in previously untreated patients, Ribas et al. reported a median OS of 11.8 months in the tremelimumab group and 10.7 months in the chemotherapy group. However, tremelimumab as a single agent failed to demonstrate an improvement in OS [77]. Tremelimumab was also tested in combination with IFN-α-2b achieving an OS of 21 months and a PFS of 6.4 months [78].
Anti-PD-1
Programmed death-1 (PD-1) is a negative regulator of the immune system that causes immune tolerance through apoptosis of the activated lymphocyte responsible for the evasion of melanoma cells [79, 80]. In a Phase I study conducted by Brahmer et al., MDX-1106, a fully human immunoglobulin G4 monoclonal antibody anti-PD-1, demonstrated antitumour activity [81]. Moreover, it caused fewer immune-related adverse events than ipilimumab [81]. Topalian et al. reported that anti-PD-1 antibody produced objective responses in approximately 1-in-4 to 1-in-5 patients with melanoma [82]. To verify new possibilities, a dose-escalation study combining MDX-1106 and ipilimumab in subjects with unresectable Stage 3 or Stage 4 malignant melanoma is currently recruiting patients (ClinicalTrials.gov Identifier: NCT01024231).
Agonistic antibodies OX44 and anti CD137 (4-1BB)
Antigen-specific memory T cells (Tms) are essential in the immune surveillance of residual and metastatic tumours, and the activation of Tms can be achieved by the administration of agonistic anti-CD137 monoclonal antibody [83–85]. The antibodies anti-OX44 and anti-4-1BB have an agonist action on T-cell activation and in combination these antibodies have demonstrated a promising new therapy against melanoma [86]. A Phase I study in patients with advanced cancer (including 54 patients with melanoma) demonstrated that BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, had clinical activity justifying its further evaluation both as a single agent and in combination therapy [87]. A study combining anti-CD137 and ipilimumab in patients with melanoma was designed but was subsequently withdrawn prior to enrolment (ClinicalTrials.gov Identifier: NCT00803374).
v-Raf murine sarcoma viral oncogene homologue B1 (BRAF) inhibitors
Non-selective BRAF inhibitors
Sorafenib
Rapidly accelerated fibrosarcoma (RAF) is a proto-oncogene, and BRAF somatic missense mutations have been identified in 66% of malignant melanomas analysed [88–90]. Moreover, up to 82% of benign nevi have been shown to possess activating BRAF mutations, suggesting that activation of the mitogen-activated protein kinase (MAPK) pathway is necessary for melanoma development but not sufficient for malignant transformation [91]. BRAF mutations and subsequent MAPK cascade activation have an oncogenic role in melanoma development, making BRAF inhibition a potential target for new therapies.
Sorafenib (BAY 43-9006) is a RAF tyrosine kinase inhibitor that also targets vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR). Sorafenib has been shown to inhibit MAPK pathway in vitro and in vivo[92]. As an inhibitor of the VEGFR, PDGFR and MAPK pathways, sorafenib may inhibit tumour growth by a dual mechanism, acting directly on the tumour and/or on tumour angiogenesis [93]. As a single-agent, sorafenib is well tolerated but it has not shown significant anti-tumour activity in advanced melanoma patients [94]. Neither has the addition of sorafenib to carboplatin and paclitaxel improved response rates and PFS [95]. However, sorafenib in combination with dacarbazine resulted in a significant improvement in PFS in patients with advanced melanoma [96]. Additionally, preliminary Phase II study results demonstrated that sorafenib in combination with temozolomide has anti-tumour activity, with an overall response rate of 24% [97].
Studies are currently being conducted to confirm the efficacy of sorafenib combined with new drugs for the treatment of melanoma: i) a Phase I/II study combining sorafenib and temsirolimus has been conducted but the results have not yet been reported (ClinicalTrials.gov Identifier: NCT00349206); ii) a Phase I trial combining riluzole and sorafenib is currently recruiting participants (ClinicalTrials.gov Identifier: NCT01303341); iii) a randomised Phase II trial combining sorafenib with either temsirolimus or tipifarnib is ongoing but not recruiting participants (ClinicalTrials.gov Identifier: NCT00281957); and iv) an expanded cohort trial of bortezomib and sorafenib is currently recruiting participants (ClinicalTrials.gov Identifier: NCT01078961).
RAF265
RAF265 is a small molecular inhibitor of mutant BRAF V600E and VEGFR-2, which can be taken orally, causing dose dependent inhibition of tumour growth [98, 99]. In the first-in-human Phase I study of RAF265, overall response rates were 16% in mutant BRAF patients and 13% in BRAF WT patients [100]. Additionally, Su et al. showed that RAF265 inhibits the growth of advanced human melanoma tumours – 41% of tumours implanted in mice responded to RAF265 treatment with more than 50% reduction in tumour growth [101]. However, more studies are needed to confirm the efficacy and safety of RAF265 and a study is already ongoing, although not recruiting participants (ClinicalTrials.gov Identifier: NCT00304525).
Selective BRAF inhibitors
Vemurafenib
Vemurafenib is an oral, reversible and selective inhibitor of BRAF V600E [102] that is approved by the FDA for the treatment of patients with nonresectable or metastatic melanoma.
Vemurafenib was first evaluated by Flaherty et al. in a Phase I study (BRIM-1). Amongst the 32 patients with the BRAF V600E mutation, 24 (75%) had a partial response and 2 (6.25%) had a complete response, with a median PFS amongst all patients of more than 7 months [103]. A Phase II study (BRIM-2) in 132 patients with BRAF V600E mutation subsequently showed an overall response rate of 53% (6% of patients achieved a complete response and 47% a partial response) [104]. The Phase III study (BRIM-3) conducted by Chapman et al. compared vemurafenib to dacarbazine in 675 patients with previously untreated, metastatic melanoma with the BRAF V600E mutation [105]. At 6 months, OS was 84% in the vemurafenib arm compared with 64% in the dacarbazine arm and the median PFS was 5.3 months with vemurafenib compared with 1.6 months with dacarbazine [105]. The overall response rate was also 53% and the PFS was 6.8 months in a Phase II trial in patients with previously treated BRAF V600E mutant metastatic melanoma [106].
The most common adverse events of vemurafenib were arthralgia, rash, photosensitivity, diarrhoea, mild-to-moderate nausea, fatigue and alopecia [105, 106].
Vemurafenib also has activity in brain metastases in patients with BRAF V600E mutation melanoma, correlated with extra-cranial tumour response [107, 108]. Although vemurafenib is associated with a relative risk reduction of 63% in the risk of death and 74% in the risk of death or disease progression compared with dacarbazine [109], resistance to BRAF inhibition has already been observed [110]. Melanomas avoid BRAF inhibition through receptor tyrosine kinase (RTK)-mediated activation of an alternative survival pathway or activated RAS-mediated reactivation of the MAPK pathway [111]. However, combinations of BRAF/mitogen- activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) inhibitors may potentially attenuate the problem [110, 112].
Dabrafenib (GSK2118436)
Dabrafenib (GSK2118436) is a selective inhibitor of mutant BRAF. In a Phase I/II study Kefford et al. reported response rates around 60% in patients with mutated BRAF melanoma [113]. Additionally, a Phase III trial concluded that dabrafenib improved PFS when compared with dacarbazine (median PFS 5.1 vs 2.7 months, respectively) [114].
Dabrafenib also has activity against brain metastases as it leads to reduction in the size of brain lesions [115, 116]. One of the problems of selective BRAF inhibitors is the resistance acquired to treatment. Clinical trials combining BRAF, MEK and PI3K/mTOR inhibitors are ongoing or planned to overcome this issue [117].
Side-effects of dabrafenib are very similar to those observed with vemurafenib, although dabrafenib is also associated with induction of keratinocytic proliferation [118].
Mitogen-activated protein kinase (MEK) inhibitors
MEK is a member of the MAPK signalling cascade that is commonly activated in melanoma [119]. These mutations render melanoma cells independent of the normal RTK-mediated pathway regulation and constitutively drive melanoma cells to oncogenic proliferation and survival [67]. Direct inhibition of MEK may therefore block cell proliferation and induce apoptosis.
Kirkwood et al. carried out a Phase II, open-label, multicentre, randomised, parallel-group trial, comparing the MEK1/2 inhibitor, selumetinib, with temozolomide, and reported no significant difference in PFS between patients with unresectable Stage 3/4 cutaneous melanoma unselected for BRAF/neuroblastoma RAS viral oncogene homologue (NRAS) mutations [120]. However, selumetinib combined with standard chemotherapeutic agents resulted in enhanced tumour growth inhibition in human tumour xenograft models [121]. Moreover, combination of selumetinib and Rous sarcoma oncogene (SRC) kinase inhibitor saracatinib suppressed melanoma cell growth and invasion [122].
Trametinib is a reversible and selective MEK1 and MEK2 inhibitor that has activity against melanoma. Following a Phase I dose-escalation trial of trametinib in patients with advanced melanoma, Falchook et al. reported a 33% response rate and a median PFS of 5.7 months [123]. Trametinib also improved rates of PFS and OS when compared with chemotherapy (PFS 4.8 vs 1.5 months and OS at 6 months 81% vs 67% in the trametinib and chemotherapy groups, respectively) [124].
Many new MEK inhibitors are being developed. Von Euw et al. reported that the MEK inhibitor TAK733 has antitumour properties in cutaneous and uveal melanoma cell lines with different oncogenic mutations [125]. Inhibition of the MEK pathway seems to be promising in the treatment of melanoma, although more studies are needed.
Antiangiogenic therapy
Bevacizumab
Angiogenesis is essential to tumour growth and VEGF plays an important role in angiogenesis, regulating the proliferation and migration of endothelial cells. Bevacizumab is a humanised monoclonal antibody that inhibits vascular endothelial growth factor-A (VEGF-A). Rationally, blocking angiogenesis would be expected to enhance tumour inhibition, so recently bevacizumab and other anti-angiogenic agents have been tested in melanoma.
In a Phase II trial combining bevacizumab with dacarbazine and daily low-dose IFN-α-2a, Vihinen et al. reported a response rate of 23% but the regimen was associated with remarkable vascular events [126]. Moreover, Grignol et al. reported a clinical response in 24% and stabilisation of disease in 20% of patients with metastatic melanoma in a Phase II trial of bevacizumab and high-dose IFN-α-2b [127].
Bevacizumab in combination with carboplatin plus paclitaxel does not appear to improve PFS in patients with untreated advanced melanoma [128]. However, von Moos et al. reported promising activity of bevacizumab in combination with temozolomide, with a median PFS and OS of 4.2 and 9.6 months, respectively [129].
Bevacizumab may be useful in the treatment of American Joint Committee on Cancer (AJCC) Stage 3 patients after therapeutic lymph node dissection [130]. New studies are needed to confirm if bevacizumab has activity when combined with new agents such as ipilimumab.
Axitinib
Axitinib is an oral tyrosine kinase inhibitor against the vascular epithelial growth factor receptors (VEGFR)-1, VEGFR-2, VEGFR-3 and PDGFR [131, 132]. Fruehauf et al. reported that in a Phase II trial axitinib was well tolerated and had single-agent activity in metastatic melanoma, achieving a response rate of 18.8% and a 6-month PFS of 33.9% [133]. Axitinib plus a specific peptide-based vaccine has also been shown to enhance antitumour efficacy [134]. Further investigation of this new drug is needed, and a two-arm trial of axitinib and carboplatin/paclitaxel is currently recruiting participants (ClinicalTrials.gov Identifier: NCT01174238).
Lenvatinib (E7080)
Lenvatinib (E7080) is a multi-targeted tyrosine kinase inhibitor against VEGFR, PDGFR and fibroblast growth factor receptor (FGFR) [135]. It has been shown to inhibit tumour angiogenesis [135] and so can be useful in melanoma treatment. Boss et al. reported a Phase I study of lenvatinib in patients with advanced solid tumours in which 46% of the patients had stable disease as best response [136]. A Phase I/ Ib, multicentre, open-label, dose escalation study of lenvatinib in patients with advanced and/or metastatic melanoma has been completed but no results have been published yet (ClinicalTrials.gov Identifier: NCT00121680). Additionally, a Phase Ib/II study of lenvatinib in combination with dacarbazine compared with dacarbazine alone as first line therapy in patients with Stage 4 melanoma is ongoing but not recruiting participants (ClinicalTrials.gov Identifier: NCT01133977). These trials will help to evaluate the possible role of lenvatinib in the treatment of melanoma.
Etaracizumab (MEDI-522)
Etaracizumab (MEDI-522) is a monoclonal antibody against alphavbeta3, an important molecule for tumour-induced angiogenesis that is upregulated in metastatic melanoma [137]. In a randomised Phase II study of etaracizumab with or without dacarbazine in patients with Stage 4 metastatic melanoma, the median OS was 12.6 months for the etaracizumab group and 9.4 months for the etaracizumab plus dacarbazine group [138]. However, more studies are needed to evaluate the use of etaracizumab in melanoma.
Anti-B-cell lymphoma (BCL)2 antisense oligonucleotide
Oblimersen
Oblimersen is an anti-B-cell lymphoma (BCL) 2 antisense oligonucleotide. An antisense drug is a short sequence of RNA which hybridises with and inactivates mRNA, preventing the protein formation. As expression of BCL2 has been associated with chemoresistance by malignant melanoma cells, down-regulation of BCL2 by oblimersen may offer a new approach to the treatment of melanoma [139]. In fact, compared with dacarbazine alone, oblimersen plus dacarbazine significantly improved PFS (1.6 vs 2.6 months, respectively) and overall response (7.5 vs 13.5%, respectively) in patients with advanced melanoma [140]. Moreover, electroporation may optimise the response of melanoma to chemotherapy, as it has shown to improve the delivery of oblimersen within tumour cells in vivo in a human melanoma xenograft [141].
Proteasome inhibitors
Bortezomib
Bortezomib is a tripeptide that binds the catalytic site of the 26S proteasome with high specificity and affinity, inhibiting proteasome activity [67]. Proteasome inhibitors may represent a new treatment option for melanoma as they block the nuclear factor kappa-light-chain-enhancer of the activated B cells (NF-κB) pathway, inhibiting tumour cell proliferation and sensitising them to chemotherapy [142].
Amiri et al. reported impressive results with temozolomide combined with bortezomib in an human melanoma xenograft model [143]. Moreover, in a Phase I trial of bortezomib with temozolide in patients with advanced melanoma (3 patients with primary ocular melanoma and 16 patients with primary cutaneous melanoma), Su et al. observed inhibition of proteasome activity in peripheral blood mononuclear cells [144]. However, inhibition was for a limited time period only and effects on NF-κB activation were not consistent.
Bortezomib has also been tested in combination with paclitaxel and carboplatin in patients with metastatic melanoma, although this combination was of limited clinical benefit and was associated with significant toxicity [145]. Nevertheless, new agents may enhance bortezomib activity. Sunitinib arrests growth and sensitises melanoma cells to bortezomib, so combining sunitinib with bortezomib may provide therapeutic benefit [146]. Further studies of bortezomib are needed to evaluate the potential of this drug.
Mechanistic target of rapamycin (mTOR) inhibitors
Mechanistic target or rapamycin (mTOR) is a serine/threonine kinase downstream of protein kinase B (AKT) [147] (the major downstream effector of the phosphatidylinositol-3-kinase [PI3K] pathway) that modulates protein synthesis, angiogenesis and cell cycle progression [148, 149]. As mTOR is important for tumour growth. mTOR inhibitors are plausible agents for the treatment of melanoma.
Everolimus and temsirolimus
Everolimus is an mTOR inhibitor. In a Phase II trial of everolimus in metastatic melanoma, 7 (35%) of the 20 patients had stable disease at 16 weeks, with a PFS of 3 months [150]. Everolimus combined with bevacizumab has been shown to have moderate activity and be well tolerated in the treatment of patients with metastatic melanoma [151]. Moreover, everolimus and vatalanib (a pan-VEGFR tyrosine kinase inhibitor) in combination have additive effects, increasing anti-tumour activity in the orthotopic BL16/BL6 murine melanoma model compared to either drug monotherapy, without increasing toxicity [152]. Daily everolimus plus low-dose weekly cisplatin also has anti-tumour activity in several tumour types, including melanoma [153].
However, in a Phase II study of the mTOR inhibitor, temsirolimus, only 1 of the 33 patients with metastatic melanoma had a partial response lasting 2 months; the median time to disease progression and OS were 10 weeks and 5 months, respectively [154]. Moreover, in a Phase I study in patients with metastatic melanoma, temsirolimus combined with sorafenib resulted in significant toxicity at higher dose levels, did not have any clinical benefit in genetically unselected patients and did not inhibit P-extracellular signal-regulated kinase (PERK) [155]. Additionally, in a Phase II study of temsirolimus in combination with sorafenib in patients with untreated metastatic melanoma, only 3 (4.8%) of the 63 patients achieved partial response [156].
Targeting v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (c-KIT or CD117)
c-KIT or CD117 encodes a receptor tyrosine kinase whose ligand is stem cell factor (SCF or KIT ligand). c-KIT signalling is important for melanocyte development, differentiation, proliferation, survival and migration [157, 158]. Mutations in c-KIT in melanoma have been reported [159]. Curtin et al. reported somatic activation of c-KIT in 39% of mucosal, 36% of acral and 28% of melanomas on chronically sun-damaged skin [160]. Because of its potentially important role in melanoma oncogenesis, c-KIT may be an effective target in the treatment of melanoma.
Imatinib
Imatinib is a receptor tyrosine kinase inhibitor against Abelson proto-oncogene-breakpoint cluster region (BCR-ABL), c-KIT and PDGFR. In a Phase II trial of imatinib, 28 of the 295 patients with melanoma screened for the presence of c-KIT mutations and amplifications were treated with imatinib. The overall durable response rate was 16%, with a median time to progression of 12 weeks and a median OS of 46.3 weeks [161]. In another Phase II study of imatinib in patients with metastatic melanoma and c-KIT mutations or amplification, the overall response rate was 23.3% [162].
Imatinib seems to have clinical benefit in a subset of patients who have c-KIT mutation or amplification. However, more studies are needed to confirm if imatinib is a viable treatment for such patients.
Sunitinib
Sunitinib is an oral, small molecule, multi-targeted receptor tyrosine kinase inhibitor against PDGFR, VEGFR and c-KIT. Minor et al. reported one complete remission for 15 months and two partial responses in the four evaluable patients with c-KIT mutations, and one partial response in the six patients with either c-KIT amplification or overexpression, concluding that sunitinib may have activity in patients with melanoma and c-KIT mutations [163]. Moreover, a strategy combining sunitinib and bortezomib has shown therapeutic benefits [146].
Discussion
Melanoma is the fifth and seventh leading cause of cancer deaths in males and females, respectively, in the USA [164].
There are 4 drugs currently approved by the FDA for the treatment of melanoma: dacarbazine, IL-2, ipilimumab and vemurafenib. Ipilimumab and vemurafenib were approved in 2011 and have raised many hopes in melanoma treatment. However, they have many limitations and their side effects have limited their use and redirected investigations towards new targets.
Dacarbazine, one of the oldest available drugs (approved in 1975), remains the gold standard in chemotherapy, achieving complete responses in 2.7–4.1% of the patients (Table 1). Combining dacarbazine or other chemotherapy agents with new pharmacological agents may represent a new way to achieve better responses in patients with metastatic melanoma. For example, high doses of IL-2 can be effective but are associated with greater toxicity. However, new data suggest that the addition of famotidine to IL-2 may decrease its toxicity as it enhances lymphokine-activated killer cell activity, whilst maintaining its activity against melanoma [58]. The CTL response is another example of the beneficial effects brought by the addition of new drugs. Although it represents a promising option for the treatment of metastatic melanoma, it is not a feasible treatment for all patients as tumour cells often escape CTL attack. However, new studies have shown that the addition of bortezomib can enhance the susceptibility of melanoma cells toward redirected CTL attack [67, 68]. Currently, ipilimumab is the most promising drug. Although it has effective response rates, its use is limited by the severe and fatal immune-related adverse reactions. However, adverse reactions can now be managed by protocol-specific guidelines, including administration of systemic corticoids and/or dose interruption/ discontinuation.
Table 1.
Current drugs and potential drugs in metastatic melanoma.
| Study reference | Drug | Number of patients included | Mean age (years) | PR | CR | OS (median months) | HR (95% CI) | PFS (median months) | HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Middleton et al. [11] | Dacarbazine | n=149 | 58.8 | 9.4% | 2.7% | 6.4 | 1.18 (0.92–1.52) | 1.5 | 1.37 (1.07–1.75) |
| Lui et al. [12] | Dacarbazine | n=1390 | 52.5 | 11.2% | 4.1% | - | 1.31 (1.06–1.61) | – | – |
| McDermott et al. [96] | Dacarbazine | n=50 | 60 | 12% | 0% | 12.83 | (10.12–18) | 2.93 | (1.53–4.48) |
| Dacarbazine + sorafenib | n=51 | 55 | 24% | 0% | 11.4 | (8.75–17.58) | 5.73 | (4–7) | |
| Robert et al. [73] | Dacarbazine + ipilimumab | n=250 | 57.5 | 13.6% | 1.6% | 11.2 | (9.4–13.6) | – | – |
| Bedikian et al. [140] | Dacarbazine + oblimersen | n=386 | 59 | 10.6% | 2.8% | 9 | 0.87 (0.75–10.01) | 2.6 | – |
| Hersey et al. [138] | Dacarbazine + etaracizumab | n=55 | 59.9 | 0% | 12.7% | 9.4 | (7.6–13.1) | 2.6 | (1.6–3.4) |
| Vihinen et al. (abstract) [126] | Dacarbazine + IFN-α + bevacizumab | n=26 | – | 15.4% | 7.7% | 11.5 | – | 2.3 | – |
| Bottoni et al. [16] | BOLD + G-CSF | n=8 | 57 | 0% | 37.5% | 12.5 | – | 8.5 | – |
| Seigler et al. [165] | BOLD | n=91 | – | 31% | 9% | 7.75 | – | – | – |
| Bajetta et al. [17] | CVD | n=72 | 51.5 | 21% | - | 12 | – | 8 | – |
| CVD + IL2 + IFN-α | n=72 | 46.5 | 29% | 4% | 11 | – | 8 | – | |
| Su et al. [20] | BCDT | n=40 | 54 | 27.5% | 5% | 11.3 | (7.0–15.6 month) | 6.2 | (2.9–9.6) |
| Middleton et al. [11] | Temozolomide | n=156 | 58.5 | 10.9% | 2.6% | 7.7 | 1.18 (0.92–1.52) | 1.9 | 1.37 (1.07–1.75) |
| Kaufmann et al. [22] | Temozolomide | n=134 | 56 | 11.2% | 2.2% | 8.4 | (7.07–9.72) | 2.4 | (1.48–3.28) |
| Temozolomide + IFN-α | n=137 | 54.5 | 16.1% | 8% | 9.7 | (8.26–11.18) | 3.3 | (2.73–3.82) | |
| Clark et al. [24] | Temozolomide + Thalidomide | n=62 | 62 | 13% | – | 8 | (6–12) | 2 | (2–4) |
| von Moos et al. (abstract) [129] | Temozolomide + bevacizumab | n=62 | 59 | 14.5% | 1.6% | 9.6 | – | 4.2 | – |
| Atkins et al. [50] | IL-2 | n=270 | 42 | 10% | 6% | – | – | 13.1 | – |
| Smith et al. [53] | IL-2 | n=305 | 45 | 9% | 4% | 12.8 | – | – | – |
| IL-2 + gp100 vaccine | n=379 | 45 | 12% | 3% | 14.2 | – | – | – | |
| Hodi et al. [71] | Ipilimumab | n=137 | 56.8 | 9.5% | 1.5% | 10.1 | (8.0–13.8) | 2.86 | (2.76–3.02) |
| Ipilimumab + gp100 | n=403 | 55.6 | 5.5% | 0.2% | 10 | (8.5–11.5) | 2.76 | (2.73–2.79) | |
| gp100 | n=136 | 57.4 | 1.5% | 0% | 6.4 | (5.5–8.7) | 2.76 | (2.73–2.83) | |
| Quagliana et al. (abstract) [27] | Vindesine | n=42 | – | 17.5% | 2.5% | – | – | – | – |
| Whitehead et al. [31] | Vinorelbine | n=21 | 58 | 0% | 0% | 6 | (3.7–8.3) | 2 | (1.5–3.3) |
| Hersh et al. [34] | Abraxane | n=37 | 61.2 | 21.6% | 0% | 9.6 | (6.7–23.7) | 4.5 | (3.4–−6.7) |
| Kottschade et al. [35] | Abraxane + carboplatin | n=39 | 59 | 23.04% | 2.56% | 11.1 | – | 4.3 | – |
| Ribas et al. [77] | Tremelimumab | n=324 | – | – | – | 11.8 | (10.4–13.9) | – | – |
| Tarhini et al. (abstract) [78] | Tremelimumab + IFN-α-2b | n=37 | – | – | – | 21 | (9.5–not reached) | 6.4 | (3.3–12.1) |
| Sosman et al. [106] | Vemurafenib | n=132 | 51.5 | 47% | 6% | 15.9 | (11.6–18.3) | 6.8 | (5.6–8.1) |
| Hauschild et al. [114] | Dabrafenib | n=187 | 53 | 47% | 3% | – | – | 5.1 | 0.30 (0.18–0.51) |
| Flaherty et al. [124] | Trametinib | n=214 | 55 | 20% | 2% | – | – | 4.8 | 0.45 (0.33–0.63) |
| Fruehauf J (abstract) [133] | Axitinib | n=32 | – | 15.6% | 3.1% | 6.6 | – | – | – |
| Hersey et al. [138] | Etaracizumab | n=57 | 56.7 | 0% | 0% | 12.6 | (6.8–14.2) | 1.8 | (1.3–2.8) |
| Croghan et al. [145] | Paclitaxel + carboplatin+ Bortezomib | n=17 | 59 | 11.8% | 0% | 7 | – | 3.2 | – |
| Hainsworth et al. [151] | Bevacizumab + everolimus | n=57 | 70 | 10.5% | 1.8% | 8.6 | – | 4 | (2.8–5.3) |
| Margolin et al. (abstract) [156] | Temsirolimus + sorafenib | n=63 | – | 4.8% | 0% | 7 | – | 2.1 | – |
| Carvajal et al. [161] | Imatinib | n=28 | 71 | 7.1% | 7.1% | 10.7 | (6.5–not achieved) | – | – |
| Guo et al. [162] | Imatinib | n=43 | 57 | 23.3% | 0% | 14 | (10.8–17.2) | 3.5 | (1.3–5.7) |
Abbreviations
BCDT, carmustine, cisplatin, dacarbazine, tamoxifen and interleukin-2; BOLD, bleomycin, vincristine, lomustine and dacarbazine; CR, complete response; CVD, cisplatin, vinblastine and dacarbazine; G-CSF, granulocyte-colony stimulating factor; HR, hazard ratio; IFN-α, interferon-alfa; IL-2, interleukin-2; OS, overall survival; PFS, progression-free survival; PR, partial response
doi: 10.7573/dic.212242.t001
Understanding oncogenesis, growth, proliferation, survival and migration pathways of melanoma cells has provided both new targets and insights in melanoma therapy. In fact, current advances in the knowledge of melanoma oncogenesis may represent our best hope to tackle melanoma. Vemurafenib is one of the best examples in this field. It is a new possibility in patients with BRAF V600E mutations, with excellent response and survival rates. However, resistance to vemurafenib has been described. New insights in molecular knowledge and combinations of drugs like BRAF/MEK/ERK inhibitors may attenuate the problem.
Despite all the efforts made to develop new drugs, few of them have demonstrated clinical benefits. Several of them have activity in vitro or in animal models, but they achieve poor response and survival rates when they are tested in clinical trials. In the next years this problem will likely be overcome with better understanding of the suitability of patients for the different therapies.
There are some limitations to comparing clinical trials and different approaches to melanoma. The heterogeneity of the populations enrolled in the studies, the different approaches to treatment and the different types and localisations of melanoma included in the trials made it impossible to compare some studies. The absence of clinical trials comparing new treatment options with some of the older and established treatment options made evaluation even more difficult.
New studies must be conducted (Table 2) to confirm if any of the recently developed drugs alone or in combination can lead to promising clinical benefits.
Table 2.
Future trials in melanoma research.
| Identifier | Clinical trial description | Condition | Intervention | Trial phase | Status | Start date | Estimated completion date |
|---|---|---|---|---|---|---|---|
| NCT01497808 | A Stratified Phase I/ II Dose Escalation Trial of Stereotactic Body Radiotherapy Followed by Ipilimumab in Metastatic Melanoma |
Metastatic melanoma | Drug: ipilimumab Radiation: stereotactic body Radiation therapy |
III | Currently recruiting patients | December 2011 | June 2014 (Primary outcome measure) |
| NCT01024231 | Dose-escalation Study of Combination BMS-936558 (MDX-1106) and Ipilimumab in Subjects With Unresectable Stage III and Stage IV Malignant Melanoma |
Malignant melanoma | Drug: BMS-936558 (MDX-1106) Drug: ipilimumab |
I | Currently recruiting patients | June 2013 | August 2014 (Primary outcome measure) |
| NCT00803374 | Combination of Anti-CD137 & Ipilimumab in Patients With Melanoma | Melanoma | Drug: antiCD137 Drug: ipilimumab |
I | Withdrawn prior to enrolment | November 2010 | Finished July 2012 |
| NCT00349206 | Sorafenib and Temsirolimus in Treating Patients With Metastatic, Recurrent, or Unresectable Melanoma | Melanoma | Drug: sorafenib tosylate Drug: temsirolimus |
I II |
Completed | April 2006 | February 2012 (Primary outcome measure) |
| NCT01303341 | Riluzole and Sorafenib Tosylate in Treating Patients With Advanced Solid Tumors or Melanoma |
Cutaneous melanoma | Drug: riluzole Drug: sorafenib tosylate Other: laboratory biomarker analysis Other: pharmacological study |
I | Currently recruiting patients | February 2011 | July 2011 (Primary outcome measure) |
| NCT00281957 | Sorafenib With Either Temsirolimus or Tipifarnib in Stage IV Melanoma That Cannot Be Removed by Surgery | Melanoma | Drug: sorafenib tosylate Drug: temsirolimus Drug: tipifarnib |
Phase II | Ongoing, but not recruiting participants | August 2007 | December 2012 (Primary outcome measure) |
| NCT01078961 | An Expanded Cohort Trial of Bortezomib and Sorafenib in Advanced Malignant Melanoma | Melanoma | Drug: bortezomib Drug: sorafenib |
I | Currently recruiting patients | September 2010 | September 2012 (Primary outcome measure) |
| NCT00304525 | A Study to Evaluate RAF265, an Oral Drug Administered to Subjects With Locally Advanced or Metastatic Melanoma (CHIR-265-MEL01) | Metastatic melanoma | Drug: RAF265 | II | Ongoing, but not recruiting participants | April 2006 | July 2014 (Primary outcome measure) |
| NCT01174238 | A Two Arm Trial of Axitinib and Carboplatin/Paclitaxel in Melanoma (CC# 10852) | Melanoma | Drug: axitinib Drug: carboplatin Drug: paclitaxel |
II | Currently recruiting patients | July 2010 | July 2015 (Primary outcome measure) |
| NCT00121680 | A Phase I/Ib, Multicenter, Open-Label, Dose Escalation Study of E7080 in Patients With Solid Tumors and in Combination With Temozolomide in Patients With Advanced and/or Metastatic Melanoma | Metastatic melanoma | Drug: E7080 | I | Completed | July 2005 | November 2011 (Primary outcome measure) |
| NCT01133977 | E7080 in Combination With Dacarbazine Versus Dacarbazine Alone as First Line Therapy in Patients With Stage IV Melanoma | Stage 4 melanoma | Drug: dacarbazine Drug: E7080 |
I II |
Ongoing, but not recruiting participants | March 2010 | June 2013 (Primary outcome measure) |
doi: 10.7573/dic.212242.t002
Footnotes
Funding: The Author received an honorarium from the Publisher.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance Epidemiology and End Results 2012. Available from: http://seer.cancer.gov/statfacts/html/melan.html#ref11 [Last : 7 August 2012].
- 3.Florell SR, Boucher KM, Garibotti G, et al. Population-based analysis of prognostic factors and survival in familiar melanoma. J Clin Oncol. 2005;23(28):7168–77. doi: 10.1200/JCO.2005.11.999. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein AM, Chan M, Harland M, et al. Melanoma Genetics Consortium (GenoMEL): Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44(2):99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspinwall L, Leaf S, Dola ER, Kohlmann W, Leachman SA. CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1510–9. doi: 10.1158/1055-9965.EPI-08-0010. [DOI] [PubMed] [Google Scholar]
- 6.Ivry GB, Ogle CA, Shim EK. Role of sun exposure in melanoma. Dermatol Surg. 2006;32(4):481–92. doi: 10.1111/j.1524-4725.2006.32101.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351(10):998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 10.Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19(1):21–34. [PubMed] [Google Scholar]
- 11.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 12.Lui P, Cashin R, Machado M, Hemels M, Corey-Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev. 2007;33(8):665–80. doi: 10.1016/j.ctrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Hill GJ, 2nd, Krementz ET, Hill HZ. Dimethyl triazeno imidazole carboxamide and combination therapy for melanoma. IV. Late results after complete response to chemotherapy (Central Oncology Group protocols 7130, 7131, and 7131A) Cancer. 1984;53(6):1299–305. doi: 10.1002/1097-0142(19840315)53:6<1299::aid-cncr2820530613>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Coates AS, Segelov E. Long term response to chemotherapy in patients with visceral metastatic melanoma. Ann Oncol. 1994;5(3):249–51. doi: 10.1093/oxfordjournals.annonc.a058802. [DOI] [PubMed] [Google Scholar]
- 15.Gasent Blesa JM, Grande Pulido E, Alberola Candel V, Provencio Pulla M. Melanoma from darkness to promise. Am J Clin Oncol. 2011;34(2):179–87. doi: 10.1097/COC.0b013e3181d6b427. [DOI] [PubMed] [Google Scholar]
- 16.Bottoni U, Bonaccorsi P, Devirgiliis V, et al. Complete remission of brain metastases in three patients with stage IV melanoma treated with BOLD and G-CSF. Jpn J Clin Oncol. 2005;35(9):507–13. doi: 10.1093/jjco/hyi141. [DOI] [PubMed] [Google Scholar]
- 17.Bajetta E, Del Vecchio M, Nova P, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol. 2006;17(4):571–7. doi: 10.1093/annonc/mdl007. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann MA, Hauschild A, Mohr P, et al. Prospective evaluation of supportive care with or without CVD chemotherapy as a second-line treatment in advanced melanoma by patient’s choice: a multicentre Dermatologic Cooperative Oncology Group trial. Melanoma Res. 2011;21(6):516–23. doi: 10.1097/CMR.0b013e3283485ff0. [DOI] [PubMed] [Google Scholar]
- 19.Chiarion Sileni V, Nortilli R, Aversa SM, et al. Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients. Melanoma Res. 2001;11(2):189–96. doi: 10.1097/00008390-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Su PJ, Chen JS, Liaw CC, et al. Biochemotherapy with carmustine, cisplatin, dacarbazine, tamoxifen and low dose interleukin-2 for patients with metastatic malignant melanoma. Chang Gung Med J. 2011;34(5):478–86. [PubMed] [Google Scholar]
- 21.Tatar Z, Thivat E, Planchat E, et al. Temozolomide and unusual indications: Review of literature. Cancer Treat Rev. 2012 Jul 18; doi: 10.1016/j.ctrv.2012.06.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann R, Spieth K, Leiter U, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. J Clin Oncol. 2005;23(35):9001–7. doi: 10.1200/JCO.2005.01.1551. [DOI] [PubMed] [Google Scholar]
- 23.Quirt I, Verma S, Petrella T, Bak K, Charette M. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12(9):1114–23. doi: 10.1634/theoncologist.12-9-1114. [DOI] [PubMed] [Google Scholar]
- 24.Clark JI, Moon J, Hutchins LF, et al. Phase 2 trial of combination thalidomide plus temozolomide in patients with metastatic malignant melanoma: Southwest Oncology Group S0508. Cancer. 2010;116(2):424–31. doi: 10.1002/cncr.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann M, Kiecker F, Wurm R, et al. Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J Neurooncol. 2006;76(1):59–64. doi: 10.1007/s11060-005-2914-0. [DOI] [PubMed] [Google Scholar]
- 26.Boogerd W, de Gast GC, Dalesio O. Temozolomide in advanced malignant melanoma with small brain metastases: can we withhold cranial irradiation? Cancer. 2007;109(2):306–12. doi: 10.1002/cncr.22411. [DOI] [PubMed] [Google Scholar]
- 27.Quagliana JM, Stephens RL, Baker LH, Costanzi JJ. Vindesine in patients with metastatic malignant melanoma: a southwest Oncology Group study. J Clin Oncol. 1984;2(4):316–9. doi: 10.1200/JCO.1984.2.4.316. [DOI] [PubMed] [Google Scholar]
- 28.Wiernik PH, Einzig Taxol in malignant melanoma. J Natl Cancer Inst Monogr. 1993;(15):185–7. [PubMed] [Google Scholar]
- 29.Bedikian AY, Weiss GR, Legha SS, et al. Phase II trial of docetaxel in patients with advanced cutaneous malignant melanoma previously treated with chemotherapy. J Clin Oncol. 1995;13(12):2895–9. doi: 10.1200/JCO.1995.13.12.2895. [DOI] [PubMed] [Google Scholar]
- 30.Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs. 1991;9(1):59–64. doi: 10.1007/BF00194546. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead RP, Moon J, McCachren SS, et al. A Phase II trial of vinorelbine tartrate in patients with disseminated malignant melanoma and one prior systemic therapy: a Southwest Oncology Group study. Cancer. 2004;100(8):1699–704. doi: 10.1002/cncr.20183. [DOI] [PubMed] [Google Scholar]
- 32.Nathan FE, Berd D, Sato T, Mastrangelo MJ. Paclitaxel and tamoxifen: An active regimen for patients with metastatic melanoma. Cancer. 2000;88(1):79–87. doi: 10.1002/(sici)1097-0142(20000101)88:1<79::aid-cncr12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106(2):375–82. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 34.Hersh EM, O’Day SJ, Ribas A, et al. A Phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naïve patients with metastatic melanoma. Cancer. 2010;116(1):155–63. doi: 10.1002/cncr.24720. [DOI] [PubMed] [Google Scholar]
- 35.Kottschade LA, Suman VJ, Amatruda T, 3rd, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group Study, N057E(1) Cancer. 2011;117(8):1704–10. doi: 10.1002/cncr.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans LM, Casper ES, Rosenbluth R. Phase II trial of carboplatin in advanced malignant melanoma. Cancer Treat Rep. 1987;71(2):171–2. [PubMed] [Google Scholar]
- 37.Mohammed MQ, Retsas S. Oxaliplatin is active in vitro against human melanoma cell lines: comparison with cisplatin and carboplatin. Anticancer Drugs. 2000;11(10):859–63. doi: 10.1097/00001813-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Kleeberg UR, Engel E, Israels P, et al. Palliative therapy of melanoma patients with fotemustine. Inverse relationship between tumour load and treatment effectiveness. A multicentre phase II trial of the EORTCMelanoma Cooperative Group (MCG) Melanoma Res. 1995;5(3):195–200. doi: 10.1097/00008390-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Calabresi F, Aapro M, Becquart D, et al. Multicenter phase II trial of the single agent fotemustine in patients with advanced malignant melanoma. Ann Oncol. 1991;2(5):377–8. doi: 10.1093/oxfordjournals.annonc.a057960. [DOI] [PubMed] [Google Scholar]
- 40.Jacquillat C, Khavat D, Banzet P, et al. Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminatd malignant melanoma including patients with cerebral metastases. Cancer. 1990;66(9):1873–8. doi: 10.1002/1097-0142(19901101)66:9<1873::aid-cncr2820660904>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Khavat D, Giroux B, Berille J, et al. Fotemustine in the treatment of brain primary tumors and metastases. Cancer Invest. 1994;12(4):414–20. doi: 10.3109/07357909409038234. [DOI] [PubMed] [Google Scholar]
- 42.Quéreux G, Dréno B. Fotemustine for the treatment of melanoma. Expert Opin Pharmacother. 2011;12(18):2891–904. doi: 10.1517/14656566.2011.633513. [DOI] [PubMed] [Google Scholar]
- 43.Hancock BW, Wheatley K, Harris S, et al. Adjuvant interferon in high-risk melanoma: the AIM HIGH Study—United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon Alfa-2a in high-risk resected malignant melanoma. J Clin Oncol. 2004;22(1):3–61. doi: 10.1200/JCO.2004.03.185. [DOI] [PubMed] [Google Scholar]
- 44.Eggermont AM, Sociu S, MacKie R, et al. EORTC Melanoma Group: Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation on patients with stage IIb/III melanoma (EORTC 18952): randomized controlled trial. Lancet. 2005;366(9492):1189–96. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- 45.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20(7):1818–25. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 46.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 47.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18(12):2444–58. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 48.Dummer R, Garbe C, Thompson JA, et al. Randomized dose-escalation study evaluating peginterferon alfa-2a in patients with metastatic malignant melanoma. J Clin Oncol. 2006;24(7):1188–94. doi: 10.1200/JCO.2005.04.3216. [DOI] [PubMed] [Google Scholar]
- 49.Liu P, Zhang C, Chen J, et al. Combinational therapy of interferon-α and chemotherapy normalizes tumor vasculature by relugating pericytes including the novel marker RGS5 in melanoma. J Immunother. 2011;34(3):320–6. doi: 10.1097/CJI.0b013e318213cd12. [DOI] [PubMed] [Google Scholar]
- 50.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 51.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 52.Keiholz U, Conradt C, Legha SS, et al. Results of interleukin-2-based treatment in advanced melanoma: a case record-based analysis of 631 patients. J Clin Oncol. 1998;16(9):2921–9. doi: 10.1200/JCO.1998.16.9.2921. [DOI] [PubMed] [Google Scholar]
- 53.Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14(17):5610–8. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston SR, Constenla DO, Moore J, et al. Randomized phase II trial of BCDT [carmustine (BCNU), cisplatin, dacarbazine (DTIC) and tamoxifen] with or without interferon alpha (IFN-alpha) and interleukin (IL-2) in patients with metastatic melanoma. Br J Cancer. 1998;77(8):1280–6. doi: 10.1038/bjc.1998.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirchner HH, Atzpodien J, Poliwoda H. Chemo-/immunotherapy in advanced malignant melanoma: carboplatin and DTIC or cisplatin, dtic, bcnu and tamoxifen followed by immunotherapy with interleukin 2 and interferon alpha-2a. Med Klin (Munich) 1996;91(Suppl 3):44–9. [PubMed] [Google Scholar]
- 56.González Astorga B, Jiménez Rubiano B, Delgado Pérez JR, et al. Biochemotherapy in the treatment of metastatic melanoma in selected patients. Clin Transl Oncol. 2009;11(6):382–6. doi: 10.1007/s12094-009-0372-4. [DOI] [PubMed] [Google Scholar]
- 57.Atzpodien J, Lopez-Hänninen E, Kirchner H, et al. Chemoimmunotherapy of advanced malignant melanoma sequential administration of subcutaneous interleukin-2 and interferon-alpha after intravenous dacarbazine and carboplatin or intravenous dacarbazine, cisplatin, carmustine and tamoxifen. Eur J Cancer. 1995;31A(6):876–81. doi: 10.1016/0959-8049(94)00459-5. [DOI] [PubMed] [Google Scholar]
- 58.Quan WD, Jr, Quan FM, Perez M, Johnson E. Outpatient Intravenous Interleukin-2 with Famotidine Has Activity in Metastatic Melanoma. Cancer Biother Radiopharm. 2012 doi: 10.1089/cbr.2012.1239. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Bleackley RC. A molecular view of cytotoxic T lymphocyte induced killing. Biochem Cell Biol. 2005;83(6):747–51. doi: 10.1139/o05-146. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U.S.A. 2004;101(Suppl 2):14639–45. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernatchez C, Radvanyi LG, Hwu P. Advances in the treatment of metastatic melanoma: adoptive T-cell therapy. Semin Onco. 2012;39(2):215–26. doi: 10.1053/j.seminoncol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velho TR, Kapiteijn E, Jager MJ. New therapeutic agents in uveal melanoma. Anticancer Res. 2012;32(7):2591–8. [PubMed] [Google Scholar]
- 68.Seeger JM, Schmidt P, Brinkmann K, et al. The Proteasome Inhibitor Bortezomib Sensitizes Melanoma Cells toward Adoptive CTL Attack. Cancer Res. 2010;70(5):1825–34. doi: 10.1158/0008-5472.CAN-09-3175. [DOI] [PubMed] [Google Scholar]
- 69.Tarhini AA, Iqbal F. CTLA-4 blockade: therapeutic potential in cancer treatments. Onco Targets Ther. 2010;3:15–25. doi: 10.2147/ott.s4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14(8):848–61. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- 71.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 73.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 74.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 75.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675, 206. J Clin Oncol. 2005;23(35):8968–77. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 76.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27(7):1075–81. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 77.Ribas A, Hauschild A, Kefford R, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. J Clin Oncol. 2008;26(May 20 suppl) abstr LBA0911. [Google Scholar]
- 78.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interfero alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30(3):322–8. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of singleagent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of andit-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Narazaki H, Zhu Y, Luo L, Zhu G, Chen L. CD137 agonist antibody prevents cancer recurrence: contribution of CD137 on both hematopoietic and nonhematopoietic cells. Blood. 2010;115(10):1941–8. doi: 10.1182/blood-2008-12-192591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y, Yang S, Ye Z, et al. Tumor cells expressing anti-CD137 scFv induce a tumor-destructive environment. Cancer Res. 2007;67(5):2339–44. doi: 10.1158/0008-5472.CAN-06-3593. [DOI] [PubMed] [Google Scholar]
- 85.Pennisi M. A mathematical model of immune-system-melanoma competition. Comput Math Methods Med. 2012 doi: 10.1155/2012/850754. 850754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatoru monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7(2):95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 87.Sznol M, Hodi FS, Margolin K, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) J Clin Oncol. 2008;26(May 20 suppl) abstr 3007. [Google Scholar]
- 88.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 89.Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell. 2002;2:5–7. doi: 10.1016/s1535-6108(02)00089-2. [DOI] [PubMed] [Google Scholar]
- 90.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 91.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 92.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 93.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 94.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: A phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27(17):2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 96.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26(13):2178–85. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 97.Amaravadi RK, Schuchter LM, Kramer A, et al. Preliminary results of a randomized phase II study comparing two schedules of temozolomide in combination with sorafenib in patients with advanced melanoma. ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2006;24:18S. (June 20 Supplement). [Google Scholar]
- 98.Eggermont AM, Robert C. New drugs in melanoma: it’s a whole new world. Eur J Cancer. 2011;47(14):2150–7. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 99.Chen J, Shen Q, Labow M, Gaither LA. Protein kinase D3 sensitizes RAF inhibitor RAF265 in melanoma cells by preventing reactivation of MAPK signaling. Cancer Res. 2011;71(12):4280–91. doi: 10.1158/0008-5472.CAN-10-3761. [DOI] [PubMed] [Google Scholar]
- 100.Sharfman WH, Hodi FS, Lawrence DP, et al. Results from the first-in-human (FIH) phase I study of the oral RAF inhibitor RAF265 administered daily to patients with advanced cutaneous melanoma. J Clin Oncol. 2011;29(suppl) abstr 8508. [Google Scholar]
- 101.Su Y, Vilgelm AE, Kelley MC, et al. RAF265 inhibits the growth of advanced human melanoma tumors. Clin Cancer Res. 2012;18(8):2184–98. doi: 10.1158/1078-0432.CCR-11-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Joseph EW, Pratilas CA, Poulikakos PI, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci USA. 2010;107:14903–8. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ribas A, Kim KB, Schuchter LM, et al. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. J Clin Oncol. 2011;29(suppl) abstr 8509. [Google Scholar]
- 105.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soffietti R, Trevisan E, Rudà R. Targeted therapy in brain metastasis. Curr Opin Oncol. 2012 doi: 10.1097/CCO.0b013e3283571a1c. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 108.Dummer R, Rinderknecht J, Goldinger SM, et al. An open-label pilot study of vemurafenib in previously treated metastatic melanoma patients with brain metastases. J Clin Oncol. 2011;29(suppl) abstr 8548. [Google Scholar]
- 109.Young K, Minchom A, Larkin J. BRIM-1, -2 and -3 trials: improved survival with vemurafenib in metastatic melanoma patients with a BRAF(V600E) mutation. Future Oncol. 2012;8(5):499–507. doi: 10.2217/fon.12.43. [DOI] [PubMed] [Google Scholar]
- 110.Ravnan MC, Matalka MS. Vemurafenib in Patients With BRAF V600E Mutation-Positive Advanced Melanoma. Clin Ther. 2012;34(7):1474–86. doi: 10.1016/j.clinthera.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 111.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kudchadkar R, Paraiso KH, Smalley KS. Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J. 2012;18(2):124–31. doi: 10.1097/PPO.0b013e31824b436e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kefford R, Arkenau H, Brown MP, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28(15s (suppl)) abstr 8503. [Google Scholar]
- 114.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 115.Long GV, Kefford RF, Carr P, et al. Phase 1/2 Study of GSK2118436, a Selective Inhibitor of V600 Mutant (Mut) BRAF Kinase: Evidence of Activity in Melanoma Brain Metastases (Mets) ESMO. 2010 Abst 5016. [Google Scholar]
- 116.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012;11(4):909–20. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 118.Anforth RM, Blumetti TC, Kefford RF, et al. Cutaneous Manifestations of Dabrafenib (GSK2118436): A Selective Inhibitor of Mutant BRAF in patients with Metastatic Melanoma. Br J Dermatol. 2012 doi: 10.1111/j.1365-2133.2012.11155.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 119.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18(2):555–67. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holt SV, Logié A, Odedra R, et al. The MEK1/2 inhibitor, selumetinib (AZD6244; ARRY-142886), enhances anti-tumour efficacy when combined with conventional chemotherapeutic agents in human tumour xenograft models. Br J Cancer. 2012;106(5):858–66. doi: 10.1038/bjc.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferguson J, Arozarena I, Ehrhardt M, Wellbrock C. Combination of MEK and SRC inhibition suppresses melanoma cell growth and invasion. Oncogene. 2012 doi: 10.1038/onc.2012.25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 125.von Euw E, Atefi M, Attar N, et al. Antitumor effects of the investigational selective MEK inhibitor TAK733 against cutaneous and uveal melanoma cell lines. Mol Cancer. 2012;11(1):22. doi: 10.1186/1476-4598-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vihinen PP, Hernberg M, Vuoristo MS, et al. A phase II trial of bevacizumab with dacarbazine and daily low-dose interferon-alpha2a as first line treatment in metastatic melanoma. Melanoma Res. 2010;20(4):318–25. doi: 10.1097/CMR.0b013e3283390365. [DOI] [PubMed] [Google Scholar]
- 127.Grignol VP, Olencki T, Relekar K, et al. A phase 2 trial of bevacizumab and high-dose interferon alpha 2B in metastatic melanoma. J Immunother. 2010;34(6):509–15. doi: 10.1097/CJI.0b013e31821dcefd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30(1):34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.von Moos R, Seifert B, Simcock M, et al. First-line temozolomide combined with bevacizumab in metastatic melanoma: a multicentre phase II trial (SAKK 50/07) Ann Oncol. 2012;23(2):531–6. doi: 10.1093/annonc/mdr126. [DOI] [PubMed] [Google Scholar]
- 130.Kruijff S, Bastiaannet E, Brouwers AH, et al. Use of S-100B to evaluate therapy effects during bevacizumab induction treatment in AJCC stage III melanoma. Ann Surg Oncol. 2012;19(2):620–6. doi: 10.1245/s10434-011-2027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kelly RJ, Rixe O. Axitinib (AG-013736) Recent Results Cancer Res. 2010;184:33–44. doi: 10.1007/978-3-642-01222-8_3. [DOI] [PubMed] [Google Scholar]
- 132.Choueiri TK. Axitinib, a novel anti-angiogenic drug with promising activity in various solid tumors. Curr Opin Investig Drugs. 2008;9(6):658–71. [PubMed] [Google Scholar]
- 133.Fruehauf J, Lutzky J, McDermott D, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17(23):7462–9. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 134.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res. 2012;22(3):236–43. doi: 10.1097/CMR.0b013e3283538293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Glen H, Mason S, Patel H, Macleod K, Brunton VG. E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer. 2011;11:309. doi: 10.1186/1471-2407-11-309. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boss DS, Glen H, Beijnen JH, et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer. 2012;106(10):1598–604. doi: 10.1038/bjc.2012.154. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moschos SJ, Sander CA, Wang W, et al. Pharmacodynamic (phase 0) study using etaracizumab in advanced melanoma. J Immunother. 2010;33(3):316–25. doi: 10.1097/CJI.0b013e3181c1f216. [DOI] [PubMed] [Google Scholar]
- 138.Hersey P, Sosman J, O’Day S, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or − dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116(6):1526–34. doi: 10.1002/cncr.24821. [DOI] [PubMed] [Google Scholar]
- 139.Jansen B, Wacheck V, Heere-Ress E, et al. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet. 2000;356(9243):1728–33. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 140.Bedikian AY, Millward M, Pehamberger H, et al. Oblimersen Melanoma Study Group: Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 141.Spugnini EP, Biroccio A, De Mori R, et al. Electroporation increases antitumoral efficacy of the bcl-2 antisense G3139 and chemotherapy in a human melanoma xenograft. J Transl Med. 2011;9:125. doi: 10.1186/1479-5876-9-125. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Madonna G, Ullman CD, Gentilcore G, Palmieri G, Ascierto PA. NF-κB as potential target in the treatment of melanoma. J Transl Med. 2012;10:53. doi: 10.1186/1479-5876-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004;64(14):4912–8. doi: 10.1158/0008-5472.CAN-04-0673. [DOI] [PubMed] [Google Scholar]
- 144.Su Y, Amiri KI, Horton LW, et al. A Phase I Trial of Bortezomib with Temozolomide in Patients with Advanced Melanoma: Toxicities, Antitumor Effects, and Modulation of Therapeutic Targets. Clin Cancer Res. 2010;16(1):348–57. doi: 10.1158/1078-0432.CCR-09-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Croghan GA, Suman VJ, Maples WJ, et al. A study of paclitaxel, carboplatin, and bortezomib in the treatment of metastatic malignant melanoma: a phase 2 consortium study. Cancer. 2010;116(14):3463–8. doi: 10.1002/cncr.25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeramian A, Sorolla A, Velasco A, et al. Inhibition of activated receptor tyrosine kinases by Sunitinib induces growth arrest and sensitizes melanoma cells to Bortezomib by blocking Akt pathway. Int J Cancer. 2012;130(4):967–78. doi: 10.1002/ijc.26096. [DOI] [PubMed] [Google Scholar]
- 147.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24(2):273–85. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- 148.Sánchez-Hernández I, Baquero P, Calleros L, Chiloeches A. Dual inhibition of (V600E)BRAF and the PI3K/ AKT/mTOR pathway cooperates to induce apoptosis in melanoma cells through a MEK-independent mechanism. Cancer Lett. 2012;314(2):244–55. doi: 10.1016/j.canlet.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 149.Pópulo H, Soares P, Lopes JM. Insights into melanoma: targeting the mTOR pathway for therapeutics. Expert Opin Ther Targets. 2012;16(7):689–705. doi: 10.1517/14728222.2012.691472. [DOI] [PubMed] [Google Scholar]
- 150.Rao RD, Windschitl HE, Allred JB, et al. Phase II trial of the mTOR inhibitor everolimus (RAD-001) in metastatic melanoma. ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2006;24:18S. (June 20 Supplement). [Google Scholar]
- 151.Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma: a phase 2 trial of the Sarah Cannon Oncology Research Consortium. Cancer. 2010;116(17):4122–9. doi: 10.1002/cncr.25320. [DOI] [PubMed] [Google Scholar]
- 152.O’Reilly T, Lane HA, Wood JM, et al. Everolimus and PTK/ZK show synergistic growth inhibition in the orthotopic BL16/BL6 murine melanoma model. Cancer Chemother Pharmacol. 2011;67(1):193–200. doi: 10.1007/s00280-010-1307-z. [DOI] [PubMed] [Google Scholar]
- 153.Fury MG, Sherman E, Haque S, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;69(3):591–8. doi: 10.1007/s00280-011-1734-5. [DOI] [PubMed] [Google Scholar]
- 154.Margolin K, Longmate J, Baratta T, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104(5):1045–8. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 155.Davies MA, Fox PS, Papadopoulos NE, et al. Phase I study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Clin Cancer Res. 2012;18(4):1120–8. doi: 10.1158/1078-0432.CCR-11-2436. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Margolin KA, Moon J, Flaherty LE, et al. Randomized phase II trial of sorafenib with temsirolimus or tipifarnib in untreated metastatic melanoma (S0438) Clin Cancer Res. 2012;18(4):1129–37. doi: 10.1158/1078-0432.CCR-11-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tabone-Eglinger S, Wehrle-Haller M, Aebischer N, Jacquier MC, Wehrle-Haller B. Membrane-bound Kit ligand regulates melanocyte adhesion and survival, providing physical interaction with an intraepithelial niche. FASEB J. 2012 doi: 10.1096/fj.12-206045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 158.Yoshida H, Kunisada T, Grimm T, Nishimura EK, Nishioka E, Nishikawa SI. Review: melanocyte migration and survival controlled by SCF/c-kit expression. J Investig Dermatol Symp Proc. 2001;6(1):1–5. doi: 10.1046/j.0022-202x.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 159.Willmore-Payne C, Holden JA, Hirschowitz S, Layfield LJ. BRAF and c-kit gene copy number in mutation-positive malignant melanoma. Hum Pathol. 2006;37(5):520–7. doi: 10.1016/j.humpath.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 160.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 161.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–34. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904–9. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 163.Minor DR, Kashani-Sabet M, Garrido M, O’Day SJ, Hamid O, Bastian BC. Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res. 2012;18(5):1457–63. doi: 10.1158/1078-0432.CCR-11-1987. [DOI] [PubMed] [Google Scholar]
- 164.Centers for Disease Control and Prevention – Department of Health and Human Services: National Program of Cancer Registries (NPCR) Available from: http://apps.nccd.cdc.gov/uscs/toptencancers.aspx [Last accessed: 15 August 2012].
- 165.Seigler HF, Lucas VS, Jr, Pickett NJ, Huang AT. DTIC, CCNU, bleomycin and vincristine (BOLD) in metastatic melanoma. Cancer. 1980;46(11):2346–8. doi: 10.1002/1097-0142(19801201)46:11<2346::aid-cncr2820461104>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]