Abstract
BACKGROUND
The contribution of obesity to hypoxemia has not been reported in a community-based study. Our hypothesis was that increasing obesity would be independently associated with lower SpO2 in an ambulatory elderly population.
METHODS
The Cardiovascular Health Study ascertained resting SpO2 in 2,252 subjects over age 64. We used multiple linear regression to estimate the association of body mass index (BMI) with SpO2 and to adjust for potentially confounding factors. Covariates including age, sex, race, smoking, airway obstruction (based on spirometry), self reported diagnosis of emphysema, asthma, heart failure, and left ventricular function (by echocardiography) were evaluated.
RESULTS
Among 2,252 subjects the mean and median SpO2 were 97.6% and 98.0% respectively; 5% of subjects had SpO2 values below 95%. BMI was negatively correlated with SpO2 (Spearman R = −0.27, P < .001). The mean difference in SpO2 between the lowest and highest BMI categories (< 25 kg/m2 and ≥ 35 kg/m2) was 1.33% (95% CI 0.89–1.78%). In multivariable linear regression analysis, SpO2 was significantly inversely associated with BMI (1.4% per 10 units of BMI, 95% CI 1.2–1.6, for whites/others, and 0.87% per 10 units of BMI, 95% CI 0.47–1.27, for African Americans).
CONCLUSIONS
We found a narrow distribution of SpO2 values in a community-based sample of ambulatory elderly. Obesity was a strong independent contributor to a low SpO2, with effects comparable to or greater than other factors clinically associated with lower SpO2.
Keywords: pulse oximetry, oxygen, obesity, body mass index, waist circumference, hypoxemia, pulmonary function test
Introduction
The contribution of obesity to the resting level of SpO2 has not been reported in a population-based study or in the elderly. Such information would be useful for clinicians to gauge whether decreased SpO2 readings are in a range potentially explained by increased weight, and to researchers assessing the health consequences of obesity. If obesity is associated with even small changes in oxygen saturation, the exposure to lower oxygen levels may have important clinical consequences, particularly in physiologic processes like respiratory drive, which are dependent on PO2 in the blood, or in situations of marginally sufficient oxygen delivery to important organs like the brain, as can be seen in the elderly.1,2
The Cardiovascular Health Study ascertained resting SpO2 on 2,252 subjects. In this report we test our a priori hypothesis that increasing levels of obesity are associated with a lower SpO2 in a population-based sample of ambulatory elderly.3,4
Methods
Subjects
The Cardiovascular Health Study is a population-based study of risk factors for cardiovascular disease in older adults (ages 65 and above at their baseline exam).5 Subjects were recruited from random samples of Medicare eligibility lists in Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Pittsburgh, Pennsylvania; and from age-eligible subjects in the same household. Potential subjects were excluded if they were institutionalized, wheelchair-bound in the home, or under active treatment for cancer, including hospice care, radiation, or chemotherapy. An original cohort of 5,201 subjects was enrolled in 1989–1990, and a second cohort of 687, predominately African Americans, was enrolled in 1992–1993.3 Subjects provided written informed consent, and study methods were approved by the institutional review board at each participating center. Details of the design and recruitment have been published.3–5 The coordinating center is based in Seattle, Washington, and has approval from the University of Washington institutional review board (37714).
QUICK LOOK.
Current knowledge
Obesity causes restrictive changes to lung function, with decreased chest wall compliance and reduced lung volumes and capacities. The impact of obesity on oxygenation is less well described.
What this paper contributes to our knowledge
In a population of elderly adults, body mass index was negatively correlated with SpO2. Obesity was a strong independent contributor to a low SpO2, with effects comparable to or greater than other clinical factors commonly associated with lower SpO2. Obesity affects lung function and diminishes oxygen exchange.
A total of 3,326 Cardiovascular Health Study subjects, out of 4,413 still living and participating in the Cardiovascular Health Study, attended the 1996–1997 clinic visit, during which a standard 6-min walk test with oximetry was offered.6 Approximately one third of the subjects were excluded or chose not to perform the walk test, leaving 2,273 subjects with oximetry results. Exclusions from testing included: use of an ambulatory aid such as wheelchair, crutches, walker or cane; heart attack, angioplasty, or heart surgery within past 3 months; resting heart rate < 50 or > 110 beats/min; systolic blood pressure > 200 m Hg or diastolic blood pressure > 110 m Hg; previous echocardiographic evidence of severe aortic stenosis; new or worsening symptoms of chest pain, shortening of breath, or fainting in the past 8 weeks; electrocardiogram with ventricular fibrillation, acute injury, acute ischemia, acute myocardial infarction, or any other acute cardiac condition; subject refusal or technician’s discretion or resting oxygen saturation < 90% (n = 1). The 2,252 subjects included in this report consist of subjects with available pre-exercise SpO2 results in addition to body mass index (BMI) and covariate measures.
SpO2
While the subject was seated, a semi-disposable oximeter sensor (D-25, Nellcor/Puritan Bennett, Pleasanton, California) was applied to the index finger of the non-dominant hand, after ensuring that nail polish was not present, and was then attached to the oximeter (N-3000, Nellcor/Puritan Bennett, Pleasanton, California). After verification of good perfusion, the values for SpO2 and pulse rate were recorded.
Anthropomorphic Measurements
Anthropometric measurements were performed by trained personnel using standardized protocols. Subjects wore standard examination suits and no shoes. Height was measured with a stadiometer and weight with a balance scale, using a standard protocol during the 1996–1997 study visits. BMI was calculated as weight (kg)/height (m)2.2 Waist circumference was measured at the level of the umbilicus.
Covariates
Sex, race, smoking history, and medical history were self-reported. Subjects were allowed the following choices for race: white (n = 1,909), black (African American) (n = 332), American Indian/Alaska native (n = 3), Asian/Pacific islander (n = 2), and other (n = 6). Race for the purposes of this analyses was classified as African American (n = 332) and white/other (n = 2,010). Spirometry was measured following American Thoracic Society guidelines, using a water-sealed spirometer at the same visit that oximetry was performed.7 Airway obstruction was defined as a low FEV1/FVC and a low FEV1, defined as below the lowest 5th percentile, based on reference equations developed from a healthy sample of Cardiovascular Health Study subjects.8 Left ventricular ejection fraction was assessed by echocardiography on an exam 2 years prior, with impaired defined as < 45%.9
Statistical Analyses
Categories of BMI were defined using standard clinical definitions: normal < 25 kg/m2, overweight 25–29.99 kg/m2, obesity class I (30–34.99), and obesity class II or greater (> 35 kg/m2). The covariates are described according to these categories. Categorical variables were compared using chi-square test, and continuous variable using analysis of variance. Initial correlations of SpO2 with BMI, waist size, and other continuous covariates were assessed using the Spearman coefficient, due to the skewed nature of distributions of some variables. For categorical covariates, mean SpO2 between categories was compared using analysis of variance, t tests, and the Spearman coefficient of ordinal categories with SpO2 was assessed.
Exploratory analyses and graphs of BMI and SpO2, including spline models, established that the relationship was reasonably linear. Therefore, linear regression models were used to estimate independent associations of BMI or waist circumference with resting SpO2. The models were adjusted for covariates chosen based upon clinical knowledge, associations with SpO2, and effects on the magnitude of the association with obesity. Variables that were not possible confounders and were not significant were dropped. Multiplicative interactions of BMI (or waist size) with race and sex were evaluated. As a result of significant interactions for BMI and obstruction with race we analyzed data separately by race. P values were based upon 2-tailed tests and statistical significance was defined as P < .05. Analyses were performed with statistics software (SPSS, SPSS, Chicago, Illinois, and Stata, StataCorp, College Station, Texas.).
Results
The sample included 2,252 ambulatory subjects in the Cardiovascular Health Study. Subjects with SpO2 measures were younger, were less likely to be female and African American, had less cardiopulmonary disease by self-report and testing, and had smoked less than those who did not attempt the test or those who did not attend the clinic visit (Table 1). Although subjects with SpO2 measures had lower mean waist circumference than those without measures, mean BMI was similar among the 2 groups.
Table 1.
Comparison of Cardiovascular Health Study Subjects With and Without Oximetry Results: 1996–1997
| With SpO2 | Without SpO2 | P | |
|---|---|---|---|
| n | 2,273 | 2,139 | |
| Age, y | 77.4 ± 4.3 | 79.8 ± 5.6 | <.001 |
| Female, % | 60.4 | 63.1 | .035 |
| African American, % | 14.8 | 18.8 | .005 |
| Physician diagnosis, % | |||
| Heart failure | 5.7 | 16.5 | <.001 |
| Emphysema | 3.3 | 5.8 | <.001 |
| Asthma | 5.9 | 7.4 | .03 |
| Bronchitis | 6.3 | 7.5 | .06 |
| Pneumonia | 2.4 | 4.9 | <.001 |
| Current smoker, % | 6.9 | 8.6 | .02 |
| Pack years | 14.9 ± 23.3 | 16.5 ± 25.4 | <.001 |
| Waist circumference, cm | 96.6 ± 12.7 | 98.1 ± 14.3 | .003 |
| Body mass index, kg/m2 | 26.8 ± 4.4 | 27.1 ± 5.2 | .055 |
| FEV1, L | 1.95 ± 0.59 | 1.84 ± 0.64 | <.001 |
| FVC, L | 2.52 ± 0.73 | 2.39 ± 0.78 | <.001 |
| FEV1/FVC | 0.77 ± 0.08 | 0.77 ± 0.09 | .056 |
| Airway obstruction, % | 3.9 | 6.4 | .005 |
| Decreased left ventricular ejection fraction, % | 8.1 | 10.9 | .02 |
± Values are mean ± SD.
The median BMI was 26.3 kg/m2 (IQR 23.8–29.1). Subjects were distributed as follows across BMI categories: normal or underweight (35%), overweight (45%), obesity class I (15%), and obesity class II or greater (5%). Higher BMI categories were related to younger age, female sex, African American race, less current smoking, greater waist circumference, and higher FEV1/FVC (Table 2).
Table 2.
Characteristics of the Cardiovascular Health Study Subjects With SpO2 Values, Stratified by Body Mass Index Category*
| Normal | Overweight | Obese Class I | Obese Class II | P for Comparison Across Categories | |
|---|---|---|---|---|---|
| n (total 2,252) | 797 | 1,013 | 336 | 106 | |
| Age, mean ± SD y | 78.4 ± 4.78 | 77.3 ± 4.04 | 76.1 ± 3.82 | 76.1 ± 3.35 | < .001 |
| Female, % | 63.4 | 55.1 | 65.2 | 75.5 | < .001 |
| African American, % | 9.8 | 14.5 | 23.2 | 27.4 | < .001 |
| Heart failure, % | 6.3 | 5.0 | 6.5 | 6.6 | .60 |
| Emphysema, % | 4.4 | 2.8 | 3.0 | 1.9 | .20 |
| Asthma, % | 4.7 | 6.2 | 8.1 | 5.7 | .16 |
| Bronchitis, % | 6.5 | 5.8 | 5.9 | 9.9 | .43 |
| Pneumonia, % | 2.2 | 2.6 | 1.8 | 4.9 | .33 |
| Current smoker, % | 9.9 | 5.0 | 6.6 | 2.9 | <.001 |
| Pack years, median (IQR) | 0 (0–22.6) | 0.1 (0–24.8) | 0 (0–23.5) | 0.5 (0–0.45) | .53† |
| Waist circumference, mean ± SD cm | 86.3 ± 8.7 | 98.3 ± 7.9 | 108.3 ± 9.1 | 121.8 ± 10.5 | < .001 |
| FEV1, mean ± SD L | 1.92 ± 0.59 | 2.01 ± 0.59 | 1.89 ± 0.56 | 1.82 ± 0.53 | < .001 |
| FVC, mean ± SD L | 2.52 ± 0.74 | 2.60 ± 0.73 | 2.39 ± 0.67 | 2.30 ± 0.65 | < .001 |
| FEV1/FVC, mean ± SD | 0.76 ± 0.08 | 0.78 ± 0.07 | 0.79 ± 0.08 | 0.79 ± 0.06 | < .001 |
| Airway obstruction, % | 5.7 | 3.3 | 2.0 | 3.4 | .02 |
| Decreased left ventricular ejection fraction % | 9.4 | 8.3 | 8.3 | 6.6 | .74 |
BMI categories: normal = < 25 kg/m2, overweight = 25–29.99 kg/m2, obese class I = 30–34.99 kg/m2, obese class II = > 35 kg/m2
Log of pack-years
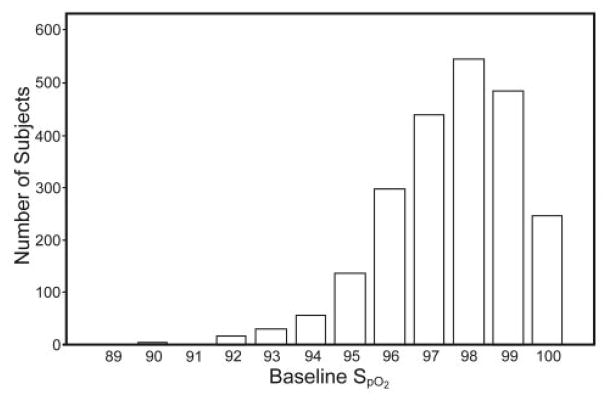
The mean and median SpO2 were 97.6 ± 1.74% and 98.0% (IQR 97–99%), respectively. The distribution of values was skewed to higher values, with a range from 81% to 100% (Fig. 1). Only 2 subjects had SpO2 values below 90%, one of which (81%) was removed from further analyses as an outlier. Five percent of subjects had SpO2 values below 95%.
Fig. 1.
The distribution of resting SpO2 in 2,252 older people.
BMI as a continuous variable was negatively correlated with SpO2 (Spearman R = −0.27, P < .001). In unadjusted analyses, mean SpO2 differed significantly by category of BMI, with a linear relationship between mean SpO2 and BMI category (Table 3). Mean SpO2 also differed significantly by race, sex, airway obstruction, obesity, smoking status, emphysema diagnosis, and left ventricular function. In addition, SpO2 was also significantly associated with the log of pack-years (r = −0.14, P < .001), deteriorating airway obstruction, as measured by FEV1/FVC (r = 0.12, P < .001), and age (r = −0.04, P = .045).
Table 3.
Mean SpO2 by Subject Characteristics
| n | SpO2 (%) mean ± SD |
P | |
|---|---|---|---|
| Body mass index | 2,252 | < .001 | |
| Normal | 98.02 ±1.6 | ||
| Overweight | 97.51 ±1.6 | ||
| Obese class I | 96.90 ±1.8 | ||
| Obese class II | 96.69 ±1.8 | ||
| Sex | 2,252 | < .001 | |
| Male | 97.32 ±1.7 | ||
| Female | 97.72 ±1.7 | ||
| Race | 2,252 | < .001 | |
| African American | 97.99 ±1.6 | ||
| White/other | 97.49 ±1.7 | ||
| Smoking | 2,222 | < .001 | |
| Never | 97.73 ±1.7 | ||
| Past | 97.40 ±1.7 | ||
| Current | 97.33 ± 1.8 | ||
| Pack years | 2,199 | < .001 | |
| ≤ 60 | 97.6 ± 1.7 | ||
| > 60 | 96.8 ± 1.9 | ||
| Airway obstruction | 1,972 | < .001 | |
| No | 97.60 ±1.7 | ||
| Yes | 96.49 ±1.9 | ||
| Emphysema | 2,247 | < .001 | |
| No | 97.59 ±1.7 | ||
| Yes | 96.83 ±1.7 | ||
| Asthma | 2,211 | .24 | |
| No | 97.57 ±1.7 | ||
| Yes | 97.45 ±1.6 | ||
| Heart failure | 2,122 | .01 | |
| No | 97.16 ± 1.7 | ||
| Yes | 97.59 ± 1.8 | ||
| Left ventricular function | 2,058 | .14 | |
| Normal | 97.60 ±1.7 | ||
| Borderline | 97.35 ±1.9 | ||
| Impaired | 96.92 ±1.8 |
In multivariable linear regression analyses the negative association between SpO2 and BMI was significant, and there was an interaction between BMI and race (P = .05). For white/other there was a −0.14 difference in SpO2 with each unit increase in BMI (Table 4). For African Americans the difference was smaller (−0.09) although still highly statistically significant (see Table 4). The unadjusted regression line between SpO2 and BMI by race is provided in Figure 2. There was also an interaction between airway obstruction and race (−2.35% decline in SpO2 in African Americans, vs −0.98% for white/other in those with obstruction, compared to those without obstruction). Other covariates significantly related to SpO2 included age and current smoking. Higher waist size was also an independent predictor of a lower SpO2 (P < .001), but there were no significant interactions between race and obesity when using waist size as the index of obesity in the model (Table 5).
Table 4.
Multivariable Analysis of Body Mass Index in Relation to SpO2, by Race Category
| Coefficient (95% CI) | P | |
|---|---|---|
| White/other | ||
| Body mass index | −0.14 (−0.16 to −0.12) | < .001 |
| Age | −0.04 (−0.06 to −0.02) | < .001 |
| Male | −0.26 (−0.42 to −0.10) | .002 |
| Smoking status | ||
| Never | ||
| Past smoker | −0.12 (−0.28 to −0.05) | .17 |
| Current smoker | −0.48 (−0.82 to −0.14) | .005 |
| Airway obstruction | −0.98 (−1.38 to −0.57) | < .001 |
| Emphysema | −0.34 (−0.77 to 0.10) | .13 |
| African American | ||
| Body mass index | −0.09 (−0.13 to −0.05) | < .001 |
| Age | −0.05 (−0.09 to −0.01) | .02 |
| Male | −0.65 (−1.05 to −0.25) | .002 |
| Smoking status | ||
| Never | ||
| Past smoker | −0.17 (−0.56 to 0.22) | .40 |
| Current smoker | −0.50 (−1.22 to −0.22) | .17 |
| Airway obstruction | −2.35 (−3.67 to −1.03) | < .001 |
| Emphysema | −0.64 (−2.04 to 0.76) | .37 |
Fig. 2.
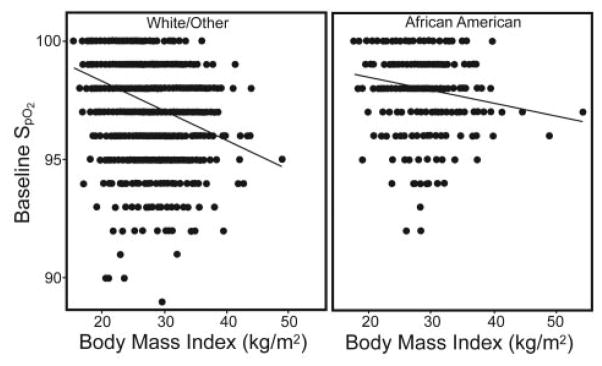
Unadjusted regression of SpO2 and body mass index by race.
Table 5.
Multivariable Analysis of Waist Circumference in Relation to SpO2 for Race Groups Combined
| Coefficient (95CI) | P | |
|---|---|---|
| Waist | −0.40 (−0.05 to −0.03) | < .001 |
| Age | −0.03 (−0.05 to −0.01) | < .001 |
| Male | −2.02 (−0.36 to −0.05) | .10 |
| African American | 0.50 (0.29 to 0.71) | < .001 |
| Smoking status | ||
| Never | ||
| Past smoker | −0.09 (−0.25 to 0.06) | .25 |
| Current smoker | −0.38 (−0.68 to −0.07) | .02 |
| Obstruction | −1.04 (−1.43 to −0.65) | < .001 |
| Emphysema | −0.42 (−0.84 to 0.00) | .05 |
Restricting regression analyses to subjects with stable weight (within 4.55 kg, n = 1,752) in the year prior to the oximetry measurement did not appreciably alter the results.
Discussion
The average resting SpO2 in a community-based population of older people who agreed to participate in a timed walk was approximately 98%, and values were rarely below 95%. To our knowledge, there is no comparable information available from a large community-based population.
Obesity was a major determinant of hypoxemia in this community-based sample. This suggests that the effects of obesity on gas exchange in adults may be under-appreciated relative to other clinical entities that are widely perceived as being associated with hypoxemia (ie, smoking, heart failure, obstructive lung disease), but had a similar magnitude of association with SpO2 levels. To our knowledge, only one other study has reported a lower SpO2 associated with obesity, in 871 emergency department patients and hospital workers with a median age of 38 and who did not have cardiopulmonary complaints.10 Our study confirms that this association is present in a much larger elderly community-based sample, and that it persists after more comprehensive adjustment for confounders, including physiologic measures of cardiac and respiratory function. The subjects in the prior study had a median SpO2 of 99%; while values below 97% were rare (5.7% prevalence) and 99–100% was the most frequent SpO2. The lower SpO2 values seen in the Cardiovascular Health Study cohort are probably due to a much higher age range.
The effects of obesity on lung function are well described.1,2 Obesity is associated with restrictive ventilatory impairment and diminished FEV1, FVC, vital capacity, total lung capacity, functional residual capacity and expiratory reserve volume.11,12 These changes are felt to be due to the added mechanical load of adipose tissue, which reduces chest wall compliance and impedes diaphragm descent. The pulmonary function changes are mild but may be more significant with severe central obesity. Also obesity may cause peripheral airways disease and air trapping. Our results show that obesity is associated with oxygen saturation, even after adjusting for airways obstruction. The most likely direct mechanism of increased BMI on reduced oxygen saturation would be increased ventilation/perfusion mismatch, with a possible contribution from decreased ventilation and increased oxygen consumption.
The magnitude of the correlation (R = −0.27) and the decrease in SpO2 associated with increasing BMI was modest (approximately 1% lower values in subjects with class II obesity than in those with normal BMI). The prognostic importance of this finding with regards to worse outcome in this cohort is uncertain, and prospective study is needed to assess its impact.
The change in SpO2 per unit change in BMI was 43% less in African Americans, relative to white/other. African Americans on average have lower abdominal visceral adipose tissue than white/other.13 Higher visceral fat has been associated with decreased lung volumes, most likely due to impaired descent of the diaphragm.14 The relationship with lower abdominal visceral adipose tissue in African Americans is supported by the absence of a significant interaction between waist size and African American race on SpO2.
Several limitations should be considered when evaluating the results of this study. There was a high rate of discretionary exclusions from the 6-min walk test. We did not independently verify the accuracy of our SpO2 measurements using arterial blood gases in a subsample of the cohort. We relied on the long-term accuracy of the pulse oximeters (stated by the manufacturer to be within 4%), and did not use an SpO2 validation instrument, but this is rarely done in clinical practice. The oximetry measurements were made in 1996–1997, and the results do not reflect improvements in accuracy and reliability of pulse oximetry technology that may have occurred since then. Echocardiography was performed 2 years prior to the SpO2 measurement and may have led to measurement error regarding left ventricular impairment at the time of oximetry. Echocardiography did not include assessment of diastolic dysfunction, which is common in the elderly and may lead to decreased SpO2. We did not consider use of medications (ie, inhalers) that may have influenced SpO2. Carboxyhemoglobin can cause elevation of SpO2 readings and may have led to overestimation of SpO2 in heavy smokers. The study results do not apply to the supine position or exercise, but these settings would be very useful to explore.
Conclusions
In conclusion, we found a narrow distribution of SpO2 values in a community-based sample of ambulatory elderly in whom SpO2 values below 95% were rare. Obesity was a strong independent contributor to a low SpO2, with effects comparable to or greater than other clinical factors commonly associated with lower SpO2. This suggests that the effects of obesity on lung function diminish oxygen exchange. The prognostic implications of this finding with regards to long-term outcomes are unclear and require further prospective evaluation,
Acknowledgments
This research was partly supported by National Institutes of Health grants N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01-HC-55222, N01-HC-75150, N01-HC-45133, National Heart, Lung, and Blood Institute grant HL080295, the National Institute of Neurological Disorders and Stroke, and National Institute on Aging grants AG-023629, AG-15928, AG-20098, and AG-027058. The Cardiovascular Health Study principal investigators and institutions are listed at http://www.chs-nhlbi.org/pi.htm.
Footnotes
Dr Fan has disclosed a relationship with Uptake Medical. Dr Au has disclosed a relationship with Gilead Sciences. The other authors have disclosed no conflicts of interest. The views herein are those of the authors and do not represent the Department of Veterans Affairs.
Contributor Information
Dr. Vishesh K. Kapur, Division of Pulmonary and Critical Care Medicine, University of Washington, Seattle, Washington.
Mr. Anthony G. Wilsdon, Collaborative Health Studies Coordinating Center, Department of Biostatistics, University of Washington, Seattle, Washington.
Dr. David Au, Health Services Research and Development Service, Veterans Affairs Puget Sound Health Care System, and with the Division of Pulmonary and Critical Care Medicine, University of Washington, Seattle, Washington.
Dr. Mark Avdalovic, Division of Pulmonary and Critical Care Medicine, University of California Davis School of Medicine, Davis, California.
Dr. Paul Enright, Respiratory Science Center, College of Medicine, University of Arizona, Tucson, Arizona.
Dr. Vincent S Fan, Health Services Research and Development Service, Veterans Affairs Puget Sound Health Care System, and with the Division of Pulmonary and Critical Care Medicine, University of Washington, Seattle, Washington.
Dr. Nadia N. Hansel, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University, Baltimore, Maryland.
Dr. Susan R. Heckbert, Department of Epidemiology, University of Washington, Seattle, Washington.
Dr. Rui Jiang, Department of Medicine, Department of Epidemiology, Columbia University Medical Center, New York, New York.
Dr. Jerry A. Krishnan, Division of Pulmonary, Critical Care, Sleep, and Allergy, University of Illinois Hospital and Health Sciences System, Chicago, Illinois.
Dr. Kenneth Mukamal, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Dr. Sachin Yende, Clinical Research, Investigation, and Systems Modeling of Acute Illness Center, Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Dr. R Graham Barr, Department of Medicine, Department of Epidemiology, Columbia University Medical Center, New York, New York.
References
- 1.Dempsey JA. Crossing the apnoeic threshold causes and consequences. Exp Physiol. 2004;90(1):13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 2.Carlson BW, Neelon VJ, Carlson JR, Hartman M, Bliwise DL. Cerebral oxygenation in wake and during sleep and its relationship to cognitive function in community-dwelling older adults without sleep disordered breathing. J Gentol A Biol Sci. 2011;66(1):150–156. doi: 10.1093/gerona/glq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 4.Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65(1):63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 6.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB. Cardiovascular Health Study. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 7.Griffith KA, Sherrill DL, Siegel EM, Manolio TA, Bonekat HW, Enright PL. Predictors of loss of lung function in the elderly: the Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163(1):61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 8.Enright PL, Kronmal RA, Higgins M, Schenker M, Haponik EF. Spirometry reference values for women and men 65 to 85 years of age. Cardiovascular Health Study. Am Rev Respir Dis. 1993;147(1):125–133. doi: 10.1164/ajrccm/147.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Rautaharju PM, Manolio TA, Siscovick D, Zhou SH, Gardin JM, Kronmal R, et al. Utility of new electrocardiographic models for left ventricular mass in older adults. The Cardiovascular Health Study Collaborative Research Group. Hypertension. 1996;28(1):8–15. doi: 10.1161/01.hyp.28.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Witting MD, Scharf SM. Diagnostic room-air pulse oximetry: effects of smoking, race, and sex. Am J Emerg Med. 2008;26(2):131–136. doi: 10.1016/j.ajem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Harrington J, Lee-Chiong T. Obesity and aging. Clin Chest Med. 2009;30(3):609–614. doi: 10.1016/j.ccm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.McCool FD, Rochester DF. The lungs and chest wall diseases. In: Murray JF, Nadel JA, editors. The textbook of respiratory medicine. 2. WB Saunders; 1994. pp. 2537–2539. [Google Scholar]
- 13.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in White/Others and African American adults. Am J Clin Nutr. 2010;91(1):7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi A, Fantin F, Di Francesco V, Guariento S, Giuliano K, Fontana G, et al. Body composition and pulmonary function in the elderly: a 7-year longitudinal study. Int J Obes (Lond) 2008;32(9):1423–1430. doi: 10.1038/ijo.2008.103. [DOI] [PubMed] [Google Scholar]