Abstract
Objective
To investigate the role of Pyk2, a proline-rich non-receptor tyrosine kinase, in GPCR agonist, thrombin-induced HASMC growth and migration and injury-induced vascular wall remodeling.
Methods and results
Thrombin, a GPCR agonist, activated Pyk2 in a time-dependent manner and inhibition of its stimulation attenuated thrombin-induced HASMC migration and proliferation. Thrombin also sequentially activated Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 and interference with stimulation of these molecules attenuated thrombin-induced HASMC migration and proliferation. In addition, adenovirus-mediated expression of dominant negative Pyk2 inhibited thrombin-induced Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 stimulation. Balloon injury also caused activation of Pyk2, Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 in the carotid artery of rat and these responses were sensitive to inhibition by dominant negative Pyk2. Furthermore, inhibition of Pyk2 activation resulted in reduced recruitment of SMC onto the luminal surface and their proliferation in the intimal region leading to suppression of neointima formation.
Conclusion
Together, these results demonstrate for the first time that Pyk2 plays a crucial role in GPCR agonist thrombin-induced HASMC growth and migration as well as balloon injury-induced neointima formation.
INTRODUCTION
Thrombin is generated at the sites of vascular injury due to exposure of platelets to sub-endothelial collagen and subsequent activation of blood clotting process (1). Thrombin, in addition to its indispensable role in blood clotting, acts as a mitogen and chemotactic factor to a variety of cell types, including fibroblasts and vascular smooth muscle cells (VSMCs) (2–5). Thrombin mediates its effects via protease-activated receptors 1–4 (PAR1–4) that are coupled to various G proteins (2, 5–7). Many studies have also demonstrated that G protein-coupled receptor (GPCR) agonists, such as angiotensin II (AngII), lysophosphatidic acid (LPA) and thrombin, transactivate receptor tyrosine kinases (RTKs) in mediating their growth and/or chemotactic effects (8, 9). Among the G proteins, Gs, Gi, Gq/11 and G12/13 are coupled to PAR 1–4 and mediate thrombin’s effects depending on the cell type (10–12). Activation of these G proteins via coupling to various phospholipase Cs (PLCs) leads to generation of second messenger molecules, DAG and IP3 which in turn results in increased intracellular calcium levels and protein kinase C (PKC) activation (13). One of the signaling molecules whose activation process depends on calcium is Pyk2 (14). Pyk2 is a proline-rich non-receptor tyrosine kinase whose function has been linked to cell motility (15). It was also reported that Pyk2 bridges the GPCR agonist, AngII-mediated calcium-PKC signaling with mitogen-activated protein kinases in VSMCs (16). Pyk2 has also been shown to be involved in VSMC growth in response to PDGF-BB (17). However, nothing is known in regard to the role of Pyk2 in thrombin-induced human aortic smooth muscle cell (HASMC) growth and migration and injury-induced vascular wall remodeling. Therefore, in the present investigation, we sought to address the role of Pyk2 in thrombin-induced HASMC growth and migration. Our findings reveal that thrombin activates Pyk2 in HASMCs and mediates their growth and migration. In addition, activation of Pyk2 is essential for thrombin-induced Gab1-p115 RhoGEF-Rac1-RhoA-Pak1 stimulation leading to HASMC growth and migration. Furthermore, balloon injury caused activation of Pyk2, Gab1, p115 RhoGEF, Rac1, RhoA and Pak1. Blockade of Pyk2 attenuated balloon injury-induced Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 activation resulting in reduced SMC migration/proliferation and neointima formation.
MATERIALS AND METHODS
The construction of adenoviral vectors, cell culture, cell growth and migration, double immunofluorescence staining, histological staining, immunoprecipitation, pull-down assays, transfections and transductions, Western blotting, common carotid artery balloon injury and in vivo smooth muscle cell migration were previously described (18–23). All the experiments involving animals were performed in accordance with the relevant guidelines and regulations approved by the Institutional Animal Care & Use Committee of the University of Tennessee Health Science Center, Memphis, TN. For detailed methods, please refer to online-only Data Supplement.
RESULTS
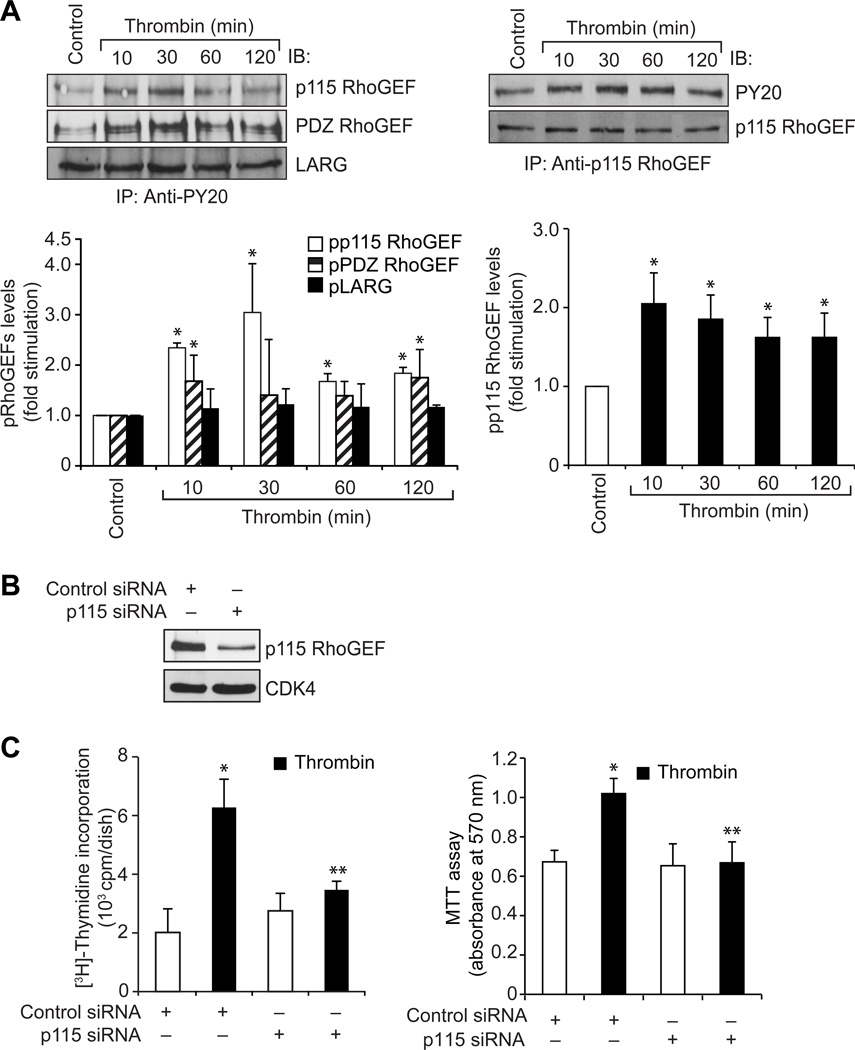
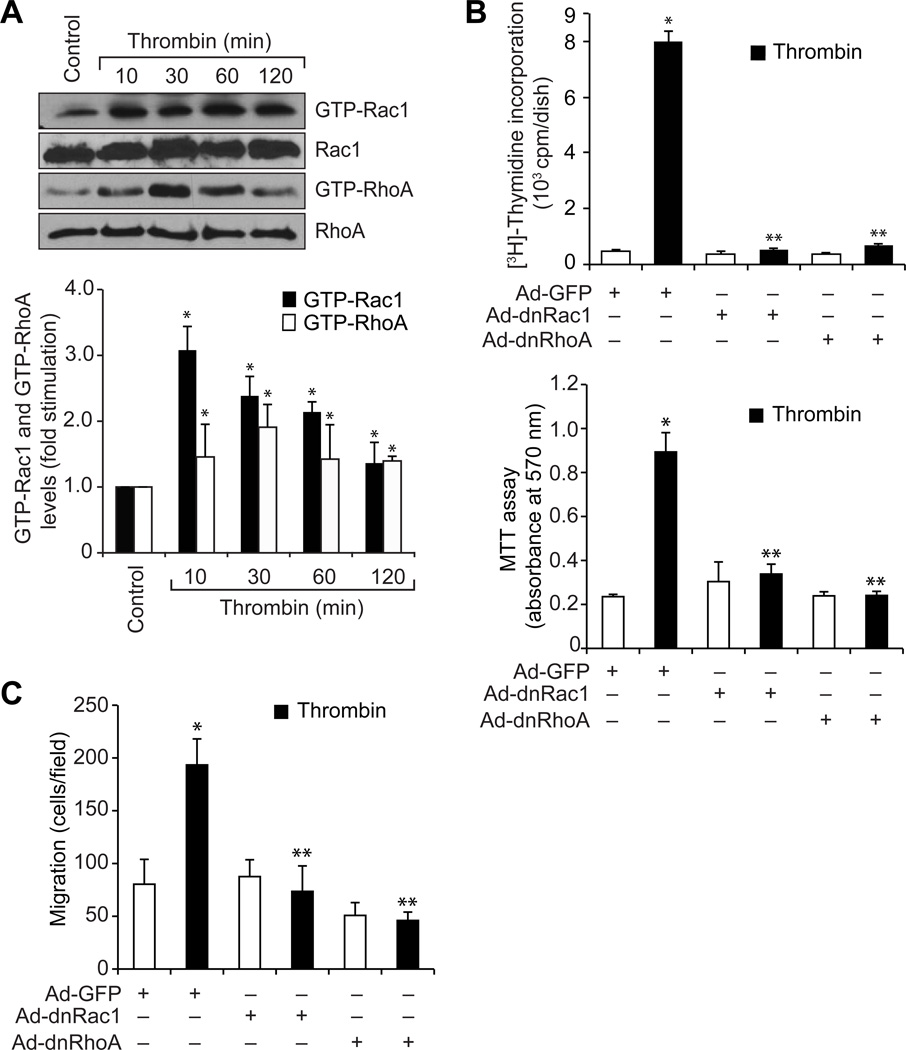
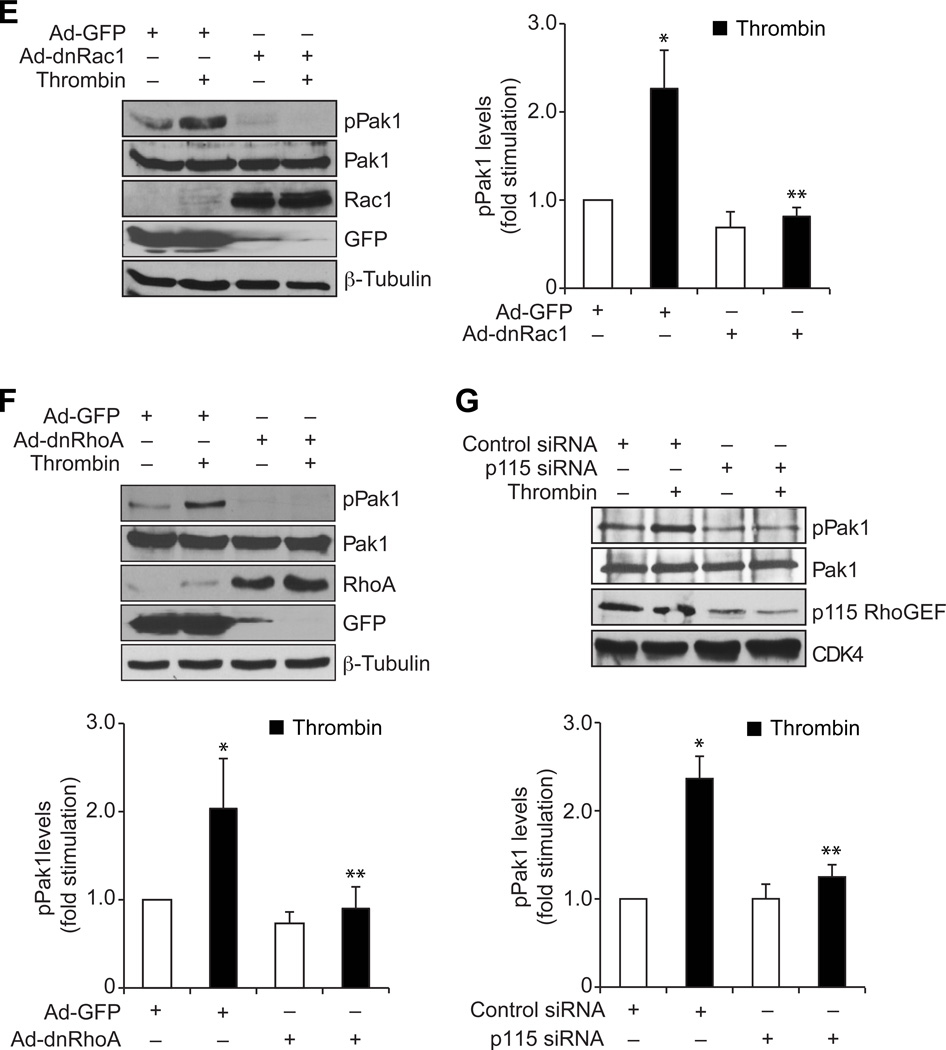
To understand the role of GPCR signaling events in vascular wall remodeling, we have studied the role of Pyk2 in thrombin-induced HASMC growth and migration. Thrombin stimulated Pyk2 tyrosine phosphorylation in a time-dependent manner (Figure 1A). Interference with Pyk2 activation by adenovirus-mediated expression of its dominant negative mutant or siRNA-mediated depletion attenuated thrombin-induced HASMC growth and migration (Figure 1B & C). To explore the signaling events of how Pyk2 mediates HASMC growth and migration, we studied the role of Gab1, a scaffolding adopter protein (24). Thrombin induced Gab1 tyrosine phosphorylation in a time-dependent manner (Figure 2A). Inhibition of Gab1 by its dominant negative mutant blocked thrombin-induced HASMC growth and migration (Figure 2B & C). In addition, inhibition of Pyk2 activation attenuated thrombin-induced Gab1 tyrosine phosphorylation (Figure 2D). To find whether Pyk2-Gab1 signaling leads to activation of any RhoGEFs, we have tested the effect of thrombin on the stimulation of p115 RhoGEF, PDZ RhoGEF and LARG, the RhoA-specific GEFs (25). Thrombin stimulated the tyrosine phosphorylation of p115 RhoGEF and PDZ RhoGEF but not LARG in HASMCs (Figure 3A). Since p115 RhoGEF activation was found to be robust, we next focused on the role of this RhoGEF in thrombin-induced HASMC growth and migration. Small interfering RNA-mediated down regulation of p115 RhoGEF substantially inhibited thrombin-induced HASMC growth and migration (Figure 3B–D). Furthermore, adenovirus-mediated expression of either dnPyk2 or dnGab1 inhibited thrombin-induced p115 RhoGEF tyrosine phosphorylation (Figure 3E & F). Since Gab1 is an adaptor molecule, we asked the question whether it directly interacts with p115 RhoGEF. In response to thrombin, p115 RhoGEF was found to be associated with Gab1 in a time-dependent manner (Figure 3G). In addition, inhibition of Gab1 activation attenuated thrombin-induced association of p115 RhoGEF with Gab1 (Figure 3H). RhoGEFs mediate activation of Rho GTPases by facilitating the exchange of GDP for GTP (26, 27). To identify the Rho GTPases activated by p115 RhoGEF, first we have studied the time course effect of thrombin on Rac1 and RhoA activation. Thrombin stimulated Rac1 and RhoA in a time-dependent manner (Figure 4A). Interestingly, dominant negative mutant-mediated blockade of either Rac1 or RhoA inhibited thrombin-induced HASMC growth and migration (Figure 4B & C). Down regulation of p115 RhoGEF by its siRNA prevented thrombin-induced Rac1 and RhoA activation (Figure 4D). Similarly, inhibition of either Gab1 or Pyk2 by adenovirus-mediated expression of their respective dominant negative mutants also attenuated both Rac1 and RhoA activation (Figure 4E & F). Previously, we have reported that RhoA mediates Rac1 activation downstream to LARG in response to thrombin in RASMCs (18). So, we asked the question whether there is any interaction between Rac1 and RhoA in HASMCs in response to thrombin. Inhibition of Rac1 blocked thrombin-induced RhoA activation (Figure 4G). On the other hand, blockade of RhoA stimulation had no effect on Rac1 activation (Figure 4H). Since both the Rho GTPases target Pak1 (26–29), we next wanted to find whether thrombin activates Pak1 in HASMCs. Thrombin induced Pak1 activation in a time-dependent manner (Figure 5A). Furthermore, down regulation of Pak1 levels by its siRNA inhibited thrombin-induced HASMC growth and migration (Figure 5B–D). In addition, adenovirus-mediated expression of either dnRac1 or dnRhoA attenuated thrombin-induced Pak1 activation (Figure 5E & F). Down regulation of p115 RhoGEF levels by its siRNA or adenovirus-mediated expression of either dominant negative Gab1 or dominant negative Pyk2 also blocked thrombin-induced Pak1 activation (Figure 5G–I). The observed effects with the use of adenoviral vectors for the delivery of dominant negative mutants of the indicated molecules into cells were not due to cell death as transduction with Ad-GFP had little (<5%) effect on HASMC viability (Online Figure I).
Figure 1. Pyk2 mediates thrombin-induced HASMC growth and migration.
A. An equal amount of protein from control and the indicated time periods of thrombin (0.5 U/ml)-treated HASMCs was analyzed by Western blotting for pPyk2 using its phosphospecific antibodies and the blot was normalized for total Pyk2 levels. B & C. HASMCs that were transduced with Ad-GFP or Ad-dnPyk2 and quiesced were subjected to thrombin (0.5 U/ml)-induced cell growth (B) or migration (C) assays. Cell growth was measured by [3H]-thymidine incorporation and MTT assay and cell migration was measured by modified Boyden chamber method. The bar graphs represent means ± SD of three independent experiments. *p < 0.01 vs control or Ad-GFP; **p < 0.01 vs thrombin, or Ad-GFP + thrombin.
Figure 2. Thrombin-induced tyrosine phosphorylation of Gab1 requires Pyk2 activation.
A. An equal amount of protein from control and each time period of thrombin (0.5 U/ml)-treated HASMCs was immunoprecipitated with anti-Gab1 antibodies and the immunocomplexes were analyzed by Western blotting using anti-PY20 antibodies. The blot was normalized for total Gab1 levels. B & C. HASMCs that were transduced with Ad-GFP or Ad-dnGab1 and quiesced were subjected to thrombin (0.5 U/ml)-induced cell growth (B) or migration (C) assays. D. HASMCs that were transduced with Ad-GFP or Ad-dnPyk2 were treated with and without thrombin (0.5 U/ml) for 30 min and an equal amount of protein from control and each treatment was immunoprecipitated with anti-Gab1 antibodies and the immunocomplexes were analyzed by Western blotting using anti-PY20 antibodies. The blot was reprobed sequentially with anti-Pyk2 and anti-Gab1 antibodies to show over expression of dnPyk2 and normalization of Gab1 levels, respectively. The bar graphs represent means ± SD of three independent experiments. * p < 0.01 vs control or Ad-GFP; ** p < 0.01 vs thrombin or Ad-GFP + thrombin.
Figure 3. Pyk2 and Gab1 mediate thrombin activation of p115 RhoGEF.
A. An equal amount of protein from control and each time period of thrombin (0.5 U/ml)-treated HASMCs was immunoprecipitated with anti-PY20 or anti-p115 RhoGEF antibodies and the immunocomplexes were analyzed by Western blotting using p115 RhoGEF, PDZ RhoGEF, LARG or PY20 antibodies. The blot in the right panel was normalized for p115 RhoGEF levels. B. An equal amount of protein from cell extracts of HASMCs that were transfected with control or p115 RhoGEF siRNA was analyzed by Western blotting for p115 RhoGEF and CDK4 levels using their specific antibodies to show the effect of the indicated siRNA on its target and off-target molecules. C & D. HASMCs that were transfected with control or p115 RhoGEF siRNA and quiesced were subjected to thrombin (0.5 U/ml)-induced cell growth (B) or migration (C) assays. E & F. An equal amount of protein from HASMCs that were transduced with Ad-GFP, Ad-dnPyk2 or Ad-dnGab1, quiesced and treated with and without thrombin (0.5 U/ml) for 30 min was immunoprecipitated with anti-p115 RhoGEF or anti-PY20 antibodies and the immunocomplexes were analyzed by Western blotting using anti-PY20 or anti-p115 RhoGEF antibodies. The blot in panel E was reprobed sequentially with anti-p115 RhoGEF and anti-Pyk2 antibodies for normalization of p115 RhoGEF levels and to show the over expression of dnPyk2, respectively. The blot in panel F was reprobed with anti-Gab1 antibodies to show the over expression of dnGab1. G. An equal amount of protein from control and each time period of thrombin (0.5 U/ml)-treated HASMCs was immunoprecipitated with anti-Gab1 or anti-p115 RhoGEF antibodies and the immunocomplexes were analyzed by Western blotting using anti-p115 RhoGEF or anti-Gab1 antibodies. The blots were normalized for Gab1 or p115 RhoGEF levels. H. All the conditions were same as in F except that immunoprecipitation was performed with anti-Gab1 antibodies and the immunocomplexes were analyzed by Western blotting using anti-p115 RhoGEF antibodies. The blot was reprobed with anti-Gab1 antibodies to show the over expression of dnGab1. The bar graphs represent means ± SD of three independent experiments. * p < 0.01 vs control or Ad-GFP; ** p < 0.01 vs thrombin or Ad-GFP + thrombin.
Figure 4. Pyk2 and Gab1 via p115 RhoGEF mediate thrombin-induced Rac1 and RhoA activation.
A. An equal amount of protein from control and each time period of thrombin (0.5 U/ml)-treated HASMCs was subjected to pull-down assay using GST-Pak or GST-Rhotekin conjugated Sepharose CL4B beads and the resultant GST-Pak and GST-Rhotekin-bound proteins were analyzed by Western blotting for Rac1 or RhoA levels using their specific antibodies. An equal amount of protein from the same samples was analyzed by Western blotting for total Rac1 and RhoA levels using their specific antibodies. B & C. After transduction with Ad-GFP, Ad-dnRac1 or Ad-dnRhoA and quiescence, HASMCs were subjected to thrombin (0.5 U/ml)-induced cell growth (B) or migration (C) assays. D. An equal amount of protein from HASMCs that were transfected with control or p115 RhoGEF siRNA and treated with and without thrombin (0.5 U/ml) for 30 min were analyzed for Rac1 and RhoA activation as described in panel A. An equal amount of protein from the same samples was analyzed by Western blotting for total Rac1, RhoA, p115 RhoGEF and CDK4 levels to show the effect of the indicated siRNA on its target and off-target molecules. E-H. An equal amount of protein from HASMCs that were transduced with Ad-GFP, Ad-dnGab1, Ad-dnPyk2, Ad-dnRac1 or Ad-dnRhoA and treated with and without thrombin (0.5 U/ml) for 30 min were analyzed for Rac1 and RhoA activation as described in panel A. An equal amount of protein from the same samples was analyzed by Western blotting for Rac1, RhoA, Gab1, Pyk2, GFP and/or β-tubulin levels to show the over expression of their respective dominant negative mutants, control vector or normalization. The bar graphs represent means ± SD of three independent experiments. * p < 0.01 vs control or Ad-GFP; ** p < 0.01 vs thrombin or Ad-GFP + thrombin.
Figure 5. Pyk2, Gab1, p115 RhoGEF, Rac1 and RhoA mediate thrombin-induced Pak1 activation.
A. An equal amount of protein from control and each time period of thrombin (0.5 U/ml)-treated HASMCs was analyzed by Western blotting for pPak1 levels using its specific antibodies and the blot was normalized for total Pak1 levels. B. An equal amount of protein from cell extracts of HASMCs that were transfected with control or Pak1 siRNA was analyzed by Western blotting for Pak1 and β-tubulin levels using their specific antibodies to show the effect of the indicated siRNA on its target and off-target molecules. C & D. HASMCs that were transfected with control or Pak1 siRNA and quiesced were subjected to thrombin (0.5 U/ml)-induced cell growth (C) or migration (D) assays. E-I. An equal amount of protein from HASMCs that were transduced with Ad-GFP, Ad-dnRac1, Ad-dnRhoA, Ad-dnGab1, Ad-dnPyk2 or transfected with control or p115 RhoGEF siRNA and treated with and without thrombin (0.5 U/ml) for 30 min were analyzed by Western blotting for pPak1 levels using its specific antibodies. The blots were reprobed with anti-Rac1, anti-RhoA, anti-p115 RhoGEF, anti-Gab1, anti-Pyk2, anti-GFP, anti-β-tubulin or anti-CDK4 antibodies to show the over expression of their respective dominant negative mutants, the siRNA effect on its target molecule level, control vector or for normalization. The bar graphs represent means ± SD of three independent experiments. * p < 0.01 vs control or Ad-GFP; ** p < 0.01 vs thrombin or Ad-GFP + thrombin.
To confirm the role of Pyk2 in thrombin-induced phosphorylation and/or activation of Gab1, p115 RhoGEF, Rac1, RhoA and Pak1, we also used siRNA approach. Pyk2 depletion by its siRNA substantially inhibited thrombin-induced Gab1 and p115 RhoGEF tyrosine phosphorylation and Rac1, RhoA and Pak1 activation (Online Figure II). Consistent with these observations, siRNA-mediated down regulation of Pyk2 also attenuated thrombin-induced HASMC growth and migration (Online Figure III). Furthermore, the blockade of Pyk2 activation by its dominant negative mutant had no effect on thrombin-induced PKCδ phosphorylation, suggesting that the role of Pyk2 on Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 stimulation by thrombin is selective (Online Figure IV).
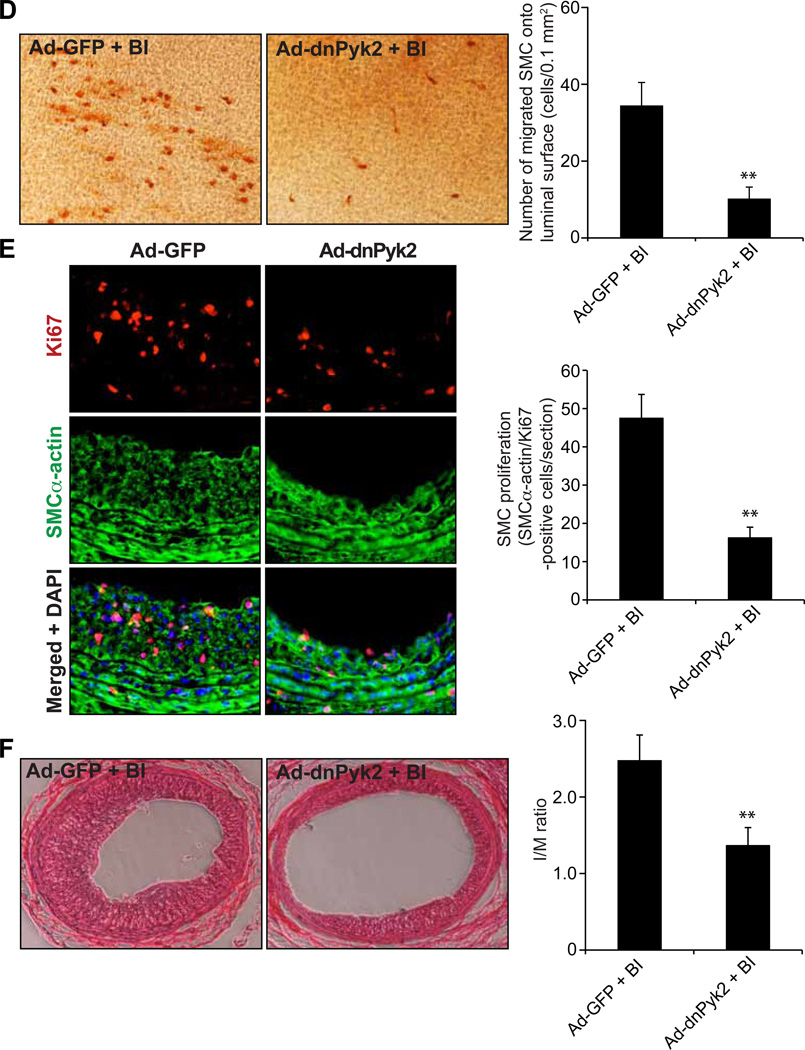
To validate these signaling events in vivo, we used balloon injury (BI) of rat carotid artery model. Balloon injury induced tyrosine phosphorylation of Pyk2, Gab1 and p115 RhoGEF as well as caused Rac1, RhoA and Pak1 activation (Figure 6A–C). In addition, adenovirus-mediated transduction of dnPyk2 blocked BI-induced Pyk2, Gab1 and p115 RhoGEF phosphorylation as well as Rac1, RhoA and Pak1 activation (Figure 6A–C). In order to understand the role of Pyk2 in neointima formation, its activation was blocked by its dominant negative mutant and tested its effects on BI-induced SMC migration and proliferation. Adenovirus-mediated expression of dnPyk2 substantially inhibited BI-induced SMC recruitment to luminal surface and their proliferation in intimal region leading suppression of neointima formation (Figure 6D–F).
Figure 6. Blockade of Pyk2 activation suppresses BI-induced neointima formation.
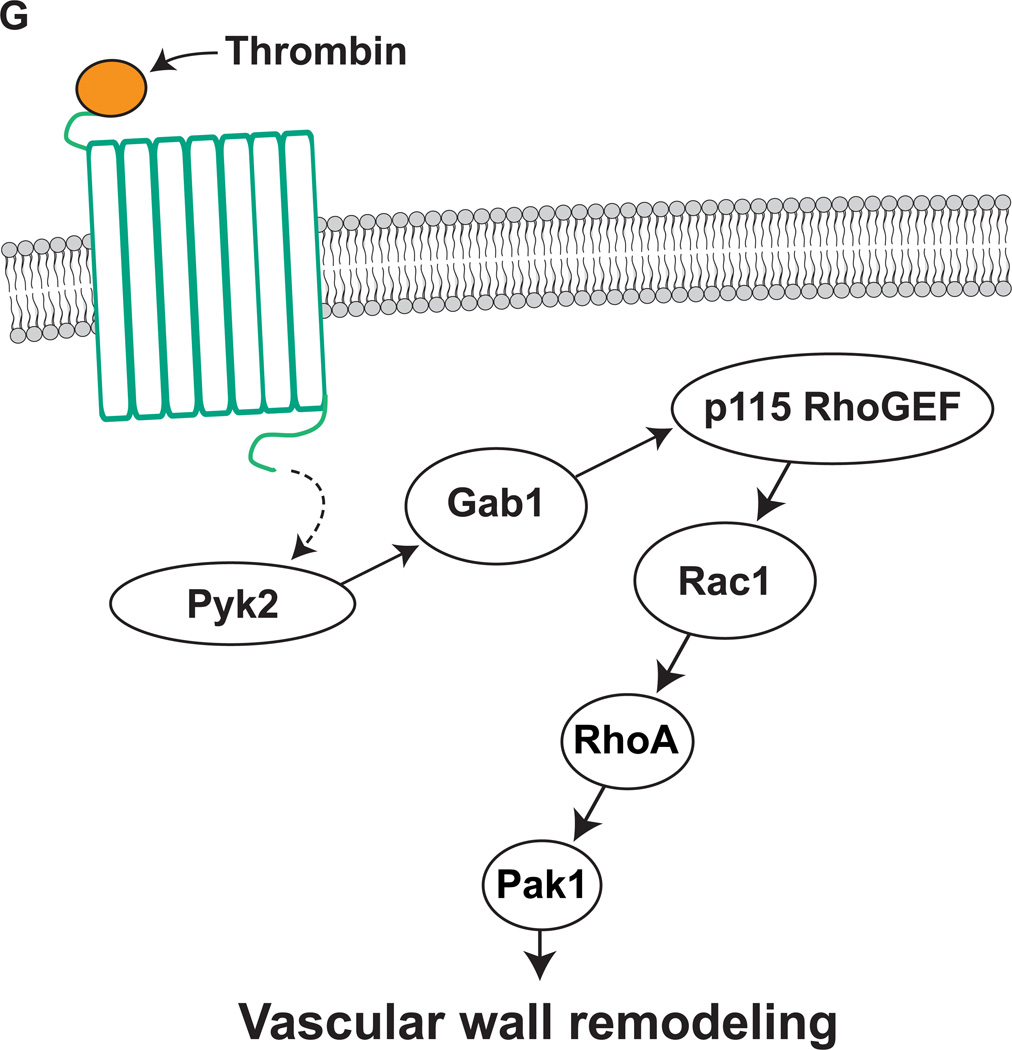
A–C. Common carotid arteries were dissected out at 12 hrs after BI and transduction with Ad-GFP or Ad-dnPyk2 and tissue extracts were prepared. Tissue extracts containing an equal amount of protein were analyzed by Western blotting for pPyk2 levels using its specific antibodies (A), immunoprecipitated with anti-PY20 antibodies and the immunocomplexes were immunoblotted for Gab1 or p115 RhoGEF levels using their specific antibodies (B) or subjected to pull-down assay for Rac1 and RhoA activation (C). For testing Pak1 activation, tissue extracts were analyzed by Western blotting for pPak1 levels using its phosphospecific antibodies and blot was normalized for total Pak1 and/or β-tubulin levels (C). The blot in panel A was reprobed sequentially with anti-Pyk2 and anti-β-tubulin antibodies to show the over expression of dnPyk2 and normalization, respectively. The bar graphs in panels A–C represent the mean ± SD values of three experiments each involving 2 to 4-pooled arteries. D. Three days after BI and transduction with Ad-GFP or Ad-dnPyk2, injured common carotid arteries were dissected out, fixed, opened longitudinally and stained with anti-SMCα-actin antibodies. The SMCα-actin-positive cells were counted and SMC migration was expressed as the number of SMCα-actin-positive cells migrated onto a unit luminal surface area. E. At 1 week post BI and transduction with Ad-GFP or Ad-dnPyk2, injured common carotid arteries were isolated, fixed, cryo-sections made and stained for SMCα-actin and Ki67 using their specific antibodies. The SMCα-actin and Ki67-positive cells were counted to measure neointimal SMC proliferation. F. At 2 weeks post BI and transduction with Ad-GFP or Ad-dnPyk2, injured common carotid arteries were isolated, fixed, cross-sections made, stained with H & E and the I/M ratios were calculated to measure neointima formation. The bar graphs in panels D&F represent the quantitative analysis of six animals. * p<0.05 vs uninjured; ** p < 0.05 vs Ad-GFP + BI. G. Schematic diagram depicting the signaling events activated by thrombin in HASMCs and/or injury in the artery.
DISCUSSION
Pyk2 is a calcium-dependent proline-rich tyrosine kinase and plays a role in the regulation of cell migration and/or proliferation (14, 15, 30). In addition, it was reported that GPCR agonists such as Ang II and RTK agonists such as PDGF-BB activate Pyk2 in VSMCs, mediating the migration and/or proliferation of these cells (16, 17). Furthermore, a recent study showed that Pyk2 plays a role in the early inflammatory reactions during atherogenesis (31). Despite this information, the mechanisms by which Pyk2 influences vascular diseases are not well understood. In this aspect, in the present study we demonstrate that thrombin activates Pyk2 as well as Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 in HASMCs (Figure 6G). In addition, thrombin-induced activation of Gab1, p115 RhoGEF, Rac1, RhoA and Pak1 requires Pyk2 stimulation. Furthermore, the present observations clearly show that activation of Pyk2 is required for thrombin-induced HASMC growth and migration. Since both GPCR and RTK agonists-induced VSMC growth and migration appear to require Pyk2 activation, it might have a role in VSMC dedifferentiation in response to cues that provoke vessel wall perturbation leading to vascular wall remodeling. Studies from our laboratory as well as others have previously reported that Gab1 plays a role in the regulation of VSMC growth/migration and cardiac hypertrophy (18, 32, 33). However, there appears to be no information on how Gab1 mediates VSMC migration and proliferation. In this regard our studies show that Gab1 physically interacts with RhoGEFs, particularly, p115 RhoGEF and facilitates its Pyk2-mediated tyrosine phosphorylation in response to thrombin. A large body of literature suggests that p115 RhoGEF exhibits specificity for RhoA activation (26, 27). However, our findings indicate that p115 RhoGEF mediates the activation of Rac1, which in turn, leads to RhoA stimulation in response to thrombin. It is interesting to note that in contrast to the antagonism reported between RhoA and Rac1 in the regulation of cell migration (34), the present observations reveal an interaction between these two Rho GTPases in thrombin-induced HASMC growth and migration. Although a similar interaction was observed between these two Rho GTPases in the modulation of RASMC migration in response to thrombin, in these cells LARG-dependent RhoA stimulation is needed for Rac1 activation (18). Thus, these findings point out clear species differences between human and rat VSMCs in the interaction of Rac1 and RhoA and the mode of their activation by their upstream RhoGEFs in response to thrombin. The Rho GTPase effector Pak1 plays a vital role in cell migration (28, 29). However, in the present study, we show that Pak1 in addition to its role in cell migration also plays a role in cell proliferation. Recently, it was demonstrated that Gab1 interacts with Pak4 in the regulation of Met receptor-mediated cell migration (35). It was also reported that Gab1 facilitates Ras-dependent MAPK activation in epidermal growth factor-induced epidermal cell proliferation (36). Based on all the these observations as well as our previous (18) and present findings, it may be suggested that Gab1 associates with many signaling molecules, including Rho and Ras-specific RhoGEFs and mediates the activation of their respective downstream signaling events such as MAPKs in enhancing the agonist-induced cell migration and proliferation. In this aspect, we would like to point out that we have previously reported a correlation between JNK1 activation and VSMC growth in response to thrombin (37). In addition, many reports showed that small GTPases, Ras, Rac1, Cdc42 and RhoA mediate the activation of JNK1 in response to various stimulants (38, 39). In view of these observations, we may speculate that Pyk2-dependent Gab1-mediated p115 RhoGEF-Rac1/RhoA-Pak1 signaling activation may target JNK1 stimulation in facilitating thrombin-induced HASMC growth and migration.
The role of Pyk2 in Gab1 and p115 RhoGEF phosphorylation leading to Rac1-RhoA-Pak1 activation in vitro in HASMCs may be validated in vivo in the carotid artery injury model. BI induced Pyk2 tyrosine phosphorylation very acutely. In addition, we observed that BI activates p115 RhoGEF, Rac1, RhoA and Pak1 with a time period that correlates with Pyk2 stimulation. The finding that blockade of Pyk2 activation attenuates BI-induced activation of all these molecules suggest that Pyk2 acts upstream to these signaling molecules. Furthermore, inhibition of Pyk2 substantially reduced the presence of SMC on luminal surface as well as their proliferation in the intimal region resulting in decreased neointima formation. Based on these findings, we can conclude that Pyk2 plays an important role in the activation of RhoGEFs such as p115 RhoGEF, which in turn, mediates Rac1 and RhoA-dependent Pak1 activation. The connection between Pyk2 and p115 RhoGEF appears to be Gab1. The previous work from our as well as other laboratories reported that local expression or intravenous delivery of recombinant hirudin inhibits neointima formation (18, 40). In addition, we have shown that recombinant hirudin attenuates BI-induced activation of RhoA, Rac1 and Pak1 (18). Because thrombin activates Pyk2-Gab1-p115 RhoGEF-Rac1-RhoA-Pak1 in HASMCs and blockade of Pyk2 negates BI-induced Gab1, p115 RhoGEF, Rac1, RhoA and Pak1, it is likely that thrombin-mediated GPCR signaling to Pyk2 activation plays a role in vascular wall remodeling. Whatever the cues that might be produced at the site of vascular injury, the present findings nonetheless suggest a prominent role for Pyk2 in vascular wall remodeling following injury.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grants HL103575 and HL069908 to GNR.
Footnotes
DISCLOSURES: None
REFERENCES
- 1.Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–1040. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 2.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, 2nd, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rauch BH, Millette E, Kenagy RD, Daum G, Fischer JW, Clowes AW. Syndecan-4 is required for thrombin-induced migration and proliferation in human vascular smooth muscle cells. J Biol Chem. 2005;280:17507–17511. doi: 10.1074/jbc.M410848200. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shavit R, Kahn A, Fenton JW, 2nd, Wilner GD. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983;96:282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabri A, Short J, Guo J, Steinberg SF. Protease-activated receptor-1- mediated DNA synthesis in cardiac fibroblast is via epidermal growth factor receptor transactivation: distinct PAR-1 signaling pathways in cardiac fibroblasts and cardiomyocytes. Circ Res. 2002;91:532–539. doi: 10.1161/01.res.0000035242.96310.45. [DOI] [PubMed] [Google Scholar]
- 6.Vergnolle N, Derian CK, D'Andrea MR, Steinhoff M, Andrade-Gordon P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential proinflammatory role for proteinase-activated receptor-4. J Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- 7.Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol. 2003;162:1503–1513. doi: 10.1016/S0002-9440(10)64283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signaling by G protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 9.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 10.Ayoub MA, Trinquet E, Pfleger KD, Pin JP. Differential association modes of the thrombin receptor PAR1 with Galphai1, Galpha12, and beta-arrestin 1. FASEB J. 2010;24:3522–3535. doi: 10.1096/fj.10-154997. [DOI] [PubMed] [Google Scholar]
- 11.McCoy KL, Traynelis SF, Hepler JR. PAR1 and PAR2 couple to overlapping and distinct sets of G proteins and linked signaling pathways to differentially regulate cell physiology. Mol Pharmacol. 2010;77:1005–1015. doi: 10.1124/mol.109.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem. 2008;283:29888–29896. doi: 10.1074/jbc.M803880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunney TD, Katan M. PLC regulation: emerging pictures for molecular mechanisms. Trends Biochem Sci. 2011;36:88–96. doi: 10.1016/j.tibs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Watson JM, Harding TW, Golubovskaya V, Morris JS, Hunter D, Li X, Haskill JS, Earp HS. Inhibition of the calcium-dependent tyrosine kinase (CADTK) blocks monocyte spreading and motility. J Biol Chem. 2001;276:3536–3542. doi: 10.1074/jbc.M006916200. [DOI] [PubMed] [Google Scholar]
- 15.Sanjay A, Houghton A, Neff L, DiDomenico E, Bardelay C, Antoine E, Levy J, Gailit J, Bowtell D, Horne WC, Baron R. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J Cell Biol. 2001;152:181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabri A, Govindarajan G, Griffin TM, Byron KL, Samarel AM, Lucchesi PA. Calcium- and protein kinase C-dependent activation of the tyrosine kinase PYK2 by angiotensin II in vascular smooth muscle. Circ Res. 1998;83:841–851. doi: 10.1161/01.res.83.8.841. [DOI] [PubMed] [Google Scholar]
- 17.Perez J, Torres RA, Rocic P, Cismowski MJ, Weber DS, Darley-Usmar VM, Lucchesi PA. PYK2 signaling is required for PDGF-dependent vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2011;301:C242–C251. doi: 10.1152/ajpcell.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Paria BC, Zhang Q, Karpurapu M, Li Q, Gerthoffer WT, Nakaoka Y, Rao GN. A role for Gab1/SHP2 in thrombin activation of PAK1: gene transfer of kinase-dead PAK1 inhibits injury-induced restenosis. Circ Res. 2009;104:1066–1075. doi: 10.1161/CIRCRESAHA.109.196691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dechert MA, Holder JM, Gerthoffer WT. p21-activate kinase 1 participates in tracheal smooth muscle cell migration by signaling to p38 MAPK. Am J Physiol Cell Physiol. 2001;281:C123–C132. doi: 10.1152/ajpcell.2001.281.1.C123. [DOI] [PubMed] [Google Scholar]
- 20.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102:1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–1470. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- 22.Berkner KL. Development of adenovirus vectors for the expression of heterologous genes. Biotecniques. 1988;6:616–629. [PubMed] [Google Scholar]
- 23.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol Pharmacol. 2010;77:111–125. doi: 10.1124/mol.109.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 27.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase 1 (PAK1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 29.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (PAK1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180(1):187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsume A, Okigaki M, Matsui A, Che J, Adachi Y, Kishita E, Yamaguchi S, Ikeda K, Ueyama T, Matoba S, Yamada H, Matsubara H. Early inflammatory reactions in atherosclerosis are induced by proline-rich tyrosine kinase/reactive oxygen species-mediated release of tumor necrosis factor-alpha and subsequent activation of the p21Cip1/Ets- 1/p300 system. Arterioscler Thromb Vasc Biol. 2011;31:1084–1092. doi: 10.1161/ATVBAHA.110.221804. [DOI] [PubMed] [Google Scholar]
- 32.Mood K, Saucier C, Bong YS, Lee HS, Park M, Daar IO. Gab1 is required for cell cycle transition, cell proliferation, and transformation induced by an oncogenic met receptor. Mol Biol Cell. 2006;17:3717–3728. doi: 10.1091/mbc.E06-03-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, Hirano T, Kawase I, Hirota H. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 34.Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology, opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paliouras GN, Naujokas MA, Park M. Pak4, a novel Gab1 binding partner, modulates cell migration and invasion by the Met receptor. Mol Cell Biol. 2009;29:3018–3032. doi: 10.1128/MCB.01286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai T, Nishida K, Hirano T, Khavari PA. Gab1 and SHP-2 promote Ras/MAPK regulation of epidermal growth and differentiation. J Cell Biol. 2002;159:103–112. doi: 10.1083/jcb.200205017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao GN, Runge MS. Cyclic AMP inhibition of thrombin-induced growth in vascular smooth muscle cells correlates with decreased JNK1 activity and c-Jun expression. J Biol Chem. 1996;271:20805–20810. doi: 10.1074/jbc.271.34.20805. [DOI] [PubMed] [Google Scholar]
- 38.Auer KL, Contessa J, Brenz-Verca S, Pirola L, Rusconi S, Cooper G, Abo A, Wymann MP, Davis RJ, Birrer M, Dent P. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes. Mol Biol Cell. 1998;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YN, Malbon CC, Wang HY. G alpha 13 signals via p115RhoGEF cascades regulating JNK1 and primitive endoderm formation. J Biol Chem. 2004;279:54896–54904. doi: 10.1074/jbc.M407581200. [DOI] [PubMed] [Google Scholar]
- 40.Rade JJ, Schulick AH, Virmani R, Dichek DA. Local adenoviral-mediated expression of recombinant hirudin reduces neointima formation after arterial injury. Nat Med. 1996;2:293–298. doi: 10.1038/nm0396-293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.