Abstract
Cell adhesion to the extracellular matrix (ECM) is necessary for development of the mammary gland, and to maintain the normal architecture and function of the gland. Cells adhere to the ECM via the integrin family of trans-membrane receptors, which signal to control mammary-specific gene expression and regulate cell proliferation and survival. During tumor formation, the ECM is extensively remodeled and signaling through integrins is altered such that cells become proliferative and invasive. A key regulator of whether integrin-mediated adhesion will promote tumor suppression or tumor formation is the stiffness of the stromal ECM. The normal mammary gland is typically surrounded by a loose collagenous stroma. An increase in the deposition of collagen and other stromal components is associated with mammographic density, which is one of the greatest risk factors for developing breast carcinoma. Several groups have demonstrated that increased stromal ECM density results in a matrix that is stiffer. Cells sense the stiffness of their surrounding ECM by Rho-mediated contraction of the actin-myosin cytoskeleton. If the surrounding ECM is stiffer than the cell's ability to contract it, then the tensile forces that result are able to drive the clustering of integrins and assemble adhesion signaling complexes. The result is subsequent activation of signaling pathways including FAK, ERK, and PI3K that drive cell proliferation and survival. In contrast, focal complexes are not formed in a compliant matrix, and activation of FAK and pERK is diminished, resulting in control of proliferation. Signaling from FAK moreover regulates p53 and miR-200 members, which control apoptosis and epithelial phenotype, such that a compliant matrix is predicted to promote normal mammary gland architecture and suppress tumor formation.
Introduction
There are several hallmarks of tumor formation that have been proposed and include evading apoptosis, uncontrolled proliferation, self-sufficiency in growth, angiogenesis, and tissue invasion and metastasis (Hanahan and Weinberg, 2000). These hallmarks all represent a disruption of normal control mechanisms and lead to a loss of normal tissue architecture. Of relevance to this chapter, the ECM impinges upon the regulation of each of these hallmark processes, and to the is poised to be a regulator of whether cells exhibit a normal or a transformed phenotype.
The normal architecture of the mammary gland is comprised of an epithelium organized into a branching ductal tree. This branching ductal tree is surrounded by a loose stromal connective tissue deposited by stromal fibroblasts and comprised predominantly of fibrillar collagens, fibronectin, and proteoglycans. The mammary gland and its stroma exists in the context of adipose tissue, the mammary fat pad. The adipose tissue contributes much to regulating the metabolism and hormonal milieu of the gland, but will not be further discussed here (for a review, see (Hovey et al., 1999)). The mammary gland is unique in that much of its differentiation and formation of an extensive ductal tree occurs only in response to a set of hormonal cues at puberty, pregnancy, and during lactation. The mammary epithelium is organized into two layers: the luminal epithelial cells that make milk proteins, and the highly contractile basal, or myo-epithelial, cells surrounding the luminal cells. The myoepithelial cells contact the basal lamina, and regulate the function and polarity of the apical cells. Much evidence supports the notion that adhesion to the basal lamina helps to establish cellular polarity (Barcellos-Hoff et al., 1989; Bissell et al., 1982; Parry et al., 1985; Wicha et al., 1982). Proteins comprising the basal lamina include collagen IV, laminin-1 and laminin-5 (epiligrin), entactin and proteoglycans (Kleinman et al., 1982; Laurie et al., 1982).
Attachment of Individual cells to the basal lamina occurs through integrins, a family of heterodimeric receptors for the ECM. Several alpha and beta subunits exist, and combine in different pairings to provide both ligand and signaling specificity (reviewed in (Berman et al., 2003)). In particular, adhesion to the basal lamina occurs through the α6β4 integrin, which is uniquely found at hemidesmosomes. Adhesion to the basal lamina regulates apical secretion, mammary-specific gene expression, and control of proliferation and apoptosis (Parry et al., 1987). (Streuli et al., 1995). Tissue structure is further maintained by cell-cell attachments through E-cadherin, and evidence is emerging that suggests that there is cross-talk between E-cadherin and integrins (Tsai and Kam, 2009).
Upon transformation, it is well established that cell-cell and cell–matrix attachments are lost or altered, with a resulting loss in tissue architecture. Tumor formation is moreover marked by an increase in cell proliferation, changes in gene expression, and upregulation of several signaling pathways. At the tissue level, tumor progression is accompanied by disruption and proteolysis of the basement membrane, and an increase in the deposition of the stromal ECM. It is the purpose of this chapter to consider the ways in which the ECM works to hold cells in the differentiated state, and thereby act as a tumor suppressor. In order to understand this process fully, we will also consider mechanisms by which altered ECM architecture affects tumor formation or progression.
Regulation of the ECM during normal mammary development
The mammary gland is unique in that the majority of ductal development occurs in response to hormonal stimuli at puberty and subsequent alveolar development with pregnancy, with full terminal differentiation occurring only if lactation ensues. Each developmental state has a unique ECM protein content, which regulates mammary gland differentiation and gene expression (Keely et al., 1995b; Schedin et al., 2004; Silberstein and Daniel, 1984; Warburton et al., 1982). The changes in gene expression include not only laminins, but also fibrillar collagens type I, III, and V, bead-filament collagen VI, collagen IX, basal lamina collagen IV and collagen-associated proteins known to effect cross linking such as elastin, fibrillin 1, decorin, lumican, and biglycan (Schedin et al., 2007). Loss of lobuloalveolar development occurs when FN is knocked-out in the mammary epithelium and is linked to diminished integrin signaling events (Liu et al., 2010), suggesting an intriguing autocrine regulation of morphogenesis by local epithelial secretion of fibronectin.
A role for fibrillar collagen in regulating development of the ductal mammary tree is suggested by findings that collagen deposition occurs along the sides of developing ducts, where epithelial cell proliferation is minimal (Silberstein and Daniel, 1982). In contrast, relatively little collagen is present at the terminal end buds (TEBs), which are the mitotically active and motile structures that drive ductal elongation and penetration through the fat pad. Application of exogenous TGF-β at the tip of the growing ductal tree inhibits ductal growth and coordinately results in the deposition of thick fibrillar collagen around the TEBs (Silberstein and Daniel, 1987).
The control of not only ECM deposition, but also degradation, is an important regulator of the formation of the ductal tree, as appropriate stromelysin-1, -3, gelatinase, and matrilysin are necessary for branching morphogenesis to occur (Rudolph-Owen et al., 1998; Sympson et al., 1994; Witty et al., 1995) (Wiseman et al., 2003). These findings support the idea that the growth of the mammary ductal tree is a process of controlled proliferation and invasion at the TEB. This process needs to be highly regulated, as inappropriate degradation of the basal lamina components by stromelysin/MMP3 causes disruption of cellular polarization and promotes tumor cell invasion (Lochter et al., 1997). The effect of degrading the basal lamina is so profound that induced expression of stromelysin-1 in mouse mammary gland promotes the formation of de novo invasive tumors (Sternlicht et al., 1999), demonstrating that an intact basal lamina suppresses tumor formation.
The finding that proteases such as stromelysin-1 regulate normal branching morphogenesis during development as well as tumor formation demonstrates the delicate balance that must be struck between an intact ECM composed of the appropriate proteins, and the degradation of the ECM. In general, in normal mammary tissue, degradation of ECM and protease activity corresponds to tissue remodeling: invasion of the ductal tree and branching morphogenesis during development and involution following lactation. The fact that similar degradation accompanies tumor formation suggests this is the co-opting of the developmental program by breast carcinoma cells, which also remodel and invade the breast stroma and fat pad.
Involution and insights for tumor suppression
The mammary gland is further unique, in that it undergoes extensive remodeling upon weaning, when the gland involutes. Involution is accompanied by extensive remodeling of the ECM and degradation of laminins, entactin, and basal lamina collagen IV (Alexander et al., 2001; Werb et al., 1996) (see Fig 1). As the normal ECM is degraded, production of milk proteins is inhibited (Schedin et al., 2000). Moreover, the increase in other proteins likely contributes to this effect, as inhibition of milk protein expression has been linked to an increase in the ECM protein, Tenascin-C, during involution (Jones et al., 1995) (Schedin et al., 2004), and increased level of fibrillar collagen become deposited (O'Brien et al., 2010).
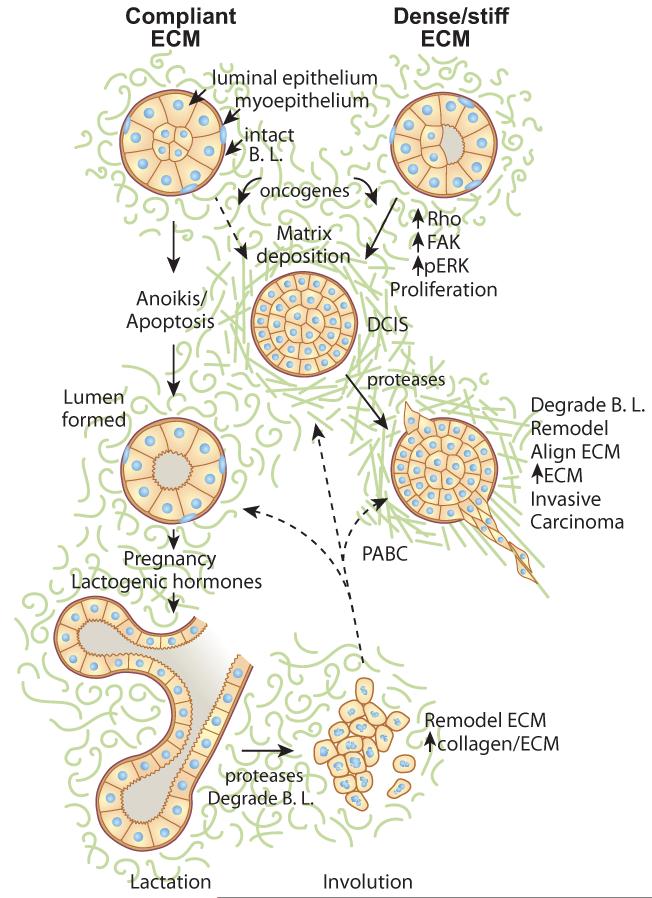
Figure 1.
Regulation of mammary development and tumorigenesis by the ECM. The mammary gland contains a luminal epithelium surrounded by myoepithelial cells and contained within the basal lamina (B.L.). The entire structure is surrounded by the stromal matrix, a loose connective tissue. The formation of the lumen occurs for cells that are non-adherent and die via anoikis, a specialized apoptosis due to loss of adhesion. Hormonal input results in full alveolar and lactogenic differentiation during pregnancy and lactation. Following weaning, involution results in remodeling of the mammary gland and changes in matrix composition. These changes are similar to those observed in tumor formation, and have been linked to pregnancy-associated breast cancer (PABC). In conditions of stiff matrices or increased ECM density, increased signaling through FAK, Rho, and ERK, increased proliferation, and decreased apoptosis can result in a filled lumen (ductal carcinoma in situ, DCIS). Degradation of the basal lamina (B.L.) by proteases and subsequent alignment of collagen results in tumor invasion.
It has recently been demonstrated that the involuting mammary stroma promotes breast carcinoma de-differentiation and metastasis (McDaniel et al., 2006). This suggests that the extensive remodeling that occurs in the involuting stroma may destroy or override some tumor-suppressive property of the normal mammary gland ECM. Consistent with this idea, during tumor formation and progression, there are several changes to the mammary stroma that mimic the changes observed during involution. One similarity is the degradation of the basal lamina that accompanies tumor cell invasion, which mimics the proteolysis observed in involution (McDaniel et al., 2006). In addition to the loss of the tumor suppressive roles of the basal lamina, there is evidence that the degraded ECM components can have unique function. For example, laminin-5 is a major component of the mammary basal lamina, and its cleavage by MMP-2 exposes a cryptic site in laminin-5 that promotes cell migration and invasion (Giannelli et al., 1997) (Koshikawa et al., 2005) (Schenk et al., 2003). Surprisingly, one peptide released by laminin degradation can bind and stimulate cells through the EGFR (Schenk et al., 2003).
A second way that tumor progression parallels involution is in the increased deposition of an altered ECM. This process, termed desmoplasia, is characterized by the increased deposition of a fibrotic stromal matrix. In addition to collagen I, there is a dramatic increase in collagen V deposition into desmoplastic stroma (Barsky et al., 1982), which is mechanically significant as collagen V changes the structure of collagen I fibrils (Berendsen et al., 2006; Breuls et al., 2009; Wenstrup et al., 2004). Again, this is similar to the increases in collagens that accompany invasion and formation of the branching ductal tree (Daniel et al., 1989) and the increase in collagens that accompany involution (Schedin et al., 2004).
Integrins are involved in both tumor suppression and tumor promotion
Cells attach to the ECM through several receptors, the dominant members of which are the integrins. Integrins play important roles in promoting the normal phenotype of cells, and are necessary for mammary epithelial differentiation both in vitro and in vivo (Berdichevsky et al., 1992; D'Souza et al., 1993; Keely et al., 1995a; Li et al., 2005). Adhesion to the basement membrane through β1 integrin activates Rac, and this provides a necessary signal that regulates lactational differentiation in response to prolactin (Akhtar and Streuli, 2006). Indeed, loss of certain integrin subunits, including α6 and α2, accompanies mammary tumor progression (Tagliabue et al., 1998; Zutter et al., 1993), consistent with a model in which α2β1 and α6β4 integrins are tumor-suppressive.
The α6β4 integrin, found at the basal surface of polarized epithelial cells in the hemidesmosomes, is a necessary regulator of normal mammary epithelial polarization and mammary differentiation (Shaw et al., 1997; Weaver et al., 1997). The α6β4 integrin activates Rac, and its effector PAK1, to activate NFkB and promote survival (Friedland et al., 2007). The activation of Rac1 is not only regulated by polarity, but contributes to polarity as it drives basal assembly of laminin in MDCK cells (O'Brien et al., 2001). When β4 integrin is blocked, not only is the normal architecture of the mammary gland lost, but the cells become sensitive to apoptotic events, demonstrating that the normal function of α6β4 integrin includes cell survival (Weaver et al., 2002).
Several lines of evidence support the view that integrins are both important regulators of mammary phenotype, and are necessary for tumor formation and progression. Integrin activation of Rac promotes cell cycle progression by regulating G1 via cyclin D1, CDK4 and CDK6 (Mettouchi et al., 2001), which continues without control when cells are transformed. The tumor-promoting role of some members of the β1 integrin family is demonstrated by the finding that the malignant phenotype can be reverted using β1 integrin blocking antibodies both within 3D cultures and in vivo (Weaver et al., 1997). Knock-out of β1 integrin in mice suppresses the formation of mammary tumors (White et al., 2004), but also diminishes branching complexity during mammary gland development (Li et al., 2005; Naylor et al., 2005). Moreover, the α6β4 integrin can be co-opted in mammary tumor cells to activate PI3-Kinase and promote migration and invasion (Shaw et al., 1997).
The dual role of integrins suggests that their regulation is key to whether the ECM plays a role as a tumor suppressor or a tumor promoter. This regulation can be due to the specific composition of the ECM. For example, in normal MECs, adhesion to collagen via α2β1 integrin causes an increase in Rac activation, while adhesion to laminin via α6β4 integrin inhibits adhesion to Rac (Xie and Haslam, 2008). The regulation of signaling pathways by specific integrins is cell-type specific, as adhesion of Human Umbilical Vein endothelial cells (HUVECs) to fibronectin via the α5β1 integrin activates Rac to promote cell cycle progression in response to growth factors, while adhesion to laminin via the α2β1 integrin does not activate Rac nor lead to cell cycle progression (Mettouchi et al., 2001).
In normal mammary epithelial cells, cell proliferation is also controlled by cell-cell attachments through E-cadherin (Fournier et al., 2008), suggesting that a coordinated control of cell proliferation in polarized epithelial cells occurs by adhesion to the basement membrane as well as through cell-cell contacts. The loss of E-cadherin mediated cell-cell adhesions during tumorigenesis would release cells from growth control, and allow uncontrolled proliferation.
Regulation of integrin function also occurs through syndecans, which are trans-membrane proteoglycans that can bind to several ECM proteins including laminin and collagens (Bernfield and Sanderson, 1990). Syndecan-1 (Sdc-1) is abundant on mammary epithelia, and is often diminished in human breast cancer patients (Baba et al., 2006), suggesting it may play a tumor suppressive function. The cytoplasmic domain of Sdc-1 has signaling capability through both tyrosine kinase and PKC pathways (Lebakken et al., 2000). Recently, it was shown that Sdc-1 enhances signaling through the avβ1 integrin in mammary epithelial cells (Beauvais and Rapraeger, 2003; McQuade et al., 2006), and thus it is poised as a molecule that can direct the outcome of integrin-signaling events. The role of Sdc-1 is complex, however, as it has also been reported that Sdc-1 loss is associated with a decrease in mammary carcinogenesis in rodent models (McDermott et al., 2007). Moreover, Wnt-induced tumor formation is suppressed in mice in which syndecan-1 (Sdc-1) has been knocked out (Alexander et al., 2000). Evidence is accumulating that suggests that loss of Sdc-1 affects the stem cell niche (Alexander, 2008; Paguirigan et al., 2006).
Mechanical considerations in tumor formation
Not only does the ECM serve an important role as a biochemical ligand for integrins and proteoglycans, recently it has become clear that the the stiffness and topography of the ECM surrounding cells also regulates integrin signaling and cell phenotype. The stiffness of the ECM is affected by its composition and organization. An increase in the deposition of the ECM is one way that increased stiffness occurs (Paszek et al., 2005; Provenzano et al., 2009). Increased deposition of stromal matrix, predominantly collagen, is associated with mammographic density, and is linked to a 4–6 fold increase in development of breast carcinoma (Boyd et al., 2001) (Ursin et al., 2005). Moreover, carcinomas largely arise in the dense regions of the breast, suggesting an important link between density and tumor formation (Martin and Boyd, 2008). A more causal link is demonstrated in the finding that a rodent model containing increased collagen deposition, due to altered collagen degradation, exhibits an increase in both tumor formation and progression to metastasis (Provenzano et al., 2008b). Moreover, the stiffness of the ECM is likely a “feed forward” mechanism, as the formation of a tumor results in a region that is locally stiffer (Paszek et al., 2005; Samuel et al., 2011), and deposition of additional stromal collagen accompanies tumor progression (Provenzano et al, 2006).
In addition to an increase in the amount of collagen, surrounding tumors the structure and organization of the collagen is altered. Collagen fibrils are cross-linked by lysyl oxidase, which is increased in the stroma surrounding tumor (Decitre et al., 1998; Peyrol et al., 1997). This increased cross-linking makes the stroma stiffer (Levental et al., 2009). Moreover, collagen near tumors is reorganized from a loose connective tissue into tracks of parallel and perpendicular fibers, which promote the invasiveness of tumor cells (Provenzano et al., 2006; Provenzano et al., 2008c; Yang et al., 2011). In patients, the formation of tracts of aligned collagen is associated with poor outcome (Conklin, 2011). In contrast, in normal mammary gland, collagen fibers are less organized (Provenzano et al., 2006; Yang et al., 2011), suggesting that a randomly organized, compliant stroma is tumor suppressive.
Mechanisms are emerging by which an aligned stroma is created. While Sdc-1 is lost from human breast carcinoma cells, Sdc-1 levels increase in the stroma and on fibroblasts of mammary carcinomas (Baba et al., 2006). Carcinoma-associated fibroblasts have increased Sdc-1 levels and secrete and organize an aligned fibronectin and collagen matrix (Yang et al., 2011). Moreover, when normal mammary fibroblasts, which have low Sdc-1 levels, are transfected with Sdc-1, they acquire the ability to secrete an aligned matrix instead of the randomly organized matrix they normally secrete (Yang et al., 2011).
Recently, it was demonstrated that caveolin-1 in stromal fibroblasts contributes to the creation of an aligned matrix. Loss of caveolin-1 in stromal fibroblasts results in a tumor-associated stroma that is not organized into perpendicular tracks of collagen fibers, and that does not promote tumor progression (Goetz et al., 2011). Moreover, the non-aligned tumor associated stroma in the caveolin deficient stroma is more compliant, while the aligned stroma from the wild-type host is stiffer (Goetz et al., 2011). Consistent with a role for celluluar contractility in creating an aligned stroma, caveolin-deficient fibroblasts have a diminished ability to contract a collagen matrix and to activate Rho (Goetz et al., 2011). Thus, all these findings support the notion that a compliant matrix is one with more disorganized fibers, and that this is tumor-suppressive compared to a stiffer ECM.
Rho GTPase regulates integrins in response to matrix stiffness
The deposition of additional ECM, and its cross-linking and alignment creates an environment that is stiffer surrounding tumors (Goetz et al., 2011; Paszek et al., 2005; Provenzano et al., 2009). One important way that mammary epithelial cells respond to matrix stiffness is through Rho-mediated contractility. Rho activates contractility through its effector, Rho Kinase (ROCK), leading to subsequent phosphorylation of the regulatory chain of myosin (MLC) and myosinactin mediated contraction (Totsukawa et al., 2000). In vitro models demonstrate that within a stiff matrix, cellular contractility is insufficient to contract the ECM (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003). The hypothesis, although not proven, is that this results in isometric tension within the cell as it “holds” contraction against the matrix. Consistent with this hypothesis, cells within a stiff environment have a more extensively polymerized actin cytoskeleton (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003).
An outcome of Rho-mediated contractility when cells are on rigid 2D surfaces is the assembly of integrin-based focal adhesions (Chrzanowska-Wodnicka and Burridge, 1996). Integrin assembly into focal adhesions is also driven by force applied to the integrin from the outside of the cell (Choquet et al., 1997). In 3D, we propose that force on the integrins is generated by Rho-mediated contractility pulling against the matrix, and thus the formation of 3D focal adhesions occurs if matrix stiffness is increased (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003). Within a stiffer matrix, larger integrin-containing 3-D focal adhesions are formed than in a more compliant matrix, which results in an increase in the associated integrin-mediated signaling including activation of FAK, Src, small GTPases, ERK, and PI3K (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003; Wyckoff et al., 2006). These pathways are linked to an increase in cell proliferation and survival in stiff matrices, whereas a control of proliferation is observed in compliant matrices (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003).
Thus, the picture that emerges is that in dense breast tissue and in the stroma of a tumor, which are stiffer, we expect that Rho-mediated contractility will activate integrin-mediated pathways and promote cell proliferation and survival (See Figure 2). This hypothesis is supported by recent findings in skin carcinoma models demonstrating that ROCK activation indeed leads to increased stiffness, increased collagen deposition, and a subsequent increase in tumor growth and progression (Samuel et al., 2011). Whether this process is able to actually initiate a tumor or serves to potentiate the role of oncogenes in tumorigenesis and progression remains to be determined.
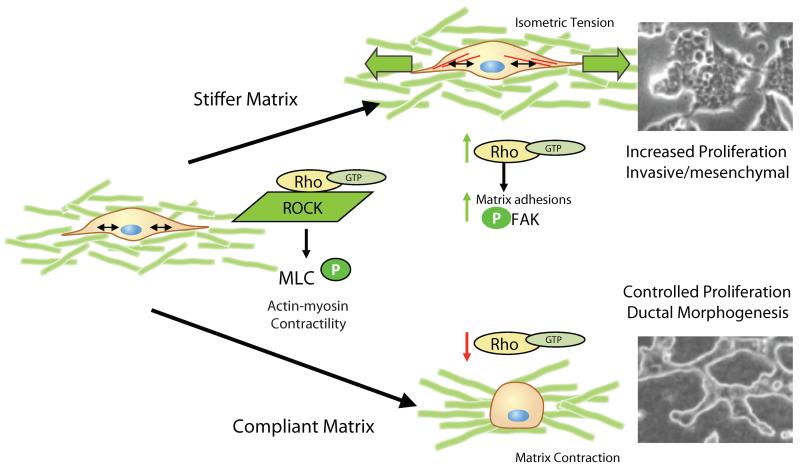
Figure 2.
Rho-ROCK mediated contractility is a sensor for matrix stiffness. Under conditions of a stiff matrix, contractility cannot overcome the stiffness of the matrix, and it is proposed that this results in isometric tension, the formation of matrix adhesions, activation of FAK, and maintenance of high Rho activation. The result is proliferation and an invasive phenotype, and a disruption of morphogenesis. Under conditions of compliance, contraction results in matrix remodeling, changes in cell shape, down regulation of Rho, down regulation of adhesive signaling events, controlled proliferation, and branching morphogenesis.
In contrast, within a compliant matrix, evidence suggests that cellular proliferation is attenuated (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003). Instead, based on in vitro work, we propose that Rho-mediated contractility results in local matrix contraction, a rounding of cell shape, and a fundamentally different signaling outcome (Figure 2). Thus, when the tissue does not exhibit increased density or stiffness, integrin signaling would be expected to be appropriately regulated, and thus contribute to the normal growth and differentiation of the mammary gland. While much is known about signaling pathways activated in the stiff condition, relatively little is known about how a compliant matrix signals to the cells to control growth, polarize, and maintain a differentiated state. The prediction is that appropriate adhesion to the basal lamina via integrins is a key aspect, but only under conditions of appropriate compliance.
Rho itself is regulated by matrix stiffness, such that it is activated to the GTP-bound form in a stiff matrix, and inactivated to the GDP-bound form in a compliant matrix in vitro (Wozniak et al., 2003). It is worth noting that inactivation of Rho is an active process dependent on a compliant ECM, as cells held in suspension in the absence of an ECM matrix, where they are rounded, have high Rho-GTP levels, while cells in a compliant matrix down-regulate Rho-GTP (Wozniak et al., 2003). A role for Rho in vivo is demonstrated by the finding that Rho/ROCK regulation by FAK is necessary for mammary branching morphogenesis (van Miltenburg et al., 2009). This may be a direct activation of Rho by FAK binding to p190RhoGEF (Zhai et al., 2003).
Evidence is accumulating for the role of p190RhoGAP-B, which regulates Rho to the inactive state (Bustos et al., 2008), in regulating Rho in the mammary gland. We find that p190RhoGAP-B down-regulates when MECs are cultured in compliant matrices, and does so by localizing Rho to cell-cell junctions where it is spatially inactivated (Ponik and Keely, in preparation). While it would be straightforward to predict that decreased Rho activation by p190-B should inhibit tumorigenesis, in fact the converse is true: increased p190-B levels enhance tumor formation (McHenry et al., 2010). Expression of p190RhoGAP-B is highest at the TEBs of the developing mammary gland, and is re-expressed in some mammary tumors (Chakravarty et al., 2000). Loss of p190-B results in decreased proliferation of the cap cells in the TEBs, and a resulting decrease in ductal morphogenesis (Chakravarty et al., 2003). The paradoxical effect of p190-B is linked to its ability to regulate Rac and IGF signaling pathways (Chakravarty et al., 2003; McHenry et al., 2010). These findings suggest that there is a balance of Rho regulation, and cross-talk with growth factor signaling that is likely to regulate whether the ECM is tumor suppressive or tumor promoting.
FAK as a mediator of proliferation and survival
Activation of focal adhesion kinase (FAK) is one of the dominant integrin-mediated signaling events, and FAK is emerging as a central regulator of mammary phenotype and mammary gland development. Targeted loss of FAK in the mammary epithelial cells results in a gland that is hypoplastic, and corresponds to diminished activation of ERK and expression of cyclin D1, suggesting the defect is due in part to diminished cell proliferation during lobuloaveolar development (Nagy et al., 2007). Loss of FAK also results in decreased Stat5 phosphorylation and resulting loss of whey acidic protein (Nagy et al., 2007), consistent with the finding that β1 integrin signaling to Stat5 is important for alveolar differentiation (Naylor et al., 2005). It is important to point out that FAK is involved in developmental events that are dependent on cell proliferation, supporting a role for an integrin-FAK-ERK pathway in regulating proliferation in response to ECM.
FAK loss does not inhibit the ultimate differentiation of the mammary gland: lactogenesis. Rather, in this case it is the integrin-binding protein, ILK, rather than FAK, that mediates lactogenic differentiation (Akhtar et al., 2009). Moreover, activated Rac rescues an ILK−/− mammary gland, pointing to an signaling axis of ECM-β1 integrin-ILK-Rac-Stat5-lactogenic genes (Akhtar et al., 2009). These results are consistent with the evolving role for Rac in promoting mammary differentiation. The role of ILK, like integrins, is balanced, however, as overexpression of ILK disrupts differentiation and promotes a carcinoma phenotype (Somasiri et al., 2001), demonstrating again that signaling pathways involved in differentiation must be appropriately controlled.
The role for FAK in regulating cell proliferation is further supported by the finding that FAK is strongly implicated in tumor formation. Several independent investigators demonstrated that loss of FAK suppresses tumor formation in mouse models (Lahlou et al., 2007; Provenzano et al., 2008a; Pylayeva et al., 2009) (Luo et al., 2009). This is consistent with the finding that FAK is often over-expressed in human breast carcinomas (Cance et al., 2000). Analysis of genes regulated by FAK loss in mouse mammary tumors link FAK to proliferation genes involved in both G1 and G2/M (Provenzano et al., 2008c; Zhao et al., 1998). FAK loss resulted in the down-regulation of several signaling pathways that link to proliferation including ERK, PI3K, and Rho/ROCK (Provenzano et al., 2008a; Pylayeva et al., 2009) (Luo et al., 2009).
FAK levels are increased in many breast carcinoma samples (Owens et al., 1995), and FAK is associated with increased progression in several tumors including breast (Lark et al., 2005; Oktay et al., 2003) colon carcinoma, and astrocytoma (Haskell et al., 2003; Matkowskyj et al., 2003). FAK transcriptional expression is increased by the action of NF-kB, which is an important survival mediator (Golubovskaya et al., 2004; Golubovskaya et al., 2009). However, the finding that FAK levels diminish in some liver and cervical carcinomas (Ayaki et al., 2001; Gabriel et al., 2006) and that FAK phosphorylation does not always correspond to tumor progression and metastasis (Lu et al., 2001) suggests that extracellular signals impinge upon FAK and determine its role in tumor suppression or progression.
The role of FAK in tumor suppression is likely not direct, but a consequence of controlling FAK activation and localization. In compliant matrices, FAK is not localized to matrix adhesions, but rather found diffuse in the cytoplasm. Under these conditions, cells are able to undergo ductal morphogenesis in vitro (Paszek et al., 2005; Provenzano et al., 2009; Wozniak et al., 2003). In contrast, within stiff matrices, FAK is phosphorylated and localized to 3D matrix adhesions, where it has enhanced binding to its downstream effectors including Src, SHC, and Grb2 (Provenzano et al., 2009). Consistent with regulation by ECM stiffness, several studies link FAK activation to the contractile action of Rho/ROCK (Chrzanowska-Wodnicka and Burridge, 1996; Pirone et al., 2006; van Miltenburg et al., 2009). Therefore, a model can be proposed in which a compliant ECM serves to suppress tumor formation in part by regulating the localization of FAK such that it is not associated in 3D focal adhesions, and cannot activate proliferative signaling pathways.
While FAK is strongly linked to tumor progression and cellular proliferation, FAK knock-out does not inhibit lactogenesis. Rather, the integrin-binding protein, ILK is linked to lactogenic differentiation (Akhtar et al., 2009) (Fig 3). Moreover, activated Rac rescues an ILK−/− mammary gland, pointing to an signaling axis of ECM-β1 integrin-ILK-Rac-Stat5-lactogenic genes (Akhtar et al., 2009). These results are consistent with the evolving role for Rac in promoting mammary differentiation. The role of ILK, like integrins, is balanced, however, as forced overexpression of ILK disrupts differentiation and promotes a carcinoma phenotype (Somasiri et al., 2001), demonstrating again that signaling pathways involved in differentiation must be appropriately controlled.
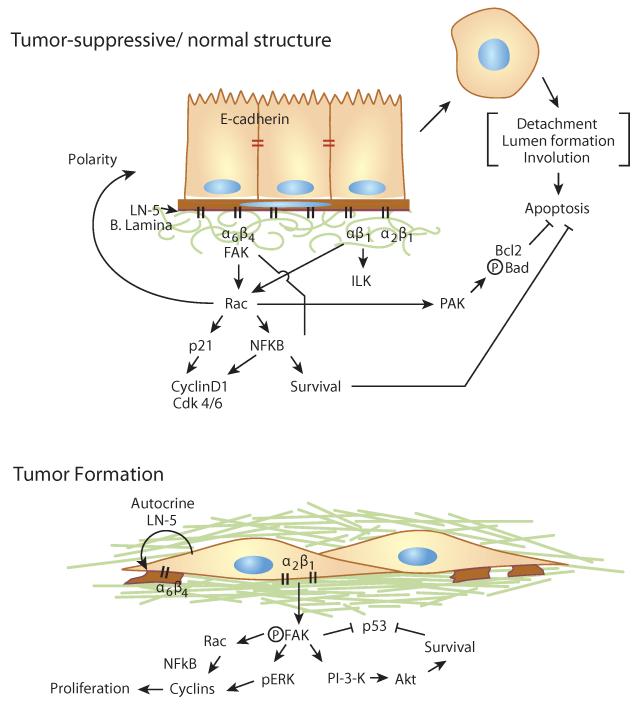
Figure 3.
Integrin-mediated signaling pathways regulate normal epithelial polarity and differentiation. Adhesion of α6β4 integrin to the basal lamina (B. Lamina) results in activation of FAK, and subsequent signaling to Rac to promote cell proliferation and survival via p21 and NFkB. Signaling through Rac to promote basal secretion of laminin-5 (LN-5) helps establish cell polarity. Adhesion to the basal lamina prevents apoptosis via PAK activation of Bcl2 and Bad. Loss of adhesion to the basal lamina results in apoptosis, necessary for luminal clearing. In the case of tumorigenesis, tumor cells degrade the basal lamina, and invade into the stromal matrix. Tumor cells can secrete their own autocrine LN-5 and prevent apoptosis. Moreover, α2β1 integrin adhesion to collagen promotes cell proliferation and survival via activation of FAK, pERK, and PI-3-K pathways. Activated pFAK antagonizes p53 to prevent apoptosis.
Proliferation and pERK
Several investigators have demonstrated that activation of FAK downstream of integrins signals directly to the Ras/ERK pathway and also regulates growth-factor signaling to this pathway (Howe and Juliano, 1998; Lin et al., 1997; Schlaepfer and Hunter, 1997; Schlaepfer et al., 1998; Sieg et al., 2000). In the context of matrix stiffness, a dense or stiff matrix activates pERK in mammary epithelial cells, while a compliant matrix does not (Paszek et al., 2005; Provenzano et al., 2009). A striking feature of cells that are cultured in compliant 3D collagen matrices is that cell proliferation is markedly attenuated in a compliant matrix compared to stiff 3D or 2D matrices, which is linked to the down-regulation of the proliferation signature downstream of pERK activation (Provenzano et al., 2009). Moreover, inhibition of MEK reverts the invasive phenotype, and promotes normal tubulogenesis in a stiff 3D collagen matrix (Provenzano et al., 2009). These findings support the notion that one way a compliant matrix is tumor-suppressive is by controlling activation of the MEK/ERK pathway.
Several growth factor receptors also signal to FAK and pERK, including PDGF, EGF, and HGF, and this may represent a means by which control of FAK and ERK by a compliant ECM can regulate growth factor signaling pathways (Hauck et al., 2000; Sieg et al., 2000)(for review see (Kamalati et al., 1999)). Although there is not space to consider this cross-regulation here, the case of HGF regulation in mammary cell phenotype is particularly appropriate for the consideration of how ECM stiffness might regulate growth factor signaling. HGF, in combination with TGFβ, regulates mammary gland branching morphogenesis (Pollard, 2001). Consistent with this finding, when HGF is added to MECs that are within a compliant 3D collagen gel, it converts ascinar structures into branching tubules (Niemann et al., 1998). In contrast, HGF added to either transformed or normal mammary epithelial cells within a stiff collagen gel instead promotes cell invasion (Provenzano et al., 2009). This demonstrates that matrix stiffness regulates the outcome of HGF signaling to promote tubulogenesis in a compliant matrix and invasion in a stiff matrix. The signaling between the ECM and HGF is likely bi-directional, as HGF enhances integrin-mediated cell adhesion, signaling to FAK and paxillin, and localization of ERK to focal complexes, all of which are required for both migration and ductal morphogenesis (Ishibe et al., 2004; Ishibe et al., 2003; Lai et al., 2000) (Trusolino et al., 2000).
Negative cross-talk between FAK and p53
The p53 protein is a complex tumor suppressor that promotes apoptosis, but is often mis-regulated in carcinomas through mutation, resulting in mutant p53 forms that gain function and contribute to tumor formation. One of the means by which the ECM inhibits apoptosis is through the inhibition of p53 by FAK (Ilic et al., 1998). The regulation is direct, as activated FAK localizes to the nucleus and binds directly to p53 to inhibit its function (Golubovskaya et al., 2008a; Golubovskaya et al., 2008b). The N-terminal FERM domain of FAK mediates this function, as this domain binds to p53 and has a nuclear localization sequence (Lim et al., 2008). Moreover, the FERM domain of FAK binds Mdm-2 and coordinates ubiquitination of p53 to cause p53 degradation (Lim et al., 2008). Under conditions of decreased adhesion, FAK is not activated, and instead links to increased phosphorylation of JNK to regulate p53 and promote apoptosis (Tafolla et al., 2005). FAK is in turn transcriptionally down-regulated by p53 (Golubovskaya et al., 2004; Golubovskaya et al., 2008a), which could serve to further suppress FAK signaling in compliant matrices. We found that many genes that are regulated by FAK have p53 binding sites in their promoter, further solidifying the link between FAK, p53, and transcriptional regulation (Provenzano et al., 2009).
Mutant p53 promotes cell transformation and invasiveness. Interestingly, one mechanism by which it effects this outcome is through increased recycling of integrin and EGFR, which serves to constitutively activate signaling downstream of EGFR (Muller et al., 2009). In MCF10A cells, mutant p53 blocks the apoptosis that results in luminal clearing, and results in expression of several mesenchymal markers (Zhang et al., 2011). Recent data suggests a mechanism for this result, by linking p53 to regulation of miR-200c, a negative regulator of the mesenchymal phenotype (Kim et al., 2011; Magenta et al., 2011).
Apoptosis and survival regulation due to the ECM
Loss of adhesion to the ECM in cells that are anchorage-dependent and express integrins leads to apoptotic cell death, which when induced by loss of adhesion is termed anoikis (Frisch and Francis, 1994). Intriguingly, an appropriately polarized epithelium that is adherent via α6β4 integrin to the basal lamina is resistant to apoptosis, but only if the cells exit the cell cycle (Boudreau et al., 1995). While this finding is linked to appropriate adhesion to the basal lamina, there is likely a mechanical component as well, since the effect requires the formation of a polarized ascinar structure in a compliant rBM, and does not occur for adhesion to rigid 2D surfaces coated with collagen or fibronectin (Boudreau et al., 1995).
During lumen formation within compliant 3D cultures with human MCF-10A cells, it was shown that the lumen initially contains cells, and then later the lumen is cleared by apoptosis (Figure 1). Lumen formation requires the pro-apoptotic regulator, Bim, a member of the BCL-2 protein family (Reginato et al., 2003), and loss of Bim in mice results in a delay in lumen formation and the formation of squamous transdifferentiation in the mammary gland (Mailleux et al., 2007). Apoptosis to form a lumen is proposed to be anoikis due to loss of adhesion to the basement membrane (Debnath et al., 2002), as adhesion via α6β4 to laminin-5 in polarized epithelial cells ensures cell survival via Rac, PAK-1, and NF-kB inhibition of apoptosis (Zahir et al., 2003) (Friedland et al., 2007) (Fig 3). The appropriate formation and maintenance of a lumen is clearly linked to tumor suppression, as luminal filling is a sign of the earliest of mammary carcinomas: Ductal or Lobular Carcinoma in situ (DCIS and LCIS, respectively).
FAK activation protects cells from anoikis (Frisch et al., 1996), and thus its regulation may be a mechanism controlling lumen formation. Consistent with this, FAK regulates several genes linked to survival including the Bcl-2 family (Provenzano et al., 2009). The regulation of FAK may help explain the loss of luminal formation that is observed when mammary epithelial cells are cultured in stiff vs compliant matrices; in a stiff matrix where FAK activation is increased, cells that detach from the basal lamina could persist and prevent clearing of the lumen. Resistance to anoikis is a property of stem cells, evidenced by their ability to form adhesion-independent mammospheres. Thus, it is exciting to note that loss of FAK leads to a loss of stem/progenitor cells (Luo et al., 2009); it is likely that a further role for FAK in determining stem cell fate will continue to emerge.
In addition to regulation by attachment to the basement membrane, Bim is regulated by EGFR (ErbB1) in vitro (Reginato et al., 2003; Wang et al., 2004), and its down-regulation may account for the luminal filling noted when ErbB2 is stimulated and artificially cross-linked (Muthuswamy et al., 2001). The complex cross-talk between integrins and EGFR family members has important clinical implications, as expression levels of ErbB2, also known as Her2 or the neu oncogene, are used to predict outcome and to determine therapy.
Tumor cells can escape anoikis by secreting autocrine laminin-5 (Zahir et al., 2003) (Fig 3). In addition, tumor cells form aggregates that allow survival by adhesion to fibronectin by neighboring cells (Zhang et al., 2004). Cells that are adherent to dense collagen evade apoptosis by regulating several apoptotic mediators including Bcl-2 family members (see supplemental material in (Provenzano et al., 2009)). Thus, tumor formation is characterized by evasion of the tumor-suppressive apoptotic pathways.
ECM regulation of tumor suppression via miRNAs
A recent and exciting finding suggests that ECM affects the expression of genes at the mRNA level through the action of miRNAs, and this mechanism needs to be considered in a discussion of how the ECM may act to suppress tumor formation. Because each miRNA has multiple targets, this allows a rapid and global control of several messages at once. miRNAs regulate genes by binding to the untranslated sequences in mRNAs and targeting them for degradation.
The miR-200 family, composed of miR-200a,b, and c forms, is emerging as a central regulator of cellular phenotype. The general finding is that miR-200 serves to suppress the mesenchymal phenotype, and promote the epithelial phenotype in several epithelial tissues, including the mammary gland (Howe et al, 2011). Targets of miR-200 include the transcription factors Zeb-1 and Zeb-2, and SUZ12, which in turn down-regulate E-cadherin (Burk et al., 2008; Howe et al., 2011; Iliopoulos et al., 2010). Thus, the result of miR-200 is to maintain E-cadherin levels and suppress tumorigenesis. Other direct targets of miR-200 are also implicated in the mesenchymal phenotype and include fibronectin, moesin, NTrk2, tubulin β3, and VEGF, all of which are increased in breast carcinomas (Cochrane et al., 2010). Other miRNAs including miR-205, miR-192, and miR-9 target Zeb1 or 2, and regulate E-cadherin levels (Kim et al., 2011; Valastyan et al., 2011). Because of the extensive regulation of cell response to the ECM by E-cadherin, whether a particular ECM is tumor suppressive or not may be affected by miRNA regulation of cell phenotype.
The regulation of these miRNAs is not fully understood. The ECM is implicated in regulating miR-200 through the TGFβ-mediated induction of TWIST and Snail, which inhibit miR-200 (Iliopoulos et al., 2010). If miR-200 is constitutively expressed, TGF-β induced EMT is blocked, pointing to the necessary role of this global regulator (Gibbons et al., 2009). Moreover, it has been noted that miR-200 allows cells to move back and forth between epithelial and mesenchymal states, and surprisingly it is the metastatic cells that retain responsiveness to the morphogenetic control of rBM, leading to the proposal that miR-200 allows cells the phenotypic flexibility to respond to the microenvironment (Gibbons et al., 2009). This idea is consistent with the observation that miR-200 targets molecules important in maintaining stem cell properties (Brabletz et al., 2011; Cochrane et al., 2010; Gibbons et al., 2009; Ma and Weinberg, 2008; Tryndyak et al., 2010; Wellner et al., 2009), and adds further support to the idea that the ECM regulates stem cell properties.
The levels of miR-200 are positively regulated by p53 (Kim et al., 2011; Magenta et al., 2011) and regulated by the balance of Akt1/Akt2 (Iliopoulos et al., 2009). Thus, a model that can be proposed is that a compliant matrix, which only minimally activates FAK, does not cause the degradation of p53, allowing the expression of miR-200 and the maintenance of the epithelial phenotype. In contrast, a matrix with increased stiffness will activate FAK, which in turn activates PI-3-kinase and Akt, which inhibits miR-200 and will allow a mesenchymal phenotype. Moreover, a stiffer ECM increases expression of TWIST-2 (Provenzano et al., 2009), which also inhibits miR-200 expression and promotes a mesenchymal phenotype (Iliopoulos et al., 2009; Ma and Weinberg, 2008).
The micro-RNA molecules participate in a feedback regulation to the ECM, as several miRNAs, including miR-29, miR-200, miR-124, miR-335, and let-7, target ECM proteins, integrins, and/or cadherin (for a complete review, see Valaystan and Weinberg (Valastyan and Weinberg, 2011)). Moreover, miR-205 targets FAK, and therefore could dramatically affect the response of cells to the ECM and regulate whether the ECM promotes tumorigenesis or is tumor-suppressive for a particular cell.
Summary: Matrix stiffness regulates whether integrins suppress or promote tumor formation
As discussed in this chapter, there are several models to explain how integrins serve to maintain cellular architecture and suppress tumor formation, yet also promote tumor progression. It is clear that while cells are adherent to an intact basal lamina, the collaboration between α6β4 integrin and other β1 integrins serves to regulate signaling pathways and promote cellular polarization. Moreover, the formation of cadherin-based cell-cell adhesions further restricts proliferative signals and maintains tumor suppression. Upon degradation of the basal lamina by several proteases, either during involution or during tumor progression, a switch in the composition of the ECM can activate different integrin isoforms, and will alter the signals that result. Tumor invasion in many ways is a recapitulation of developmental events at the proliferative and invasive TEB, which serves to invade into the mammary fat pad and result in a branched ductal tree. The matrix around the TEB, during involution, and during tumor progression is altered both in matrix composition, and in its orientation, such that aligned stromal fibronectin and collagens accompany ductal morphogenesis, involution, and tumor cell invasion. These changes to the ECM result in matrices that are stiffer, which cells can sense via Rho/ROCK mediated contraction against the matrix. Thus, in a stiffer matrix, integrin signaling is enhanced and mis-regulated. This localizes FAK to 3D matrix adhesions, where it is activated and collaborates with additional signaling pathways including pERK and PI-3-kinase to promote proliferation. Moreover, FAK activation inhibits p53 and miR-200, which blocks epithelial phenotype and apoptosis. Thus, a reasonable hypothesis is that integrin signaling is controlled in a compliant matrix by controlling the level of signaling downstream to FAK in a manner that allows cell survival, controlled proliferation, and appropriate differentiation. Currently much less is known about how the ECM might serve to prevent tumor formation, but as our understanding continues to grow, it will become more feasible to incorporate a consideration of the ECM in prevention, prognosis and treatment of breast cancer.
References
- Akhtar N, Marlow R, Lambert E, Schatzmann F, Lowe ET, Cheung J, Katz E, Li W, Wu C, Dedhar S, Naylor MJ, Streuli CH. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 2009;136:1019–1027. doi: 10.1242/dev.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia 10.1083/jcb.200601059. J. Cell Biol. 2006;17:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM. Base behavior behind budding breasts: integrins and mammary stem cell activity. Cell Stem Cell. 2008;3:5–6. doi: 10.1016/j.stem.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki M, Komatsu K, Mukai M, Murata K, Kameyama M, Ishiguro S, Miyoshi J, Tatsuta M, Nakamura H. Reduced expression of focal adhesion kinase in liver metastases compared with matched primary human colorectal adenocarcinomas. Clin Cancer Res. 2001;7:3106–3112. [PubMed] [Google Scholar]
- Baba F, Swartz K, van Buren R, Eickhoff J, Zhang Y, Wolberg W, Friedl A. Syndecan-1 and syndecan-4 are overexpressed in an estrogen receptor-negative, highly proliferative breast carcinoma subtype. Breast Cancer Res Treat. 2006;98:91–98. doi: 10.1007/s10549-005-9135-2. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky SH, Rao CN, Grotendorst GR, Liotta LA. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am J Pathol. 1982;108:276–283. [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, Rapraeger AC. Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Exp Cell Res. 2003;286:219–232. doi: 10.1016/s0014-4827(03)00126-5. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Gilbert C, Shearer M, Taylor-Papadimitriou J. Collagen-induced rapid morphogenesis of human mammary epithelial cells: the role of the alpha 2 beta 1 integrin. J Cell Sci. 1992;102:437–446. doi: 10.1242/jcs.102.3.437. [DOI] [PubMed] [Google Scholar]
- Berendsen AD, Bronckers AL, Smit TH, Walboomers XF, Everts V. Collagen type V enhances matrix contraction by human periodontal ligament fibroblasts seeded in three-dimensional collagen gels. Matrix Biol. 2006;25:515–522. doi: 10.1016/j.matbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Berman AE, Kozlova NI, Morozevich GE. Integrins: structure and signaling. Biochemistry (Mosc) 2003;68:1284–1299. doi: 10.1023/b:biry.0000011649.03634.74. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Sanderson RD. Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors. Philos Trans R Soc Lond B Biol Sci. 1990;327:171–186. doi: 10.1098/rstb.1990.0052. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. doi: 10.1007/s11912-001-0083-7. [DOI] [PubMed] [Google Scholar]
- Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. Embo J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuls RG, Klumpers DD, Everts V, Smit TH. Collagen type V modulates fibroblast behavior dependent on substrate stiffness. Biochem Biophys Res Commun. 2009;380:425–429. doi: 10.1016/j.bbrc.2009.01.110. [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos RI, Forget MA, Settleman JE, Hansen SH. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr Biol. 2008;18:1606–1611. doi: 10.1016/j.cub.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen J. p190-B RhoGAP Regulates Mammary ductal morphogenesis. Molec. Endocrin. 2003;17:1054–1065. doi: 10.1210/me.2002-0428. [DOI] [PubMed] [Google Scholar]
- Chakravarty G, Roy D, Gonzales M, Gay J, Contreras A, Rosen JM. P190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth Differ. 2000;11:343–354. [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: A Marker of Aggressiveness and Chemoresistance in Female Reproductive Cancers. J Oncol. 2010;2010:821717. doi: 10.1155/2010/821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178 doi: 10.1016/j.ajpath.2010.11.076. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza B, Berdichevsky F, Kyprianou N, Taylor-Papadimitriou J. Collagen-induced morphogenesis and expression of the alpha 2-integrin subunit is inhibited in c-erbB2-transfected human mammary epithelial cells. Oncogene. 1993;8:1797–1806. [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB, Van Horn K, Strickland P, Robinson S. TGF-beta 1-induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev Biol. 1989;135:20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Decitre M, Gleyzal C, Raccurt M, Peyrol S, Aubert-Foucher E, Csiszar K, Sommer P. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Invest. 1998;78:143–151. [PubMed] [Google Scholar]
- Fournier AK, Campbell LE, Castagnino P, Liu WF, Chung BM, Weaver VM, Chen CS, Assoian RK. Rac-dependent cyclin D1 gene expression regulated by cadherin- and integrin-mediated adhesion. J Cell Sci. 2008;121:226–233. doi: 10.1242/jcs.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel B, zur Hausen A, Stickeler E, Dietz C, Gitsch G, Fischer DC, Bouda J, Tempfer C, Hasenburg A. Weak expression of focal adhesion kinase (pp125FAK) in patients with cervical cancer is associated with poor disease outcome. Clin Cancer Res. 2006;12:2476–2483. doi: 10.1158/1078-0432.CCR-05-1867. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, Kurie JM. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJ, Garcia R, Keely PJ, Sanchez-Mateos P, Cukierman E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya V, Kaur A, Cance W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: nuclear factor kappa B and p53 binding sites. Biochim Biophys Acta. 2004;1678:111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Conway-Dorsey K, Edmiston SN, Tse CK, Lark AA, Livasy CA, Moore D, Millikan RC, Cance WG. FAK overexpression and p53 mutations are highly correlated in human breast cancer. Int J Cancer. 2009;125:1735–1738. doi: 10.1002/ijc.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. p53 regulates FAK expression in human tumor cells. Mol Carcinog. 2008a;47:373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Finch R, Zheng M, Kurenova EV, Cance WG. The 7-amino-acid site in the proline-rich region of the N-terminal domain of p53 is involved in the interaction with FAK and is critical for p53 functioning. Biochem J. 2008b;411:151–160. doi: 10.1042/BJ20071657. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haskell H, Natarajan M, Hecker TP, Ding Q, Stewart J, Jr., Grammer JR, Gladson CL. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin Cancer Res. 2003;9:2157–2165. [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Schlaepfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB- stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J Biol Chem. 2000;275:41092–41099. doi: 10.1074/jbc.M005450200. [DOI] [PubMed] [Google Scholar]
- Hovey RC, McFadden TB, Akers RM. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia. 1999;4:53–68. doi: 10.1023/a:1018704603426. [DOI] [PubMed] [Google Scholar]
- Howe AK, Juliano RL. Distinct mechanisms mediate the initial and sustained phases of integrin-mediated activation of the Raf/MEK/Mitogen-activated protein kinase cascade. J. Biol. Chem. 1998;273:27268–27274. doi: 10.1074/jbc.273.42.27268. [DOI] [PubMed] [Google Scholar]
- Howe EN, Cochrane DR, Richer JK. Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res. 2011;13:R45. doi: 10.1186/bcr2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004;16:257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol Cell. 2003;12:1275–1285. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Jamme F, Villette S, Giuliani A, Rouam V, Wien F, Lagarde B, Refregiers M. Synchrotron UV fluorescence microscopy uncovers new probes in cells and tissues. Microsc Microanal. 2010;16:507–514. doi: 10.1017/S1431927610093852. [DOI] [PubMed] [Google Scholar]
- Jones PL, Boudreau N, Myers CA, Erickson HP, Bissell MJ. Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J Cell Sci. 1995;108(Pt 2):519–527. doi: 10.1242/jcs.108.2.519. [DOI] [PubMed] [Google Scholar]
- Kamalati T, Niranjan B, Yant J, Buluwela L. HGF/SF in mammary epithelial growth and morphogenesis: in vitro and in vivo models. J Mammary Gland Biol Neoplasia. 1999;4:69–77. doi: 10.1023/a:1018756620265. [DOI] [PubMed] [Google Scholar]
- Keely P, Fong A, Zutter M, Santoro S. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense α2 integrin mRNA in mammary cells. J Cell Science. 1995a;108:595–607. doi: 10.1242/jcs.108.2.595. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 1995b;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208:875–883. doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JF, Kao SC, Jiang ST, Tang MJ, Chan PC, Chen HC. Involvement of focal adhesion kinase in hepatocyte growth factor- induced scatter of Madin-Darby canine kidney cells. J Biol Chem. 2000;275:7474–7480. doi: 10.1074/jbc.275.11.7474. [DOI] [PubMed] [Google Scholar]
- Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, Cance W. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18:1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- Laurie GW, Leblond CP, Martin GR. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982;95:340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebakken CS, McQuade KJ, Rapraeger AC. Syndecan-1 signals independently of beta1 integrins during Raji cell spreading. Exp Cell Res. 2000;259:315–325. doi: 10.1006/excr.2000.4981. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24:1942–1953. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Chen Q, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between ras and its downstream kinase. J. Biol. Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- Liu K, Cheng L, Flesken-Nikitin A, Huang L, Nikitin AY, Pauli BU. Conditional knockout of fibronectin abrogates mouse mammary gland lobuloalveolar differentiation. Dev Biol. 2010;346:11–24. doi: 10.1016/j.ydbio.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S, Wicha MS, Guan JL. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69:466–474. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Weinberg RA. MicroRNAs in malignant progression. Cell Cycle. 2008;7:570–572. doi: 10.4161/cc.7.5.5547. [DOI] [PubMed] [Google Scholar]
- Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkowskyj KA, Keller K, Glover S, Kornberg L, Tran-Son-Tay R, Benya RV. Expression of GRP and Its Receptor in Well-differentiated Colon Cancer Cells Correlates with the Presence of Focal Adhesion Kinase Phosphorylated at Tyrosines 397 and 407. J. Histochem. Cytochem. 2003;51:1041–1048. doi: 10.1177/002215540305100807. [DOI] [PubMed] [Google Scholar]
- McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, Schedin P. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. 2006;168:608–620. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott SP, Ranheim EA, Leatherberry VS, Khwaja SS, Klos KS, Alexander CM. Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene. 2007;26:1407–1416. doi: 10.1038/sj.onc.1209930. [DOI] [PubMed] [Google Scholar]
- McHenry PR, Sears JC, Herrick MP, Chang P, Heckman-Stoddard BM, Rybarczyk M, Chodosh LA, Gunther EJ, Hilsenbeck SG, Rosen JM, Vargo-Gogola T. P190B RhoGAP has pro-tumorigenic functions during MMTV-Neu mammary tumorigenesis and metastasis. Breast Cancer Res. 2010;12:R73. doi: 10.1186/bcr2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC. Syndecan-1 regulates alphavbeta5 integrin activity in B82L fibroblasts. J Cell Sci. 2006;119:2445–2456. doi: 10.1242/jcs.02970. [DOI] [PubMed] [Google Scholar]
- Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC, Vousden KH. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy T, Wei H, Shen TL, Peng X, Liang CC, Gan B, Guan JL. Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J Biol Chem. 2007;282:31766–31776. doi: 10.1074/jbc.M705403200. [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of {beta}1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation 10.1083/jcb.200503144. J. Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, Birchmeier W. Reconstitution of mammary gland development in vitro: requirement of c- met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–545. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Fornetti J, Schedin P. Isolation of mammary-specific extracellular matrix to assess acute cell-ECM interactions in 3D culture. J Mammary Gland Biol Neoplasia. 2010;15:353–364. doi: 10.1007/s10911-010-9185-x. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34:240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- Owens L, Xu L, Craven R, Dent G, Weiner T, Kornberg L, Liu E, Cance W. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- Paguirigan A, Beebe DJ, Liu B, Alexander C. Mammary stem and progenitor cells: tumour precursors? Eur J Cancer. 2006;42:1225–1236. doi: 10.1016/j.ejca.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Parry G, Cullen B, Kaetzel CS, Kramer R, Moss L. Regulation of differentiation and polarized secretion in mammary epithelial cells maintained in culture: extracellular matrix and membrane polarity influences. J Cell Biol. 1987;105:2043–2051. doi: 10.1083/jcb.105.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Lee EY, Farson D, Koval M, Bissell MJ. Collagenous substrata regulate the nature and distribution of glycosaminoglycans produced by differentiated cultures of mouse mammary epithelial cells. Exp Cell Res. 1985;156:487–499. doi: 10.1016/0014-4827(85)90556-7. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol. 1997;150:497–507. [PMC free article] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol. 2006;174:277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001;3:230–237. doi: 10.1186/bcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol. 2008a;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008b;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact Guidance Mediated Three-Dimensional Cell Migration is Regulated by Rho/ROCK-Dependent Matrix Reorganization. Biophys J. 2008c;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Rudolph-Owen LA, Cannon P, Matrisian LM. Overexpression of the matrix metalloproteinase matrilysin results in premature mammary gland differentiation and male infertility. Mol Biol Cell. 1998;9:421–435. doi: 10.1091/mbc.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, Barker N, Clevers H, Sansom OJ, Anderson KI, Weaver VM, Olson MF. Actomyosin-Mediated Cellular Tension Drives Increased Tissue Stiffness and beta-Catenin Activation to Induce Epidermal Hyperplasia and Tumor Growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5:211–225. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog. 2004;41:207–220. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- Schedin P, O'Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12:71–82. doi: 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src-and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HHF, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982;90:215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of serially aged mouse mammary ducts. Mechanisms of ageing and development. 1984;24:151–162. doi: 10.1016/0047-6374(84)90067-8. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987;237:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Somasiri A, Howarth A, Goswami D, Dedhar S, Roskelley CD. Overexpression of the integrin-linked kinase mesenchymally transforms mammary epithelial cells. J Cell Sci. 2001;114:1125–1136. doi: 10.1242/jcs.114.6.1125. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafolla E, Wang S, Wong B, Leong J, Kapila YL. JNK1 and JNK2 oppositely regulate p53 in signaling linked to apoptosis triggered by an altered fibronectin matrix: JNK links FAK and p53. J Biol Chem. 2005;280:19992–19999. doi: 10.1074/jbc.M500331200. [DOI] [PubMed] [Google Scholar]
- Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L, Cavassa S, Angelini P, Ando M, Bertotti A, Comoglio PM, Boccaccio C. HGF/scatter factor selectively promotes cell invasion by increasing integrin avidity. Faseb J. 2000;14:1629–1640. doi: 10.1096/fj.14.11.1629. [DOI] [PubMed] [Google Scholar]
- Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- Tsai J, Kam L. Rigidity-dependent cross talk between integrin and cadherin signaling. Biophys J. 2009;96:L39–41. doi: 10.1016/j.bpj.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7:R605–608. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Chang A, Benaich N, Reinhardt F, Weinberg RA. Activation of miR-31 function in already-established metastases elicits metastatic regression. Genes Dev. 2011;25:646–659. doi: 10.1101/gad.2004211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Valastyan S, Weinberg RA. Roles for microRNAs in the regulation of cell adhesion molecules. J Cell Sci. 2011;124:999–1006. doi: 10.1242/jcs.081513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Miltenburg MH, Lalai R, de Bont H, van Waaij E, Beggs H, Danen EH, van de Water B. Complete focal adhesion kinase deficiency in the mammary gland causes ductal dilation and aberrant branching morphogenesis through defects in Rho kinase-dependent cell contractility. Faseb J. 2009;23:3482–3493. doi: 10.1096/fj.08-123398. [DOI] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and Testing of a Gene Expression Signature of Invasive Carcinoma Cells within Primary Mammary Tumors 10.1158/0008-5472.CAN-04-1136. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- Warburton MJ, Mitchell D, Ormerod EJ, Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982;30:667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Werb Z, Sympson CJ, Alexander CM, Thomasset N, Lund LR, MacAuley A, Ashkenas J, Bissell MJ. Extracellular matrix remodeling and the regulation of epithelial-stromal interactions during differentiation and involution. Kidney Int Suppl. 1996;54:S68–74. [PMC free article] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Lowrie G, Kohn E, Bagavandoss P, Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982;79:3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Xie JW, Haslam SZ. Extracellular matrix, Rac1 signaling, and estrogen-induced proliferation in MCF-7 breast cancer cells. Breast Cancer Res Treat. 2008;110:257–268. doi: 10.1007/s10549-007-9719-0. [DOI] [PubMed] [Google Scholar]
- Yang N, Mosher R, Seo S, Beebe D, Friedl A. Syndecan-1 in breast cancer stroma fibroblasts regulates extracellular matrix fiber organization and carcinoma cell motility. Am J Pathol. 2011;178:325–335. doi: 10.1016/j.ajpath.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM. Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors. J Cell Biol. 2003;163:1397–1407. doi: 10.1083/jcb.200302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Lin H, Nie Z, Wu J, Canete-Soler R, Schlaepfer WW, Schlaepfer DD. Direct Interaction of Focal Adhesion Kinase with p190RhoGEF. J. Biol. Chem. 2003;278:24865–24873. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Dazin P, Kapila Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin alphav mediate survival signals through focal adhesion kinase. J Biol Chem. 2004;279:48342–48349. doi: 10.1074/jbc.M407953200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan W, Chen X. Mutant p53 disrupts MCF-10A cell polarity in three-dimensional culture via epithelial-to-mesenchymal transitions. J Biol Chem. 2011;286:16218–16228. doi: 10.1074/jbc.M110.214585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Krigman HR, Santoro SA. Altered integrin expression in adenocarcinoma of the breast. Analysis by in situ hybridization. Am J Pathol. 1993;142:1439–1448. [PMC free article] [PubMed] [Google Scholar]