Abstract
We describe an oxidative Strecker reaction that allows for direct cyanation of para-methoxyphenyl (PMP)-protected primary amines. A vanadium(V) complex was used as the catalyst and TBHP as the oxidant. The cyanation occurs at the α-C position bearing either an alkyl or an aromatic group. This method provides a direct access to α-aminonitrile from amines with one-carbon extension.
Keywords: Vanadium catalyst, C–H cyanation, Oxidative Strecker reaction, Primary amine, Schiff base ligand
Cyanide, or nitrile, is a valuable one-carbon unit. It can be converted into a variety of polar functional groups, for example, amine, aldehyde, ketone, carboxylic acid, and amide. The Strecker reaction is the most commonly used cyanation reaction, which gives α-aminonitriles from imines.1 Imines are normally prepared by condensing amines and aldehydes (Figure 1, top), but can also be generated by oxidizing amines (Figure 1, bottom). Recently, this oxidative approach has been recognized as a powerful new way to functionalize amines.
Figure 1.

Traditional vs. oxidative Strecker reactions.
The oxidative Strecker reaction is a cross-dehydrogenative coupling (CDC) reaction.2 It provides a more direct access to α-aminonitriles than the traditional Strecker reaction. It also uses the amine building blocks in a different way. Instead of cyanating aldehydes at the carbonyl position after imine formation, this one-carbon extension reaction functionalizes amines at the α-C-position. The focus of current research on this type of CDC reaction is the oxidative functionalization of tertiary amines, in particular, N,N-dialkylaniline and tetrahydroisoquinoline.3,4 Issues associated with primary and secondary amines arise from the N–H oxidation step,5,6 which often yields nitrones or N-oxides instead of imines.7
Vanadium complexes have been used widely to catalyze the oxidation of olefins, thioethers, alcohols, phenols, but not amines.8 There is only one report of vanadium-catalyzed CDC reaction, specifically, oxidative Strecker reaction of tertiary amines.9 As part of a program to expand the utility of vanadium complexes, we searched for vanadium catalyst systems that promote the N–H oxidation. We report here that vanadium-Schiff base complexes can catalyze oxidative Strecker reaction of PMP-protected primary amines.
We recently found that vanadocene dichloride (Cp2VCl2) catalyzes benzylic C–H oxidation selectively and efficiently.10 We hypothesized that high-valent vanadium complexes could also oxidize the weakly activated α-C–H group of amines and promote a Strecker reaction. We envisioned that methods for cyanating primary amines would be most versatile because the nitrogen center of the products could be further functionalized. We chose to use the electron-rich para-methoxyphenyl (PMP) protecting group to promote the oxidation and circumvent the self-condensation issue of primary amines. After C–H cyanation, the N-PMP group can be removed easily to give α-aminonitriles that bear a primary amino group.11
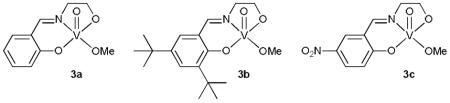
We began our study by searching for an active vanadium catalyst at 5 mol % loading. We used PMP-benzylamine (1) as the substrate, TBHP as the oxidant, and TMSCN as the cyanide source (Table 1). All the vanadium(III) and vanadium(IV) complexes we examined were not able to promote this reaction (entries 1 and 2). However, various vanadium(V) complexes were found active (entry 3), with the vanadium(V)-Schiff base complex 3a catalyzing the oxidative cyanation of 1 to give 2 in 50% yield (entry 4). Introducing two sterically bulky and slightly electron-donating tert-butyl groups to the aromatic ring of the Schiff base ligand resulted in decreased reaction rate (entry 5), whereas adding an electron-withdrawing nitro group led to a complex mixture of products (entry 6). Acetonitrile was proved to be the solvent of choice (entries 7–11). The yield of 2 could be improved to 61% by increasing the reaction concentration to 0.2 M (entry 12).
Table 1.
Development of the vanadium-catalyzed oxidative α-C-cyanation reactiona
| Entry | Catalyst | Solvent | Yield |
|---|---|---|---|
| 1 | VCl3 or VBr3 | CH3CN | 0% |
| 2 | VF4, VO2, VOSO4 or VO(acac)2 | CH3CN | 0% |
| 3 | V2O5, VO(OiPr)3, VO(OSiPh3)3 | CH3CN | <10% |
| 4 | 3a | CH3CN | 50% |
| 5 | 3b | CH3CN | 32% |
| 6 | 3c | CH3CN | <10% |
| 7 | 3a | CH2CI2 | 30% |
| 8 | 3a | DMF | <10%c |
| 9 | 3a | THF | <10% |
| 10 | 3a | dioxane | <10% |
| 11 | 3a | toluene | <10% |
| 12 | 3a | CH3CNb | 61% |
Reaction conditions: 1 (0.1 mmol), TBHP (70 wt. % in water, 0.15 mmol), TMSCN (0.12 mmol), 1 mL solvent.
0.5 mL solvent.
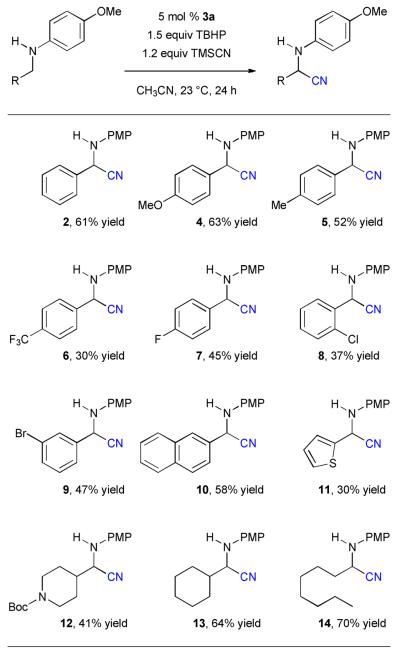
The generality of this vanadium-catalyzed oxidative Strecker reaction is shown in Table 2. A variety of PMP-protected primary amines can be cyanated easily. For substituted benzylamines, both electron-donating and withdrawing groups can be tolerated at the para, meta, or ortho position (4–9). In general, electron-rich benzylamines (4 and 5) are more reactive than the electron-deficient ones (6–9). Aromatic rings other than phenyl can also be tolerated. For example, both the PMP-protected naphthylmethylamine and thiophenylmethylamine were cyanated smoothly (10 and 11). Importantly, activation of the α-position by an aromatic group is not necessary. This oxidative Strecker reaction can be used to functionalizing PMP-protected aliphatic amines (12–14).
Table 2.
|
Reaction conditions: amine (0.2 mmol), TBHP (70 wt. % in water, 0.3 mmol), TMSCN (0.24 mmol), 1 mL CH3CN.
Isolated yields. PMP = para-methoxyphenyl (p-MeOPh).
This oxidative Strecker reaction allows us to make use of amine building blocks to prepare α-aminonitriles that cannot be easily accessed by traditional methods. For example, α-C-cyantion of the PMP-protected (+)-dehydroabietylamine (15) gave 16 in 61% yield as a 2:1 mixture of diastereomers. 15 was synthesized by copper-catalyzed N-arylation of (+)-dehydroabietylamine (see Supplementary Material for details).
Mechanistically, we believe that 3a first reacted with TBHP to give I as the active catalyst. The PMP-amine then coordinated to I and formed complex II. Similar to other metal-catalyzed amine oxidation reactions,2 a single-electron transfer (SET) from the electron-rich nitrogen center to the vanadium complex occurred to give III, a valence tautomer of II. Subsequent O–O homolysis and C–H abstraction gave IV and the iminium ion, which reacted with cyanide to give the α-aminonitrile. Support for the SET hypothesis followed from the observation that electron deficient acetyl, tosyl, and Boc-protected benzylamines did not react under these conditions. In addition, vanadium(III) complexes could not promote this oxidative Strecker reaction, suggesting that a two-electron vanadium(III/V) redox mechanism was not operative. We also believe that amine oxidation was not mediated by tert-butoxyl radical,12 because vanadium(IV) complexes did not catalyze the reaction, and vanadium(V) complexes other than 3 gave only less than 10% conversion. For the same reason, we believe that the aminium ion was coordinated to vanadium (III) when the C–H abstraction occurred. The Schiff base ligand likely served as an electron sink to facilitate the SET. We further propose that the silyl transfer from TMSCN to the oxide ligand of III or IV promoted the regeneration of I and released the cyanide for the Strecker reaction.
In summary, we have developed a vanadium-catalyzed oxidative Strecker reaction of PMP-protected primary amines. This α-C–H cyanation reaction allows for functionalization of amines at the α-C-position, and is complimentary to the traditional Strecker reaction which functionalizes aldehydes. Efforts are underway to further explore the utility of vanadium-catalyzed CDC reactions.
Supplementary Material
Figure 2.

Functionalization of natural product dehydroabietylamine. Reaction conditions: 15 (0.2 mmol), TBHP (70 wt. % in water, 0.3 mmol), TMSCN (0.24 mmol), 1 mL CH3CN. PMP = para-methoxyphenyl (p-MeOPh).
Figure 3.
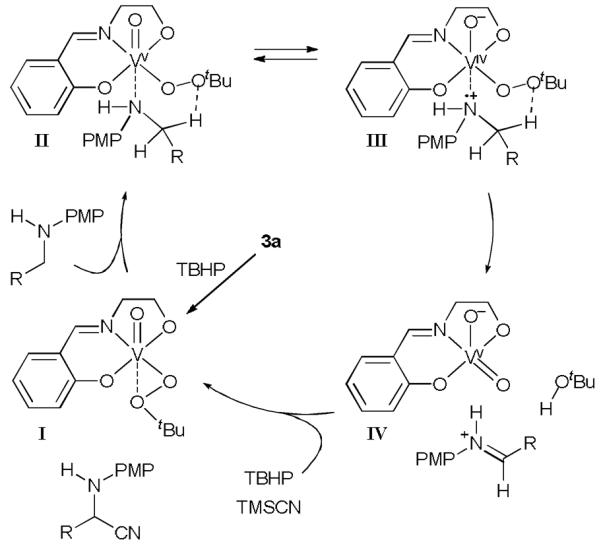
Proposed mechanism for the vanadium-catalyzed α-C-cyanation reaction.
Acknowledgements
Financial Support was provided by NIH (NIGMS R01-GM079554) and the Welch Foundation (I-1596). C. C. is a Southwestern Medical Foundation Scholar in Biomedical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material Experimental procedures and characterization data of the reaction products can be found, in the online version, at (link).
References and notes
- 1.(a) Gröger H. Chem. Rev. 2003;103:2795–2828. doi: 10.1021/cr020038p. [DOI] [PubMed] [Google Scholar]; (b) Enders D, Shilvock JP. Chem. Soc. Rev. 2000;29:359–373. [Google Scholar]; (c) Wang J, Liu X, Feng X. Chem. Rev. 2011;111:6947–6983. doi: 10.1021/cr200057t. [DOI] [PubMed] [Google Scholar]; (d) Zuend SJ, Coughlin MP, Lalonde MP, Jacobsen EN. Nature. 2009;461:968–970. doi: 10.1038/nature08484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Murahashi S-I. Pure Appl. Chem. 1992;64:403–412. [Google Scholar]; (b) Murahashi S-I. Angew. Chem. Int. Ed. 1995;34:2443–2465. [Google Scholar]; (c) Murahashi S-I, Zhang D. Chem. Soc. Rev. 2008;37:1490–1501. doi: 10.1039/b706709g. [DOI] [PubMed] [Google Scholar]; (d) Campos KR. Chem. Soc. Rev. 2007;36:1069–1084. doi: 10.1039/b607547a. [DOI] [PubMed] [Google Scholar]; (e) Li C-J. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]; (f) Yoo W-J, Li C-J. Top. Curr. Chem. 2010;292:281–302. doi: 10.1007/128_2009_17. [DOI] [PubMed] [Google Scholar]; (g) Yeung CS, Dong VM. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]; (h) Sun C-L, Li B-J, Shi Z-J. Chem. Rev. 2011;111:1293–1314. doi: 10.1021/cr100198w. [DOI] [PubMed] [Google Scholar]; (i) Liu C, Zhang H, Shi W, Lei A. Chem. Rev. 2011;111:1780–1824. doi: 10.1021/cr100379j. [DOI] [PubMed] [Google Scholar]
- 3.For examples of CDC reactions of secondary amines: Choi H, Doyle MP. Chem. Commun. 2007:745–747. doi: 10.1039/b615805f.; Zhao L, Li C-J. Angew. Chem. Int. Ed. 2008;47:7075–7078. doi: 10.1002/anie.200801367.; Zhao L, Baslé O, Li C-J. Proc. Natl. Acad. Sci. 2009;106:4106–4111. doi: 10.1073/pnas.0809052106.; Xie J, Huang Z-Z. Angew. Chem. Int. Ed. 2010;49:10181–10185. doi: 10.1002/anie.201004940.; Zhang G, Zhang Y, Wang R. Angew. Chem. Int. Ed. 2011;50:10429–10432. doi: 10.1002/anie.201105123.; Wu J-C, Song R-J, Wang Z-Q, Huang X-C, Xie Y-X, Li PDJ-H. Angew. Chem. Int. Ed. 2012;51:3453–3457. doi: 10.1002/anie.201109027.; Li K, Tan G, Huang J, Song F, You J. Angew. Chem. Int. Ed. 2012 Early View, DOI: 10.1002/anie.201306181.
- 4.For examples of oxidative Strecker reaction of secondary amines: Barton DHR, Billion A, Boivin J. Tetrahedron Lett. 1985;26:1229–1232.; Köhler V, Bailey KR, Znabet A, Raftery J, Helliwell M, Turner NJ. Angew. Chem. Int. Ed. 2010;49:2182–2184. doi: 10.1002/anie.200906655.; Sonobe T, Oisaki K, Kanai M. 2012;3:3249–3255.
- 5.(a)Ref. 2a–c; Enders D, Shilvock JP. Chem. Soc. Rev. 2000;29:359–373.; Largeron M. Eur. J. Org. Chem. 2013:5225–5235.
- 6.Martin GM, Benditt EP, Eriksen N. Nature. 1960;186:884–885. doi: 10.1038/186884a0.; Chu G, Li C. Org. Biomol. Chem. 2010;8:4716–4719. doi: 10.1039/c0ob00043d.; Murahashi S-I, Naota T, Taki H. J. Chem. Soc. Chem. Commun. 1985:613–614.; Samec JSM, Éll AH, Bäckvall J-E. Chem. Eur. J. 2005;11:2327–2334. doi: 10.1002/chem.200401082.; Murahashi S-I, Okano Y, Sato H, Nakae T, Komiya N. Synlett. 2007:1675–1678.; Nishinaga r., Yamazaki S, Matsuura T. Tetrahedron Lett. 1988;33:4115–4118.; Larsen J, Jørgensen KA. J. Chem. Soc., Perkin Trans. 2. 1992:1213–1217.; Nicolaou KC, Mathison CJN, Montagnon T. Angew. Chem. Int. Ed. 2003;42:4077–4082. doi: 10.1002/anie.200352076.; Patila RD, Adimurthy S. Adv. Synth. Catal. 2011;353:1695–1700.; Huang B, Tian H, Lin S, Xie M, Yu X, Xu Q. Tetrahedron Lett. 2013;54:2861–2864.; Largeron M, Chiaroni A, Fleury M-B. Chem. Eur. J. 2008;14:996–1003. doi: 10.1002/chem.200700876.; Largeron M, Fleury M-B. Angew. Chem. Int. Ed. 2012;51:5409–5412. doi: 10.1002/anie.201200587.; Wendlandt AE, Stahl SS. Org. Lett. 2012;14:2850–2853. doi: 10.1021/ol301095j.; (n)Ref. 3c.
- 7.(a) Murahashi S-I, Shiota T. Tetrahedron Lett. 1987;28:2383–2386. [Google Scholar]; (b) Murahashi S, Oda T, Masui Y. J. Am. Chem. Soc. 1989;111:5002–5003. [Google Scholar]; (c) Murahashi S, Mitsui H, Shiota T, Tsuda T, Watanabe S. J. Org. Chem. 1990;55:1736–1744. [Google Scholar]; (d) Murahashi S-I, Imada Y, Ohtake H. J. Org. Chem. 1994;59:6170–6172. [Google Scholar]; (e) Imada Y, Iida H, Ono S, Murahashi S-I. J. Am. Chem. Soc. 2003;125:2868–2869. doi: 10.1021/ja028276p. [DOI] [PubMed] [Google Scholar]
- 8.(a) Sharpless KB, Verhoeven TR. Aldrichimica Acta. 1979;12:63–74. [Google Scholar]; (b) Butler A, Clague MJ, Meister GE. Chem. Rev. 1994;94:625–638. [Google Scholar]; (c) Hirao T. Chem. Rev. 1997;97:2707–2724. doi: 10.1021/cr960014g. [DOI] [PubMed] [Google Scholar]; (d) Conte V, Di Furia F, Licini G. Appl. Catal., A. 1997;157:335–361. [Google Scholar]; (e) Ligtenbarg AGJ, Hage R, Feringa BL. Coord. Chem. Rev. 2003;237:89–101. [Google Scholar]
- 9.Singhal S, Jain SL, Sain B. Chem. Commun. 2009:2371–2372. doi: 10.1039/b820402k. [DOI] [PubMed] [Google Scholar]
- 10.Xia J-B, Cormier KW, Chen C. Chem. Sci. 2012;3:2240–2245. doi: 10.1039/C2SC20178J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Verkade JMM, van Hemert LJC, Quaedflieg PJLM, Alsters PL, van Delfta FL, Rutjesa FPJT. Tetrahedron Lett. 2006;47:8109–8113. [Google Scholar]; (b) Verkade JMM, van Hemert LJC, Quaedflieg PJLM, Schoemaker HE, Schümann M, van Delft FL, Rutjes FPJT. Adv. Synth. Catal. 2007;349:1332–1336. [Google Scholar]; (c) Koch K, van Weerdenburg BJA, Verkade JMM, Nieuwland PJ, Rutjes FPJT, van Hest JCM. Org. Process Res. Dev. 2009;13:1003–1006. [Google Scholar]
- 12.Ratnikov MO, Doyle MP. J. Am. Chem. Soc. 2013;135:1549–1557. doi: 10.1021/ja3113559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


