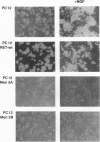
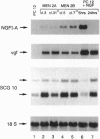
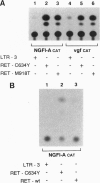
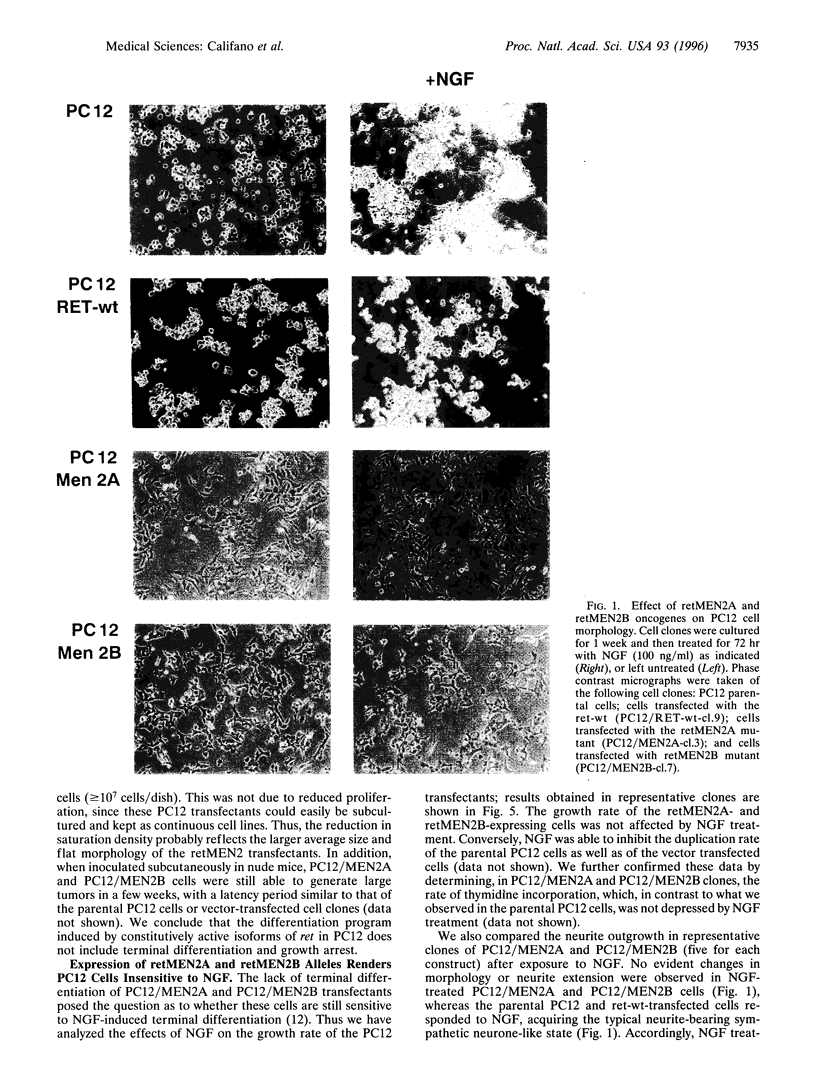
Abstract
Germ-line missense mutations of the receptor-like tyrosine kinase ret are the causative genetic event of the multiple endocrine neoplasia (MEN) type 2A and type 2B syndromes and of the familial medullary thyroid carcinoma. We have used the rat pheochromocytoma cell line, PC12, as a model system to investigate the mechanism or mechanisms by which expression of activated ret alleles contributes to the neoplastic phenotype in neuroendocrine cells. Here we show that stable expression of ret mutants (MEN2A and MEN2B alleles) in PC12 cells causes a dramatic conversion from a round to a flat morphology, accompanied by the induction of genes belonging to the early as well as the delayed response to nerve growth factor. However, in the transfected PC12 cells, the continuous expression of neuronal specific genes is not associated with the suppression of cell proliferation. Furthermore, expression of ret mutants renders PC12 cells unresponsive to nerve growth factor-induced inhibition of proliferation. These results suggest that induction of an aberrant pattern of differentiation, accompanied by unresponsiveness to growth-inhibitory physiological signals, may be part of the mechanism of action of activated ret alleles in the pathogenesis of neuroendocrine tumors associated with MEN2 syndromes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Asai N., Iwashita T., Matsuyama M., Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995 Mar;15(3):1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Baybis M., Salton S. R. Nerve growth factor rapidly regulates VGF gene transcription through cycloheximide sensitive and insensitive pathways. FEBS Lett. 1992 Aug 17;308(2):202–206. doi: 10.1016/0014-5793(92)81274-p. [DOI] [PubMed] [Google Scholar]
- Boulukos K. E., Ziff E. B. Adenovirus 5 E1A proteins disrupt the neuronal phenotype and growth factor responsiveness of PC12 cells by a conserved region 1-dependent mechanism. Oncogene. 1993 Feb;8(2):237–248. [PubMed] [Google Scholar]
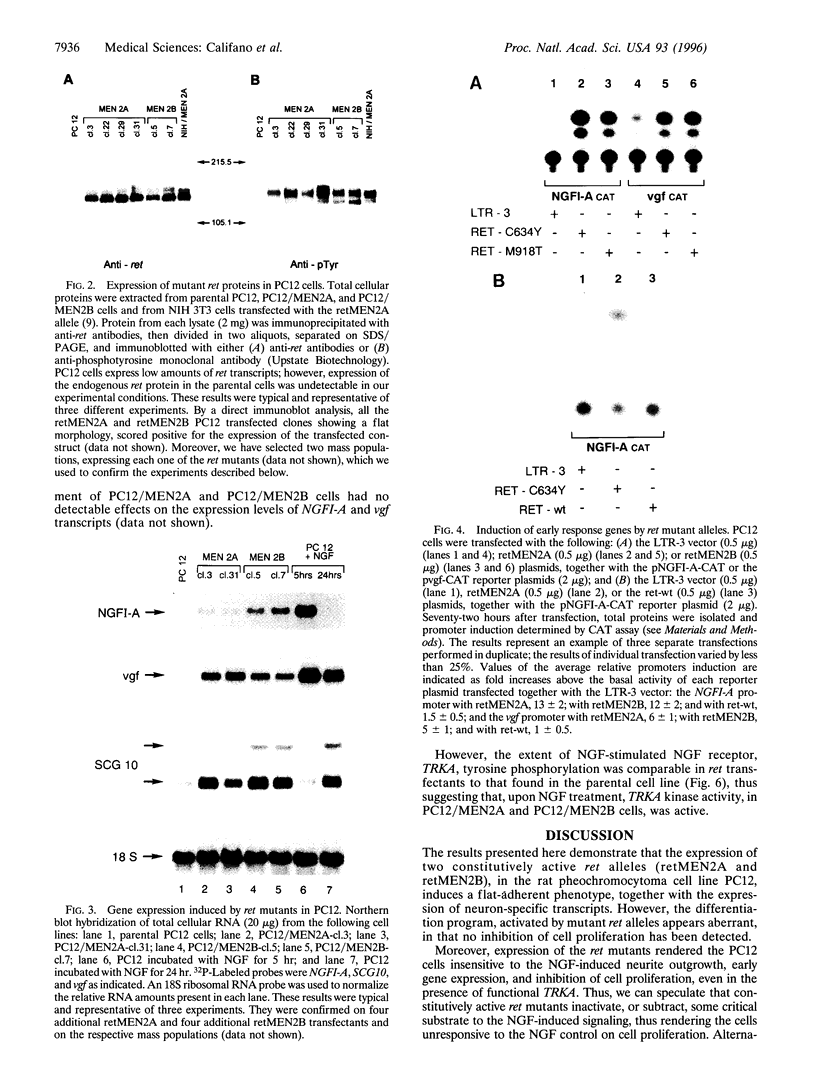
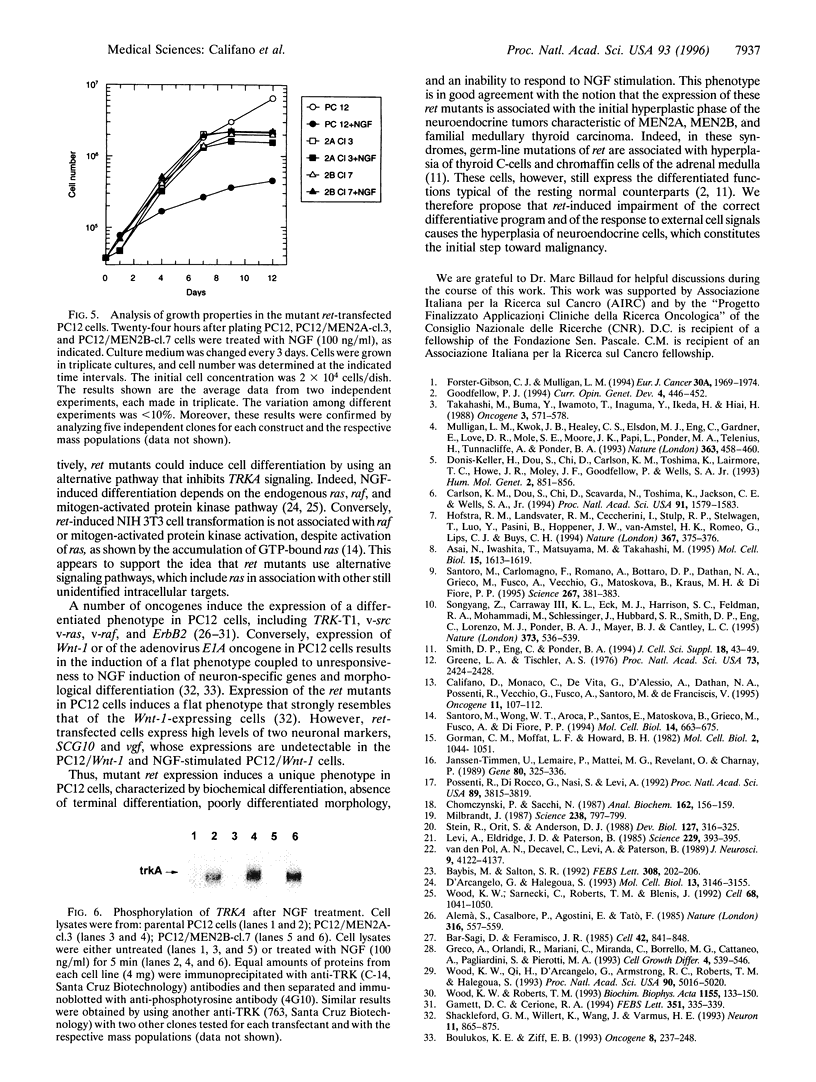
- Califano D., Monaco C., de Vita G., D'Alessio A., Dathan N. A., Possenti R., Vecchio G., Fusco A., Santoro M., de Franciscis V. Activated RET/PTC oncogene elicits immediate early and delayed response genes in PC12 cells. Oncogene. 1995 Jul 6;11(1):107–112. [PubMed] [Google Scholar]
- Carlson K. M., Dou S., Chi D., Scavarda N., Toshima K., Jackson C. E., Wells S. A., Jr, Goodfellow P. J., Donis-Keller H. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G., Halegoua S. A branched signaling pathway for nerve growth factor is revealed by Src-, Ras-, and Raf-mediated gene inductions. Mol Cell Biol. 1993 Jun;13(6):3146–3155. doi: 10.1128/mcb.13.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Dou S., Chi D., Carlson K. M., Toshima K., Lairmore T. C., Howe J. R., Moley J. F., Goodfellow P., Wells S. A., Jr Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993 Jul;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- Forster-Gibson C. J., Mulligan L. M. Multiple endocrine neoplasia type 2. Eur J Cancer. 1994;30A(13):1969–1974. doi: 10.1016/0959-8049(94)00388-l. [DOI] [PubMed] [Google Scholar]
- Gamett D. C., Cerione R. A. Oncogenically activated or ligand-stimulated neu kinase stimulates neurite outgrowth in PC12 cells. FEBS Lett. 1994 Sep 12;351(3):335–339. doi: 10.1016/0014-5793(94)00855-8. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. J. Inherited cancers associated with the RET proto-oncogene. Curr Opin Genet Dev. 1994 Jun;4(3):446–452. doi: 10.1016/0959-437x(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A., Orlandi R., Mariani C., Miranda C., Borrello M. G., Cattaneo A., Pagliardini S., Pierotti M. A. Expression of TRK-T1 oncogene induces differentiation of PC12 cells. Cell Growth Differ. 1993 Jul;4(7):539–546. [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra R. M., Landsvater R. M., Ceccherini I., Stulp R. P., Stelwagen T., Luo Y., Pasini B., Höppener J. W., van Amstel H. K., Romeo G. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994 Jan 27;367(6461):375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Janssen-Timmen U., Lemaire P., Mattéi M. G., Revelant O., Charnay P. Structure, chromosome mapping and regulation of the mouse zinc-finger gene Krox-24; evidence for a common regulatory pathway for immediate-early serum-response genes. Gene. 1989 Aug 15;80(2):325–336. doi: 10.1016/0378-1119(89)90296-5. [DOI] [PubMed] [Google Scholar]
- Levi A., Eldridge J. D., Paterson B. M. Molecular cloning of a gene sequence regulated by nerve growth factor. Science. 1985 Jul 26;229(4711):393–395. doi: 10.1126/science.3839317. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Kwok J. B., Healey C. S., Elsdon M. J., Eng C., Gardner E., Love D. R., Mole S. E., Moore J. K., Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993 Jun 3;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Possenti R., Di Rocco G., Nasi S., Levi A. Regulatory elements in the promoter region of vgf, a nerve growth factor-inducible gene. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3815–3819. doi: 10.1073/pnas.89.9.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M., Carlomagno F., Romano A., Bottaro D. P., Dathan N. A., Grieco M., Fusco A., Vecchio G., Matoskova B., Kraus M. H. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995 Jan 20;267(5196):381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Santoro M., Wong W. T., Aroca P., Santos E., Matoskova B., Grieco M., Fusco A., di Fiore P. P. An epidermal growth factor receptor/ret chimera generates mitogenic and transforming signals: evidence for a ret-specific signaling pathway. Mol Cell Biol. 1994 Jan;14(1):663–675. doi: 10.1128/mcb.14.1.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford G. M., Willert K., Wang J., Varmus H. E. The Wnt-1 proto-oncogene induces changes in morphology, gene expression, and growth factor responsiveness in PC12 cells. Neuron. 1993 Nov;11(5):865–875. doi: 10.1016/0896-6273(93)90116-9. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Eng C., Ponder B. A. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes and Hirschsprung disease. J Cell Sci Suppl. 1994;18:43–49. doi: 10.1242/jcs.1994.supplement_18.6. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995 Feb 9;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Stein R., Orit S., Anderson D. J. The induction of a neural-specific gene, SCG10, by nerve growth factor in PC12 cells is transcriptional, protein synthesis dependent, and glucocorticoid inhibitable. Dev Biol. 1988 Jun;127(2):316–325. doi: 10.1016/0012-1606(88)90318-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Buma Y., Iwamoto T., Inaguma Y., Ikeda H., Hiai H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene. 1988 Nov;3(5):571–578. [PubMed] [Google Scholar]
- Wood K. W., Qi H., D'Arcangelo G., Armstrong R. C., Roberts T. M., Halegoua S. The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: a potential role for cellular raf kinases in neuronal growth factor signal transduction. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5016–5020. doi: 10.1073/pnas.90.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. W., Roberts T. M. Oncogenes and protein kinases in neuronal growth-factor action. Biochim Biophys Acta. 1993 Aug 23;1155(2):133–150. doi: 10.1016/0304-419x(93)90002-t. [DOI] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Decavel C., Levi A., Paterson B. Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci. 1989 Dec;9(12):4122–4137. doi: 10.1523/JNEUROSCI.09-12-04122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]