Abstract
Based on the collected ethnobotanical data from the Traditional Medicine and Materia Medica Research Center (TMRC), Iran, Myrtus communis L. (myrtle) was selected for the assessment of in vitro and in vivo antimalarial and cytotoxic activities. Methanolic extract of myrtle was prepared from the aerial parts and assessed for antiplasmodial activity, using the parasite lactate dehydrogenase (pLDH) assay against chloroquine-resistant (K1) and chloroquine-sensitive (3D7) strains of Plasmodium falciparum. The 4-day suppressive test was employed to determine the parasitemia suppression of the myrtle extract against P. berghei in vivo. The IC50 values of myrtle extract were 35.44 µg/ml against K1 and 0.87 µg/ml against 3D7. Myrtle extract showed a significant suppression of parasitaemia (84.8 ± 1.1% at 10 mg/kg/day) in mice infected with P. berghei after 4 days of treatment. Cytotoxic activity was carried out against mammalian cell lines using methyl thiazol tetrazolium (MTT) assay. No cytotoxic effect on mammalian cell lines up to 100 µg/mL was shown. The results support the traditional use of myrtle in malaria. Phytochemical investigation and understanding the mechanism of action would be in our upcoming project.
1. Introduction
Malaria is a classic example of a disease that affects the productivity of individuals, families, and the whole society [1]. The number of deaths due to malaria was estimated to be 655000 in 2010 [2]. Malaria was highly endemic in the Caspian areas in the north and Persian Gulf littoral and plain areas in the south parts of Iran. It had been widely prevalent for a long time in the country [3]. At present the southern area of Iran (Figure 1), accommodating numerous emigrants including Afghanis and Pakistanis, is considered a high risk region in the area. According to the Ministry of Health of Iran the total number of malaria cases in Iran had been estimated to be 2900 cases in 2010 [4].
Figure 1.
Map of malaria risk areas in Iran.
A dramatic recrudescence of malaria is ongoing due to the increasing resistance of mosquito vectors to insecticides and resistance of parasites, mainly Plasmodium falciparum, to available modern drugs [5]. Since malaria chemotherapy is complicated by drug-resistant strains of Plasmodium, new antimalarial agents are needed [6]. Traditional treatments may well prove to be the source of new antimalarial agents in view of the success with the two important chemotherapeutic agents, quinine and artemisinin, both of which are derived from plants [1]. Iran has an honorable past in traditional medicine. One of the most significant ancient heritages is sophisticated experience of people who have tried over millennia to find useful plants for health improvement, with each generation adding its own experience to this tradition [7].
This study is based on the ethnomedicinal data of the indigenous people of Kohgiluyeh-va-Boyer Ahmad province of Iran (unpublished data), where aqueous extract of myrtle is known as antiparasitic agent. Myrtle has been previously reported for various ailments like gastric ulcer, diarrhea, dysentery, vomiting, rheumatism, hemorrhage, deep sinuses, leucorrhoea, and cosmetic purposes [8]. In this study the in vitro and in vivo antiplasmodial activities and in vitro cytotoxic effect of the plant were assessed. The methanolic extract was selected for this experiment avoiding possible elimination of active compounds.
2. Materials and Methods
2.1. Collection and Extraction
Myrtus communis L. was collected in its natural habitats. Botanical identification was performed by voucher specimen (TMRC 1169) deposited at the herbarium of the Traditional Medicine and Materia Medica Research Center (TMRC). The aerial parts of the plant were allowed to dry in shadow until desiccated. Plant sample was crushed into powder using a hammer mill and stored at room temperature in appropriate container.
10 g of powdered dried aerial parts was macerated in methanol with constant shaking at the room temperature for 24 hours. The filtrate was evaporated to dryness and used for further assessments.
2.2. In Vitro Antiplasmodial Activity
In vitro screens for compound activity require the ability to culture P. falciparum in human erythrocytes [9]. The chloroquine-resistant (K1) and chloroquine-sensitive (3D7) strains of P. falciparum were continuous subcultured in vitro from cryopreservation and maintained in human red blood cells, diluted to 7% hematocrit with RPMI1640 medium. All cultures were placed in the candle jar at 37°C under 3% O2, 6% CO2, and 91% N2 atmosphere.
In vitro antiplasmodial activity, following the lactate dehydrogenase (LDH) method was assessed [10]. Plasmodium species depend on LDH for the metabolism of carbohydrates. Parasite LDH(pLDH) is used for the conversion of lactate into pyruvate, which is the last step in glycolysis; however, only pLDH can use coenzyme 3-acetyl-pyridine adenine dinucleotide (APAD). At the presence of APAD, the detection of LDH is specific for the parasite enzyme. LDH determination is carried out in the presence of nitro blue tetrazolium (NBT) which is reduced to formazan that is detected at 630 nm.
Starting concentration of chloroquine diphosphate and artemisinin which served as positive controls was 20 mg/mL. Myrtle extract was dissolved in DMSO to produce stock solution of 20 mg/mL. The stock solutions were subsequently diluted with deionized water at twenty different concentrations. Twofold serial dilutions were made in 96-well microtitre plates in duplicate and infected erythrocytes were added to give a 2% hematocrit and 1% parasitaemia. For the infected control, parasitized red blood cells were devoid of myrtle extract, whereas only nonparasitised red blood cells were prepared for noninfected control. Test samples were incubated at 37°C for 24 hours and subsequently cooled at −20°C to lyse the red blood cells. The plates were next allowed to reach room temperature. The spectrophotometric assessment of LDH activity was facilitated by adding 20 μL nitroblue tetrazolium (NBT) and phenazine ethosulfate mixture to the 100 μL MALSTAT reagent. Absorbance was measured with an ELISA plate reader at 630 nm. The percentage inhibition at each concentration was determined and the mean of the IC50 values of parasite viability was calculated using probit analysis [11].
2.3. In Vitro Cytotoxicity Activity
Preferably, the extract for antiplasmodial investigation should have no cytotoxicity. So the cytotoxicity of myrtle was measured by the colorimetric methyl thiazol tetrazolium (MTT) assay and scored as a percentage of absorbance reduction at 570 nm of treated cultures versus untreated control cultures.
MCF7 (breast adenocarcinoma), HepG2 (hepatocellular carcinoma), WEHI (fibrosarcoma), and MDBK (normal kidney cells) were seeded into 96-well microplates at 104 cells per well and allowed to grow for 24 hours. The initial concentration of extract was 100 μg/mL in DMSO, which was serially diluted in complete culture medium with two fold dilutions. Different concentrations of the extract were added to each well. Plates were incubated at 37°C for 72 hours under 5% CO2 atmosphere. Then the 50 μL of MTT-PBS solution in culture medium was added to each well. The plates were further incubated for 4 hours at the same condition. The medium was then removed and replaced with 200 μL of DMSO to solubilize the MTT formazan product. The solutions were shaked for 20 min and the absorbance at 570 nm was measured. The IC50 values were calculated from the drug concentration-response curves. Tamoxifen was used as a positive control with concentrations from 50 to 1.56 μg/mL.
2.4. In Vivo Antimalarial Assay
The suppressive activity of the methanol extract of myrtle was assessed using the 4-day suppressive test against Plasmodium berghei infection in mice [12]. All the procedure was accepted by Shahid Beheshti University Ethics committee and in accordance with the principles for laboratory animal use and care in the European community guidelines. Since the advantage of intraperitoneal injection is to be very easy to perform in comparison with other routes of administration, this route was selected for the study. Adult male albino mice, weight 30 ± 3 g, were inoculated with P. berghei, and each mouse received 1 × 107 infected erythrocytes by intraperitoneal injection on the first day of the experiment. The mice were randomly divided into experimental test and control consisting of 5 mice per cage. Two control groups were used in this experiment: one was treated with chloroquine at dose of 20 mg/kg as a positive control while the other group was kept untreated given normal saline as placebo. The mice of test group were treated during consecutive day with 10 mg/kg of the sample by intraperitoneal injection for 4 days. On days 5, 8, and 15 of the test, thin blood smears were made from the tail blood of the mice. The blood films were fixed with methanol and stained with Giemsa and then assessed by microscope. Percentage of parasitaemia was counted based on infected erythrocytes calculated per 1000 erythrocytes.
3. Results
Myrtle extract was prepared from the aerial part of Myrtus communis and was first tested at 20 concentrations on the Plasmodium falciparum K1 and 3D7 strains using pLDH assay. The cytotoxic activity was performed against three cancer cell lines (MCF7, HepG2, and WEHI) and normal cell line (MDBK). The 4-day suppressive test against P. berghei was carried out. The results of the in vitro and in vivo antiplasmodial and cytotoxic activities of the Myrtus communis are shown in Table 1. Myrtle extract showed better antiplasmodial activity against sensitive 3D7 strain with no cytotoxic activity up to 100 μg/mL against the selected cell lines.
Table 1.
In vitro and in vivo antiplasmodial activity and cytotoxic effect on mammalian cell lines.
| Sample | In vitro antiplasmodial activity of Plasmodium falciparum IC50 (μg/mL) | Cytotoxicity IC50 (μg/mL) | In vivo antiplasmodial activity of Plasmodium berghei % inhibition | ||||
|---|---|---|---|---|---|---|---|
| K1 | 3D7 | MCF7 | HepG2 | WEHI | MDBK | ||
| Myrtus communis L. | 35.44 | 0.87 | >100 | >100 | >100 | >100 | 84.8 ± 1.1 (day 5) 63.1 ± 4.1 (day 15) |
| Chloroquine | 0.02 | 0.01 | 23.90 | 34.60 | 12.29 | >100 | 100 |
| Artemisinin | 0.001 | 0.004 | >100 | >100 | >100 | >100 | N.D |
| Tamoxifen | N.D. | N.D | 3.60 | 4.38 | 19.1 | 6.35 | N.D |
N.D.: not done.
Percentage of parasitaemia on days 5, 8, and 15 of the experiment in the test group in comparison with placebo group is shown in Figure 2. The average survival days of the test group were 18 ± 0.57 days. All the mice of placebo group died within two weeks of experiment.
Figure 2.
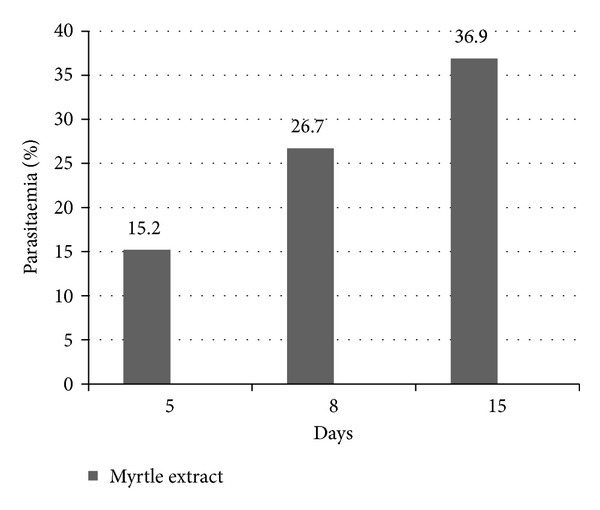
Percentage of parasitaemia in the test group (myrtle extract) on days 5, 8, and 15 in comparison with placebo.
4. Discussion
Our previous studies [13, 14] and this report lead us to carry out more surveys in Iranian folk medicine and traditional medicine which may help to detect new effective plants. The aim of this survey was the assessment of in vitro and in vivo antimalarial activities and in vitro cytotoxic effect of a plant traditionally used for treatment of parasitic infections.
Based on ethnobotanical data of some provinces of Iran that was carried out at TMRC [13, 14] and a study which revealed potential antiplasmodial activity of essential oil of Myrtus communis L. [15], myrtle was selected for this survey.
Myrtus communis L. is an aromatic and medicinal species from the Myrtaceae family. Myrtle is used in folk medicine of Iran for treatment of some diseases such as parasitic disorders and herpes [14, 16, 17].
Ideally, effective extracts at the blood stage of the malaria parasite should have strong in vitro and in vivo antimalarial activities and should be devoid of cytotoxicity at concentration up to 100 μg/mL [18]. Peters test is an appropriate method used to assessment in vivo antiplasmodial activity of plant extracts. Myrtle has strong in vitro antiplasmodial activity (IC50 = 35.44 and 0.87 μg/mL) with no cytotoxicity up to 100 μg/mL (Table 1). After four days of treatment the suppressive percentage of parasitaemia with 84.8% was obtained for the methanolic extract of myrtle. This result is interesting when it was compared with the results from other plant extracts reported in the following literatures works.
Antimalarial activity of different extract doses of Cocos nucifera was investigated in vivo against Plasmodium berghei (NK65) infections in mice. Chemosuppression effects of 44.71%, 56.86%, 79.61%, and 83.73% were, respectively, shown for the corresponding dose of extract (50, 100, 200, and 400 mg/kg) [19]. In another study ethanolic leaf extract of Verbena hastata was evaluated using chloroquine-sensitive P. berghei berghei infection in mice at various doses (200, 400, and 800 mg/kg) causing 64%, 70%, and 71% chemosuppression, respectively [20].
Chloroform extracts of Artemisia macrivera Linn. and A. maritime Linn. showed antimalarial activity in mice against P. berghei NK65 at dose of 100 mg/kg with average percentage parasitaemia 0.30 ± 0.04 and 0.40 ± 0.05, respectively, on day 5 of the test [21].
Methanolic extracts from 15 medicinal plants in Kenya were screened for their in vivo antimalarial activity in mice against a chloroquine-tolerant P. berghei NK65 at a dose of 500 mg/kg. The best percentage of suppression on day 4 was 59.3% for Toddalia asiatica [22]. Other workers have studied the in vivo antimalarial activity of the methanol extract of Annona senegalensis against P. berghei at the doses of 100, 200, 400, 600, and 800 mg/kg. Chemosuppression of parasitaemia was 57.1%, 59.3%, 76.3%, 89.8%, and 91.1%, respectively [23]. The extracts of Cassia occidentalis, Morinda morindoides, and Phyllanthus niruri were evaluated for their antimalarial activity in vivo against P. berghei ANKA in mice at dose of 200 mg/kg. The most active extract that was from Morinda morindoides reduced parasitaemia by 74% [24]. Extracts obtained from the leaf and stem of Quassia amara and Q. undulate were screened against P. berghei berghei in mice at doses of 100 and 200 mg/kg. The parasite density (%) was between 0.6 and 8.5 on day 5 [25].
For comparison among some plant extracts that were screened for their in vivo antiplasmodial activity using Peters method, myrtle was shown to have dramatic effects at a low dose (10 mg/kg) in day four. Despite the decreasing suppression of parasitaemia on days 8 and 15, still chemosuppression of myrtle extract with 63.1% was observed.
Myrtle extract has compounds such as monoterpenoids, flavonoides, triterpenoids, and phloroglucinol type compounds [26]. Phytochemical investigation and isolation of various compounds from Myrtus communis has led to the identification of some compounds like β-sitosterol, myricetin, myricitrin, myrtillin, chrysanthemin, oenin, delphidin-3-arabinoside, cyanidin-3-arabinoside, petunidin-3-glucoside, petunidin-3-arabinoside, peonidin-3-glucoside, malvidin-3-arabinoside, 3-methoxymyricetin-7-O-α-L-rhamnopyranoside, and myrtucommulones. Among these compounds myricitrin isolated from the aerial part of Euphorbia hirta exhibited antiplasmodial activity with IC50 value of 5.4 μg/mL against P. falciparum [27]. This compound isolated from the leaves of Licania octandra possessed antiplasmodial activity (IC50 = 17.37 μg/mL) [28]. Myricetin is another compound isolated from edible plants and showed antiplasmodial activity against P. falciparum 3D7 and 7G8. The IC50 values were 40 μg/mL and 76 μg/mL, respectively [29]. Furthermore, myricetin was found to have antimalarial activity when tested against P. falciparum K1 and NF54 with IC50 values of 12.9 μg/mL and 57.3 μg/mL [30]. Among the other myrtle compounds, β-sitosterol isolated from the leaves of Teclea trichocarpa displayed antiplasmodial activity with IC50 value of 8.20 μg/mL against P. falciparum K1 [31].
Although several classes of natural products are responsible for the antiplasmodial activity of many plant species used in traditional medicine for the treatment of malaria, the most important and diverse biopotency has been observed in alkaloids, quassinoids, and sesquiterpene lactone. Nonalkaloidal natural compounds from plants with antiplasmodial and antimalarial properties, belonging to the classes of terpenes, limonoids, flavonoides, chromone, xanthone, anthraquinone, and related compounds, were recently reviewed [32]. According to this, flavonoides and steroides from the myrtle might have antiplasmodial activity, and bioassay guided fractionation resulting in isolated active components of Myrtus communis is necessary.
5. Conclusion
To our knowledge, myrtle extract has not been previously studied for its antiplasmodial activity. Our evaluation of the plant against two strains of Plasmodium falciparum in vitro and P. berghei in vivo proved antimalarial activities with no cytotoxicity up to 100 μg/mL. The results suggest that the Iranian ethnic medicinal application of myrtle has a pharmacological basis. Phytochemical investigation and also understanding the mechanism of action would be the next step of this study.
Acknowledgments
This study received financial support partially by Grants 115, 117, and 123 from the Traditional Medicine and Materia Medica Research Center, Shahid Beheshti University of Medical Science. The authors wish also to thank Miss. Zahra Tavakoli for her assistance to organize the data. The authors acknowledge Ms. Atefeh Pirani for the collection and identification of the plant species and voucher specimen preparation.
References
- 1.Saxena S, Pant N, Jain DC, Bhakuni RS. Antimalarial agents from plant sources. Current Science. 2003;85(9):1314–1329. [Google Scholar]
- 2.WHO. The World Malaria Report from WHO. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3.Edrisian GH. Malaria in Iran: past and present situation. Iranian Journal of Parasitology. 2006;1:1–14. [Google Scholar]
- 4.Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, et al. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Tropica. 2012;121(2):85–92. doi: 10.1016/j.actatropica.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Koudouvo K, Karou DS, Kokou K, et al. An ethnobotanical study of antimalarial plants in Togo Maritime Region. Journal of Ethnopharmacology. 2011;134(1):183–190. doi: 10.1016/j.jep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Andrade-Neto VF, Brandão MGL, Stehmann JR, Oliveira LA, Krettli AU. Antimalarial activity of Cinchona-like plants used to treat fever and malaria in Brazil. Journal of Ethnopharmacology. 2003;87(2-3):253–256. doi: 10.1016/s0378-8741(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 7.Naghibi F, Mosaddegh M, Mohammadi Motamed S, Ghorbani A. Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iranian Journal of Pharmaceutical Research. 2005;2:63–79. [Google Scholar]
- 8.Sumbul S, Aftab Ahmad M, Asif M, Akhtar M. Myrtus communis Linn.—a review. Indian Journal of Natural Products and Resources. 2011;2(4):395–402. [Google Scholar]
- 9.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 10.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. American Journal of Tropical Medicine and Hygiene. 1993;48(2):205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 11.Chan K-L, Choo C-Y, Abdullah NR, Ismail Z. Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum. Journal of Ethnopharmacology. 2004;92(2-3):223–227. doi: 10.1016/j.jep.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Peters W, Portus JH, Robinson BL. The chemotherapy of rodent malaria, XXII. The value of drug resistant strains of P. berghei in screening for blood schizontocidal activity. Annals of Tropical Medicine and Parasitology. 1975;69(2):155–171. [PubMed] [Google Scholar]
- 13.Esmaeili S, Naghibi F, Mosaddegh M, Sahranavard S, Ghafari S, Abdullah NR. Screening of antiplasmodial properties among some traditionally used Iranian plants. Journal of Ethnopharmacology. 2009;121(3):400–404. doi: 10.1016/j.jep.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Mosaddegh M, Naghibi F, Moazzeni H, Pirani A, Esmaeili S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. Journal of Ethnopharmacology. 2012;141(1):80–95. doi: 10.1016/j.jep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Milhau G, Valentin A, Benoit F, et al. In vitro antimalarial activity of eight essential oils. Journal of Essential Oil Research. 1997;9(3):329–333. [Google Scholar]
- 16.Afshar Systani I. Iranian Traditional Medicine. 2nd edition. Tehran, Iran: Leila Publication; 1998. (in Persian) [Google Scholar]
- 17.Shams Ardakani MR, Mohebbi A, Shajari E. A Survey of Iranian Ethnomedicineedn. 1st edition. Tehran, Iran: Sahbaye danesh; 2011. (in Persian) [Google Scholar]
- 18.Willcox M, Bodeker G, Rasoanaivo P. Guidelines for the nonclinical evaluation of the efficacy of traditional antimalarials. In: Willcox M, Rasoanaivo P, Bodeker G, editors. Traditional Medicinal Plants and Malaria. Boca Raton, Fla, USA: CRC Press; 2004. [Google Scholar]
- 19.Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R. Evaluation of the use of Cocos nucifera as antimalarial remedy in Malaysian folk medicine. Journal of Ethnopharmacology. 2011;134(3):988–991. doi: 10.1016/j.jep.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Akoudor GC, Idris-Usman, Ngozi MA, et al. In vivo antimalarial activity of ethanolic leaf extract of Verbena hastata against Plasmodium berghei berghei in mice. Journal of Herbal Medicine and Toxicology. 2010;4(2):17–23. [Google Scholar]
- 21.Ene AC, Atawodi SE, Ameh DA, Kwanashie HO, Agomo PU. In vivo antiplasmodial effect of chloroform extracts of Artemisia maciverae Linn and Artemisia maritima Linn. African Journal of Biotechnology. 2009;8(23):6612–6616. [Google Scholar]
- 22.Muregi FW, Ishih A, Miyase T, et al. Antimalarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite, in mice. Journal of Ethnopharmacology. 2007;111(1):190–195. doi: 10.1016/j.jep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Ajaiyeoba E, Falade M, Ogbole O, Okpako L, Akinboye D. In vivo antimalarial and cytotoxic properties of Annona senegalensis extract. African Journal of Traditional, Complementary and Alternative Medicines. 2006;3(1):137–141. [Google Scholar]
- 24.Tona L, Mesia K, Ngimbi NP, et al. In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri . Annals of Tropical Medicine and Parasitology. 2001;95(1):47–57. [PubMed] [Google Scholar]
- 25.Ajaiyeoba EO, Abalogu UI, Krebs HC, Oduola AMJ. In vivo antimalarial activities of Quassia amara and Quassia undulata plant extracts in mice. Journal of Ethnopharmacology. 1999;67(3):321–325. doi: 10.1016/s0378-8741(99)00073-2. [DOI] [PubMed] [Google Scholar]
- 26.Atta-ur-rahman, Choudhary MI, Shaheen F, Ahmad M, Khan SN, Anjum S. New alpha-glucosidase inhibitors and antibacterial compounds from Myrtus communis L. Karachi(PK) Patent US 2008/0269510 A1, 2008.
- 27.Liu Y, Murakami N, Ji H, Abreu P, Zhang S. Antimalarial flavonol glycosides from Euphorbia hirta. Pharmaceutical Biology. 2007;45(4):278–281. [Google Scholar]
- 28.Pires AS. Investigation of antiplasmodial compounds from various plant extracts [M.S. thesis] University of Genève; 2009. [Google Scholar]
- 29.Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Research Notes. 2008;1, article 26 doi: 10.1186/1756-0500-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasdemir D, Lack G, Brun R, Rüedi P, Scapozza L, Perozzo R. Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. Journal of Medicinal Chemistry. 2006;49(11):3345–3353. doi: 10.1021/jm0600545. [DOI] [PubMed] [Google Scholar]
- 31.Mwangi ESK, Keriko JM, Machocho AK, et al. Antiprotozoal activity and cytotoxicity of metabolites from leaves of Teclea trichocarpa. Journal of Medicinal Plant Research. 2010;4(9):726–731. [Google Scholar]
- 32.Batista R, De Jesus Silva Júnior A, De Oliveira AB. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. non-alkaloidal natural products. Molecules. 2009;14(8):3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]


