Abstract
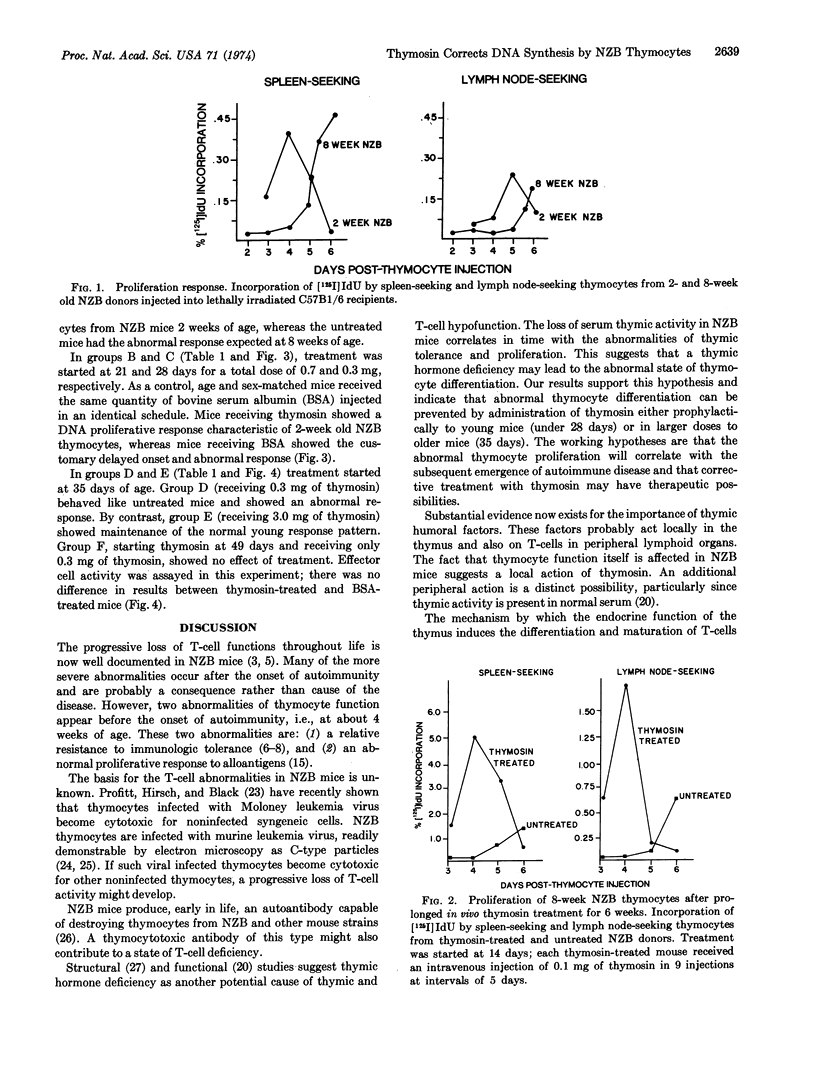
New Zealand Black (NZB) mice develop after 16 weeks of age an autoimmune and lymphoproliferative disease which is a model for systemic lupus erythematosus and lymphoid malignancy in humans. At this age, the mice manifest a progressive decline in T lymphocyte (thymus-derived lymphocyte) functions and serum thymosin levels. Thymocytes from 8-week old NZB mice exhibit an abnormal DNA synthetic response when transplanted into lethally irradiated C57B1/6 recipients. DNA synthesis (measured as the incorporation of radioactively labeled 5-iodo-2′-deoxyuridine) is delayed in onset and still increasing 6 days after cell transfer. By contrast, 2-week old NZB thymocytes show a normal response which is rapid in onset and completed by day 6.
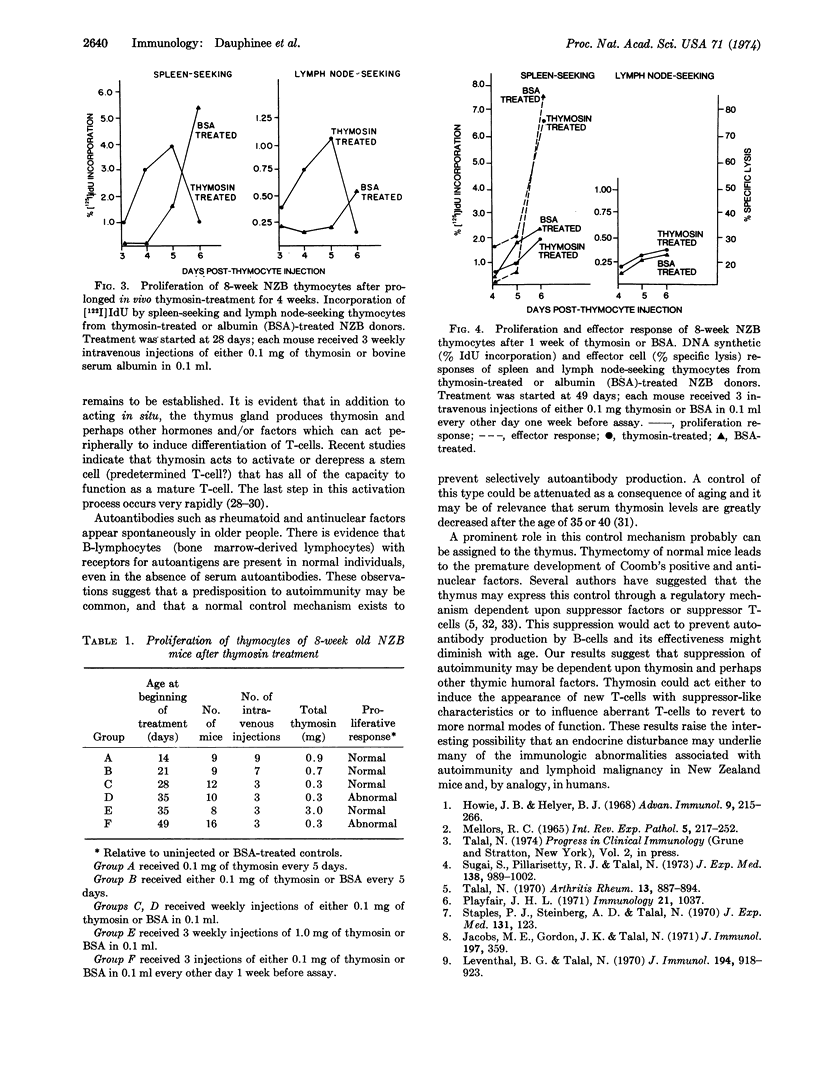
NZB mice were injected with thymosin fraction 5 or with bovine serum albumin starting at 2 weeks of age. Thymocytes from 8-week old thymosin-treated mice showed a normal DNA synthetic response, whereas the albumin-treated controls showed the abnormal response expected at this age. The ability of thymosin to correct the DNA synthetic response was related to dose and duration of treatment. These results suggest that thymosin can induce a more normal state of thymocyte differentiation in NZB mice. If abnormal thymocyte differentiation is related to the subsequent emergence of autoimmunity and lymphoid malignancy, then continuous treatment with thymosin may have therapeutic potential. These experiments suggest that an endocrine disturbance may contribute to autoimmune and lymphoproliferative disease in NZB mice and possibly in humans.
Keywords: autoimmune disease
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Salomon J. C. Studies on thymus products. IV. Absence of serum 'thymic activity' in adult NZB and (NZB x NZW) F1 mice. Clin Exp Immunol. 1973 Jun;14(2):247–256. [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Asofsky R., Talal N. Synergy among lymphoid cells mediating the graft-versus-host response. I. Synergy in graft-versus-host reactions produced by cells from NZB-Bl mice. J Exp Med. 1970 Feb;131(2):223–234. doi: 10.1084/jem.131.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee M. J., Talal N. Alteration in DNA synthetic response of thymocytes from NZB mice of different ages. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3769–3772. doi: 10.1073/pnas.70.12.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman A. M., Denman E. J. Depletion of long-lived lymphocytes in old New Zealand black mice. Clin Exp Immunol. 1970 Apr;6(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Howe M. L., White A. Ontogenesis of cell-mediated immunity in murine thymocytes and spleen cells and its acceleration by thymosin, a thymic hormone. J Immunol. 1971 Mar;106(3):773–780. [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A., Chused T. M., Steinberg A. D. Supressor cells in the graft vs host reaction. J Immunol. 1973 Aug;111(2):650–651. [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Jacobs M. E., Gordon J. K., Talal N. Inability of the NZB-NZW F 1 thymus to transfer cyclophosphamide-induced tolerance to sheep erythrocytes. J Immunol. 1971 Aug;107(2):359–364. [PubMed] [Google Scholar]
- Jacobs M. E., Gordon J. K., Talal N. Inability of the NZB-NZW F 1 thymus to transfer cyclophosphamide-induced tolerance to sheep erythrocytes. J Immunol. 1971 Aug;107(2):359–364. [PubMed] [Google Scholar]
- Leventhal B. G., Talal N. Response of NZB and NZB-NZW spleen cells to mitogenic agents. J Immunol. 1970 Apr;104(4):918–923. [PubMed] [Google Scholar]
- Mellors R. C. Autoimmune and immunoproliferative diseases of NZB/Bl mice and hybrids. Int Rev Exp Pathol. 1966;5:217–252. [PubMed] [Google Scholar]
- Mellors R. C., Huang C. Y. Immunopathology of NZB/BL mice. V. Viruslike (filtrable) agent separable from lymphoma cells and identifiable by electron microscopy. J Exp Med. 1966 Dec 1;124(6):1031–1038. doi: 10.1084/jem.124.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune responses of mice. 3. A raised tolerance threshold in NZB thymus cells. Immunology. 1971 Dec;21(6):1037–1043. [PMC free article] [PubMed] [Google Scholar]
- Proffitt M. R., Hirsch M. S., Black P. H. Murine leukemia: a virus-induced autoimmune disease? Science. 1973 Nov 23;182(4114):821–823. doi: 10.1126/science.182.4114.821. [DOI] [PubMed] [Google Scholar]
- Prosser P. R. Particles resembling murine leukaemia virus in New Zealand black mice. Clin Exp Immunol. 1968 Mar;3(3):213–226. [PMC free article] [PubMed] [Google Scholar]
- Rodey G. E., Good R. A., Yunis E. J. Progressive loss in vitro of cellular immunity with ageing in strains of mice susceptible to autoimmune disease. Clin Exp Immunol. 1971 Sep;9(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Deficient immunologic functions of NZB mice. Proc Soc Exp Biol Med. 1968 Apr;127(4):1204–1207. doi: 10.3181/00379727-127-32910. [DOI] [PubMed] [Google Scholar]
- Sugai S., Pillarisetty R., Talal N. Monoclonal macroglobulinemia in NZB-NZW F1 mice. J Exp Med. 1973 Oct 1;138(4):989–1002. doi: 10.1084/jem.138.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N. Immunologic and viral factors in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1970 Nov-Dec;13(6):887–894. doi: 10.1002/art.1780130620. [DOI] [PubMed] [Google Scholar]
- Zatz M. M., Mellors R. C., Lance E. M. Changes in lymphoid populations of ageing CBA and NZB mice. Clin Exp Immunol. 1971 Mar;8(3):491–500. [PMC free article] [PubMed] [Google Scholar]
- de Vries M. J., Hijmans W. Pathological changes of thymic epithelial cells and autoimmune disease in NZB, NZW and (NZB x NZW)F1 mice. Immunology. 1967 Feb;12(2):179–196. [PMC free article] [PubMed] [Google Scholar]


