Abstract
Objective:
To determine the associations between classes of antihypertensive medication use and the risk of cognitive impairment among elderly hypertensive men.
Methods:
The Honolulu-Asia Aging Study is a prospective, community-based cohort study of Japanese American men conducted in Honolulu, Hawaii. We examined 2,197 participants (mean age 77 years at cohort entry, 1991–1993, followed through September 2010) with hypertension and without dementia or cognitive impairment at baseline, who provided information on medication use. Cognitive function was assessed at 7 standardized examinations using the Cognitive Abilities Screening Instrument (CASI). Cognitive impairment was defined as a CASI score <74.
Results:
A total of 854 men developed cognitive impairment (median follow-up, 5.8 years). β-Blocker use as the sole antihypertensive drug at baseline was consistently associated with a lower risk of cognitive impairment (incidence rate ratio [IRR] 0.69; 95% confidence interval [CI] 0.50–0.94), as compared with men not taking any antihypertensive medications, adjusting for multiple potential confounders. The use of diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, or vasodilators alone was not significantly associated with cognitive impairment. Results were similar excluding those with cardiovascular disease or <1 year of follow-up, and additionally adjusting for pulse pressure, heart rate, baseline and midlife systolic blood pressure, and midlife antihypertensive treatment (IRR 0.65; 95% CI 0.45–0.94). The association between β-blocker use and cognitive impairment was stronger among men with diabetes, men aged >75 years, and those with pulse pressure ≥70 mm Hg.
Conclusions:
β-blocker use is associated with a lower risk of developing cognitive impairment in elderly Japanese American men.
Current treatments for dementia provide limited clinical benefit,1 highlighting the need for preventive approaches to stem the epidemic estimated to affect more than 115 million people worldwide by 2050.2 Hypertension, particularly elevated systolic blood pressure (SBP), has been associated with the development of cognitive impairment and dementia in late life,3–8 as well as the neuropathologic lesions of dementia at autopsy.6,9,10 Previous analyses from the Honolulu-Asia Aging Study (HAAS) have estimated that 27% of dementia cases may be attributed to midlife SBP ≥120 mm Hg among inadequately treated men.11
The effects of antihypertensive drug class in late life to prevent cognitive impairment, however, remain unclear. Data from mice have demonstrated neuroprotective effects with carvedilol and propranolol.12–14 While some clinical trials have shown a beneficial effect of antihypertensive treatment in reducing the risk of dementia and cognitive decline in the elderly,15 others have not.16 These trials, however, included diuretics, calcium channel blockers, and angiotensin-converting enzyme (ACE) inhibitors, and did not evaluate the effects of β-blockers (BB). Limited data from observational studies suggest that BB use may slow cognitive decline17,18 and possibly prevent dementia,19 while others have found divergent results.20
The current study examines the association between antihypertensive drug class and the risk of cognitive impairment in a large prospective cohort of elderly Japanese American men.
METHODS
Study population.
The HAAS is a prospective cohort of 3,374 Japanese American men followed since 1965 as part of the Honolulu Heart Program (HHP). Study details have been described previously.21,22 The HHP included 8,006 men of Japanese ancestry born 1900–1919 who were residing on Oahu, Hawaii, at the time of the first examination (1965–1968). Two additional HHP examinations occurred through 1975. The HAAS was established in 1991 as a continuation of the HHP to study aging-related conditions, with a focus on brain diseases (figure 1; HHP examination 4 corresponds to the baseline HAAS examination). Subsequent HAAS examination cycles occurred every 2–3 years after the baseline, with the last examination completed in 2010. Information was collected through standardized cognitive, physical, and laboratory evaluations, and structured interviews on participant characteristics, including demographics, medical history, education, and lifestyle.
Figure 1. Design of the HHP/HAAS cohorts and derivation of the analytic sample.
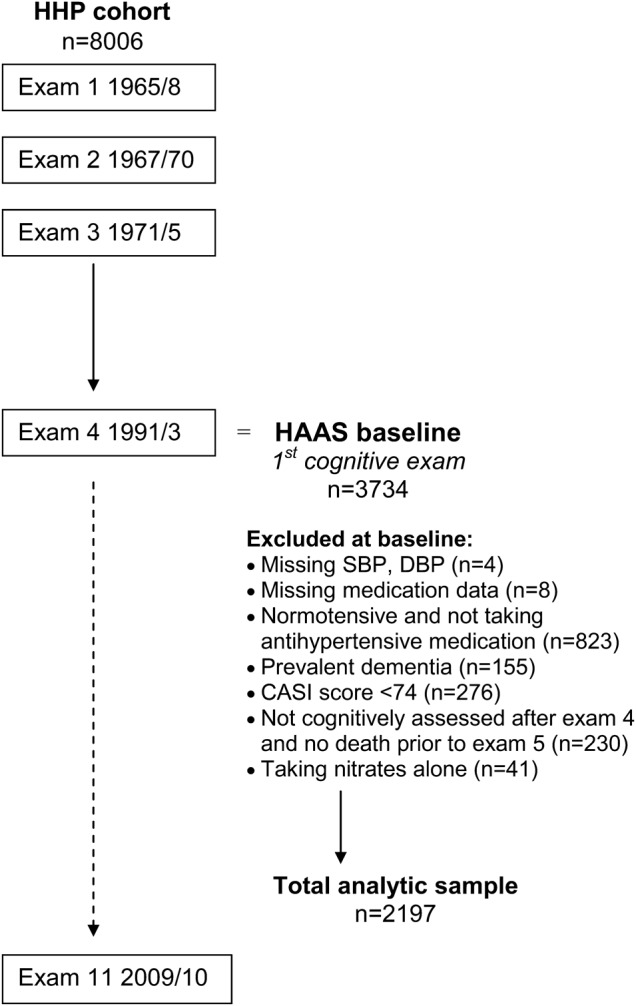
CASI = Cognitive Abilities Screening Instrument; DBP = diastolic blood pressure; HAAS = Honolulu-Asia Aging Study; HHP = Honolulu Heart Program; SBP = systolic blood pressure.
At the HAAS baseline (examination 4), 3,734 men were evaluated with the Cognitive Abilities Screening Instrument (CASI). Participating men were aged 71–93 years and represented ∼80% of the surviving cohort.23 Our analyses exclude men for whom baseline data on diastolic blood pressure (DBP) and SBP (n = 4) or medication use (n = 8) were missing. For men not taking antihypertensive medication, we excluded those who at baseline were normotensive (SBP <140 mm Hg and DBP <90 mm Hg; n = 823), since the purpose of this analysis was to compare outcomes among hypertensive participants. Also excluded were those identified at baseline as having dementia (n = 155), those with baseline CASI scores <74 (n = 276), and those without death information prior to the second examination who were also missing all follow-up cognitive evaluations (n = 230). We additionally excluded 41 men taking nitrates and no other antihypertensive medication. Men using nitrates alone were more likely to have cardiovascular disease (CVD) than others (75.6% vs 22.9%, p < 0.001) and had lower baseline blood pressure (mean SBP, 140 vs 156 mm Hg, p < 0.001; mean DBP, 76 vs 82 mm Hg, p < 0.001). The following analyses included the remaining 2,197 men.
Standard protocol approvals, registrations, and patient consents.
The Kuakini Medical Center institutional review board reviewed and approved this study. All participants or their representatives provided written informed consent.
Exposures.
Antihypertensive medication use was determined by evaluation of medications brought to the HAAS baseline visit (examination 4), supplemented by self-report and telephone follow-up when appropriate. Medications were classified according to their mechanism of action and individual or combined use: BB, ACE inhibitors, diuretics, calcium channel blockers, vasodilators (α-blockers, hydralazine, clonidine, etc.), BB with any other antihypertensive drug, and all other antihypertensive combinations. The reference group comprised hypertensive men not taking any antihypertensive medication. We used 3 drug categories when sample sizes were limited in subgroup analyses: any BB (with or without other drugs), all other antihypertensive medications, and no drug use (reference category). Medication use was also self-reported at examination 6 (1997–1999). For these analyses, categories examined were: BB used at both the fourth and sixth examinations (with or without other antihypertensive medications), any other antihypertensive medication use at both examinations, and no drug use at both examinations.
Covariates.
Covariates assessed at the HAAS baseline included age (years, continuous), SBP (mm Hg, continuous; the mean of 2 seated readings), pulse pressure (PP, mm Hg, continuous [SBP-DBP]), heart rate (HR, beats/minute, continuous; from the ventricular rate on ECG), duration of any antihypertensive medication use (</≥5 years), body mass index (BMI, kg/m2, continuous), cigarette smoking (never, past, current), alcohol consumption (0, >0–2, >2 drinks/day), physical activity index (tertile),24 diabetes (serum glucose ≥126 mg/dL fasting or ≥200 mg/dL 2 hours following a 75 g oral glucose load, or the use of insulin or oral medications for diabetes), CVD history (stroke, angina, myocardial infarction, or other coronary heart disease), and baseline CASI score (continuous). CVD history was assessed through participant interview, electrocardiography, and medical record review. Midlife SBP was estimated as the mean from the first 3 HHP examinations (<120, 120–139, ≥140 mm Hg).3 Use of any antihypertensive medications in midlife, without respect to class, was determined at the first 3 HHP examinations. Education history (years of completed schooling, continuous) and childhood years spent in Japan (</≥5 years) were obtained at the HHP baseline. Genotyping for APOE ε4 (1 or 2 alleles vs none) was performed at Duke University, Durham, North Carolina, using conventional methods.25
Outcomes.
Cognitive assessments began in 1991–1993 using a standardized protocol described previously.21,23 Cognitive function was tested using the 100-point CASI, a combination of the Hasegawa Dementia Screening Scale, the Folstein Mini-Mental State Examination, and the Modified Mini-Mental State Examination.26 The CASI was used to identify a subgroup for further dementia evaluation.21,23 Dementia is not included as an outcome for our analyses as some men with cognitive impairment declined full dementia evaluations.
Cognitive impairment was defined as a CASI score <74 (16th percentile of all baseline scores), independent of dementia classification, using the last available score. In prior HAAS analyses, a CASI score <74 had ∼80% sensitivity and 90% specificity for identifying dementia.27 Cognitive decline was defined as a decrease in CASI score of ≥9 points, using the difference between the first and last available scores (9 points representing ∼1 SD unit of decline in scores between the first 2 cognitive examinations for all men).
Statistical analyses.
We compared participant characteristics according to medication categories using analysis of variance for continuous variables and χ2 tests for categorical variables. We used Cox proportional hazards models to estimate incidence rate ratios (IRR) and 95% confidence intervals (CI) for cognitive impairment and decline according to medication categories. Person-years of follow-up were calculated as the time from the baseline HAAS examination to the development of the outcome, censoring, or end of follow-up (September 23, 2010), whichever occurred first. Available CASI scores were used to calculate the average change in score per year of follow-up. Average yearly change in score was summed across years of follow-up to define the date at which an outcome was met.
Three multivariable-adjusted models were considered. Model 1 adjusted for age, education, APOE ε4 status, childhood years in Japan, and baseline CASI score. Model 2 adjusted for the variables in model 1 plus BMI, smoking, alcohol consumption, physical activity, diabetes, and CVD. Model 3 adjusted for the variables in model 2 plus HAAS baseline PP and HR. Additional analyses adjusted for baseline SBP, midlife SBP,3 and midlife antihypertensive medication use.
Secondary analyses were performed to explore alternative explanations for the observed associations. We examined men without prior CVD to minimize potential confounding by indication for medication use. We used information from examinations 4 and 6 to evaluate consistency of BB use over ∼6 years. Separate models included joint terms between medication categories and diabetes, baseline PP (</≥70 mm Hg), SBP (</≥150 mm Hg), or age (≤/>75 years). The likelihood ratio test (LRT) was used to assess effect modification, contrasting age-adjusted models with and without interaction terms. Other analyses stratified men by duration of any antihypertensive medication use prior to baseline (</≥5 years). We excluded men with <1 year of follow-up to minimize bias related to early censoring. Two-sided p values are reported; p < 0.05 was considered statistically significant. Analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
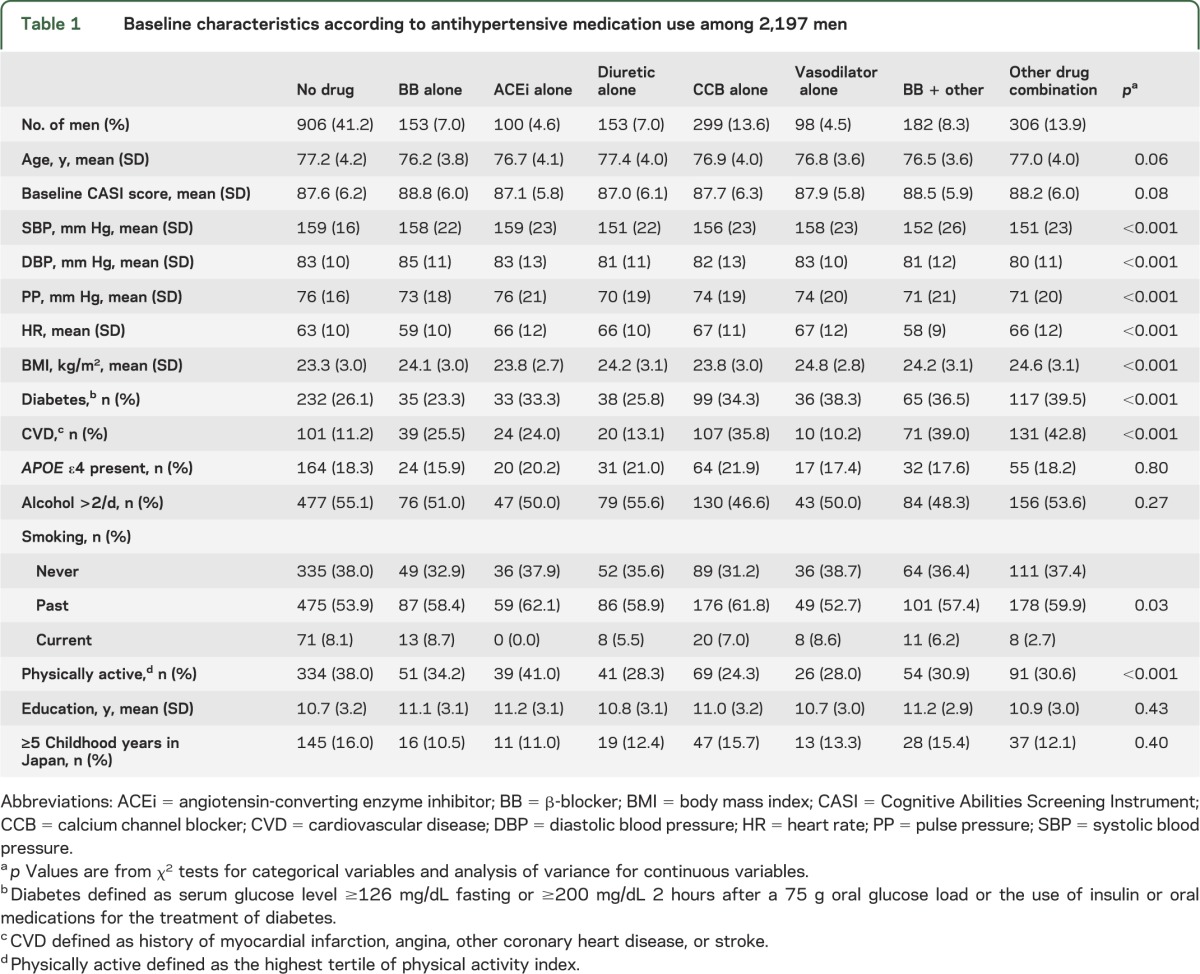
Baseline participant characteristics are shown according to categories of antihypertensive medication use in table 1. Among 2,197 men (mean age 77 years; range 71–93 years), 41.2% were hypertensive but did not report taking antihypertensive medications. Mean baseline CASI score was 87.8 (SD 6.1). Men taking BBs had a higher DBP and lower HR, and were least likely to have diabetes. Men using antihypertensive combinations and diuretics alone had the lowest SBP and DBP. Those taking non-BB combinations were most likely to have diabetes and CVD.
Table 1.
Baseline characteristics according to antihypertensive medication use among 2,197 men
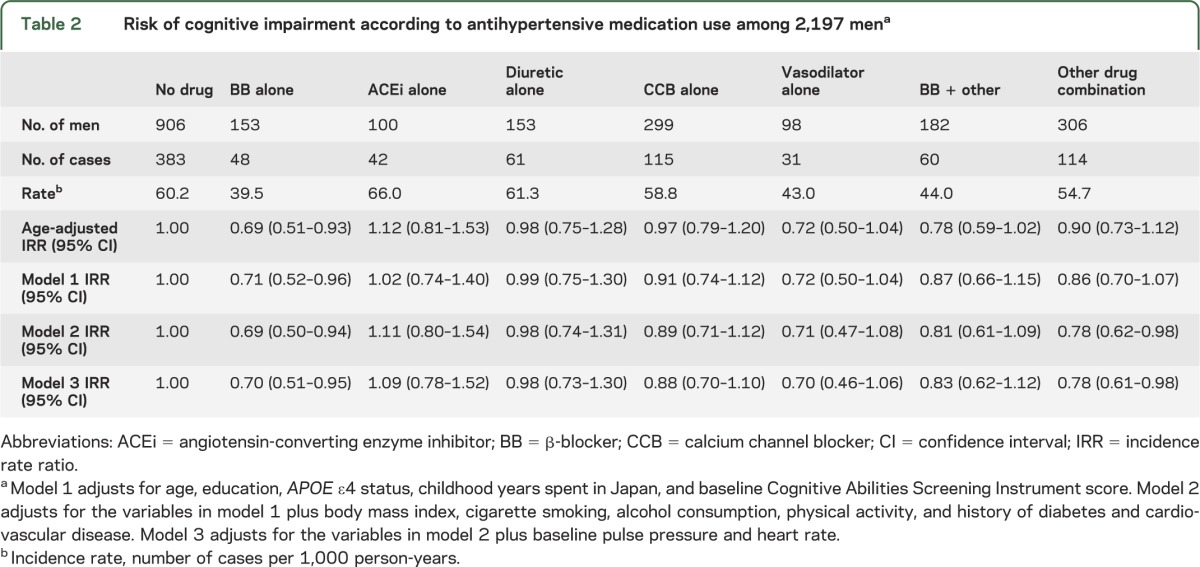
During a median follow-up of 5.8 years (SD 5.1), 854 men (38.9%) developed cognitive impairment (CASI score <74). The use of a BB as the sole antihypertensive medication was associated with a lower risk of cognitive impairment, as compared with untreated men (model 2 IRR 0.69; 95% CI 0.50–0.94; table 2). Non-BB drug combinations were also associated with a reduced risk (model 2 IRR 0.78; 95% CI 0.62–0.98). Diuretics, ACE inhibitors, calcium channel blockers, vasodilators, and drug combinations including a BB were not statistically significantly associated with the development of cognitive impairment. Additionally adjusting for baseline PP, HR, SBP, midlife SBP, and midlife antihypertensive medication use did not materially alter the findings (IRR for BB use alone 0.66; 95% CI 0.48–0.90), nor did further exclusion of those with <1 year of follow-up (IRR 0.70; 95% CI 0.51–0.98). There were no significant associations for all other drug categories when excluding men with <1 year of follow-up.
Table 2.
Risk of cognitive impairment according to antihypertensive medication use among 2,197 mena
Cognitive decline (≥9-point decrease in CASI score) occurred in 1,167 men (53.1%). BB use was also associated with a trend toward a decreased risk of cognitive decline: model 2 IRR 0.78 (95% CI 0.61–1.00) for BB use alone; 0.81 (95% CI 0.64–1.03) for BB in combination with other drugs. None of the other drug categories was significantly associated with cognitive decline (data not shown).
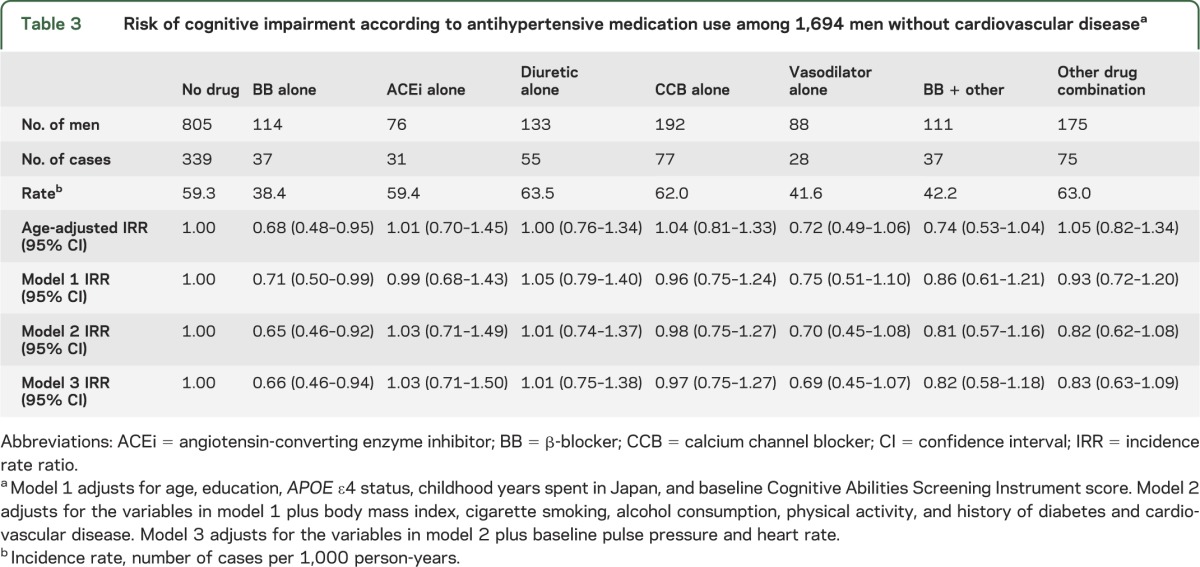
Limiting analyses to men without prior CVD at baseline, BB use remained strongly associated with a reduced risk of cognitive impairment at follow-up, as compared with no antihypertensive medication use (model 2 IRR 0.65; 95% CI 0.46–0.92; table 3). Other drug categories were not significantly associated with cognitive impairment. Further adjustment for PP, HR, SBP, midlife SBP, and midlife antihypertensive medication use, and excluding those with <1 year of follow-up, yielded similar results (IRR 0.65; 95% CI 0.45–0.94).
Table 3.
Risk of cognitive impairment according to antihypertensive medication use among 1,694 men without cardiovascular diseasea
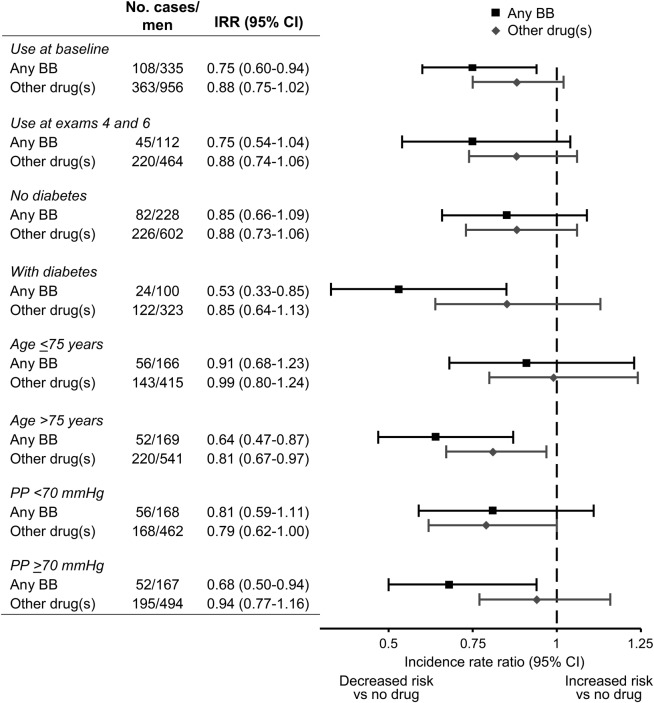
The results of secondary analyses are shown in figure 2, comparing any BB use (alone or in combination with other antihypertensive medications), other drugs, and no antihypertensive drug use (reference group). Any BB use at baseline was associated with a lower risk of cognitive impairment (IRR 0.75; 95% CI 0.60–0.94) as compared with untreated men. The IRR for all other antihypertensive drug use was 0.88 (95% CI 0.75–1.02). Results were similar limiting analyses to men with medication use reported at both examinations 4 and 6. Any BB use appeared particularly associated with a lower risk of cognitive impairment among men with diabetes at baseline, although there was no evidence for statistical effect modification (LRT, p = 0.25). There also were stronger associations between BB use and a reduced risk of cognitive impairment among men aged >75 years at baseline (LRT, p = 0.04) and those with baseline PP ≥70 mm Hg (LRT, p = 0.64). Among men with baseline SBP <150 mm Hg, the IRR for cognitive impairment was 0.69 (95% CI 0.49–0.99) for any BB use and 0.82 (95% CI 0.64–1.07) for other antihypertensive medication use, as compared with no drug use. Among men with baseline SBP ≥150 mm Hg, the IRRs (95% CI) were 0.80 (0.60–1.07) and 0.91 (0.75–1.10), respectively (LRT, p = 0.62). Limiting analyses to men with ≥5 years of any antihypertensive medication use prior to the baseline examination, the IRR for any BB use was 0.73 (95% CI 0.55–0.98; model 2), as compared with other antihypertensive medications (data not shown).
Figure 2. Sensitivity analyses of the risk of cognitive impairment according to antihypertensive medication use.
Reference group: men without antihypertensive medication use. Cognitive impairment defined as Cognitive Abilities Screening Instrument (CASI) score <74. Model adjusts for age, education, APOE ε4 status, childhood years in Japan, baseline CASI score, body mass index, smoking, alcohol consumption, physical activity, diabetes, and cardiovascular disease. BB = β-blocker; CI = confidence interval; IRR = incidence rate ratio; PP = pulse pressure.
DISCUSSION
In this large prospective cohort, BB use was consistently associated with a lower risk of developing cognitive impairment. The associations remained statistically significant after adjustment for multiple potential confounders and did not appear to be mediated by baseline HR, PP, or SBP. Furthermore, BB use remained protective against cognitive impairment when limiting analyses to men without a history of CVD, suggesting that confounding due to CVD as an indication for BB treatment did not alter the aggregate findings. Risk of cognitive impairment with BB use was particularly reduced among men with diabetes at baseline, older men (>75 years; with statistical effect modification), and those with a higher baseline PP (≥70 mm Hg). These groups may be more vulnerable to the hypertensive microvascular injury against which BB treatment may protect. Among men with higher baseline SBP (≥150 mm Hg), BB use was not significantly associated with a lower risk of cognitive impairment, suggesting that medication class may be less relevant if the SBP is not adequately controlled.
Previous studies provide limited and conflicting information regarding the effects of antihypertensive medication use on cognition. Any antihypertensive drug use, and particularly diuretic use, was associated with a lower risk of Alzheimer disease in a prospective study, although follow-up was 3 years and included 104 cases.28 BB use was associated with delay of functional decline in a small study of persons with Alzheimer disease.18 In a retrospective analysis, lower rates of cognitive decline were observed with diuretic, ACE inhibitor, and BB use in the elderly, and improved cognition was seen with angiotensin receptor blockers (ARB).17 Another retrospective, case-control study showed decreased odds of dementia with BB use during the preceding 3 years.19 However, a small, 6-year follow-up study suggested worse cognitive decline among those with BB and ACE inhibitor use, and improved cognition with calcium channel blocker use in the elderly.20 ARB treatment has also been linked to a reduced risk of Alzheimer disease among persons with a history of CVD, as compared with other cardiovascular drugs; however, this analysis used administrative data without validated outcome assessment and did not assess BB use specifically.29 In an autopsy series of hypertensive subjects, ARB use was associated with less Alzheimer-related pathology but more strokes, as compared with other antihypertensive and no drug treatment, although BB use alone was not evaluated.30
Clinical trial data are similarly limited. In a small trial of 31 participants, ARB treatment was associated with greatest improvement in executive function, comparing lisinopril, candesartan, and hydrochlorothiazide; however, BBs were not studied, and follow-up was 12 months.31 Larger trials of diuretics, calcium channel blockers, and ACE inhibitors, which also did not evaluate BBs, have provided conflicting results regarding cognitive decline during 2-year follow-up.15,16
Potential mechanisms for a protective effect of BB on cognition remain unclear. In mouse models of Alzheimer disease, carvedilol appeared to reestablish basal synaptic transmission, enhance neuronal plasticity, suppress neuronal hyperexcitability,12 and attenuate brain β-amyloid content and cognitive deterioration.13 Another study found improved cognitive measures and fewer brain amyloid and tau pathologies with propranolol treatment in a senescence-accelerated mouse model.14 In prior HAAS analyses, lower plasma β-amyloid was associated with an increased risk of Alzheimer disease and cerebral amyloid angiopathy at autopsy, particularly among those with elevated midlife blood pressure, suggesting that hypertension may lead to microvascular injury, promoting amyloid angiopathy and impaired β-amyloid clearance from the brain.32 It has also been suggested that, among the elderly, aortic stiffness may increase pulsatile energy in the cerebral vasculature, with subsequent microvascular damage and thereby worsening of cognitive function.33 We hypothesize that, among individuals with hypertension, BB use may provide neuroprotection through alterations in cerebral blood perfusion, improvement of microvascular integrity, and possibly reduction of subsequent neuropathology, including cerebral angiopathy, amyloid deposition, widening of periarteriolar spaces, microinfarcts, and atrophy. Adjustment for PP, HR, and SBP did not attenuate the association between BB use and cognitive impairment in our analyses, perhaps because pretreatment measurements were not available to evaluate changes in these parameters with treatment. Alternatively, BB use may provide cognitive protection through mechanisms other than those mediated by these hemodynamic measures.
Strengths of our study include its prospective design, large sample size, duration of follow-up, information on multiple covariates assessed with standardized methods in mid and late life, evaluation of multiple drug categories, and validated outcome assessment. These strengths support the validity of our study and distinguish it from the limitations of prior studies. Certain limitations should be considered in the interpretation of our findings. The study sample was restricted in participant race and sex, possibly limiting the generalization of results, although the demographics also restrict potential confounding by these factors. Secondly, we did not have complete information on duration of specific medication use or adherence. Although BB use at both examinations 4 and 6 appeared to confer a lower risk of cognitive impairment, the time of medication initiation was not available, so that changes in blood pressure with treatment could not be examined. However, previous analyses from the HAAS have shown that lowering midlife SBP can prevent late-life dementia.11 Third, these analyses did not account for the competing risk of death prior to the outcomes of interest. We did not, however, find substantial differences in the results after excluding men with <1 year of follow-up. Fourth, ARB use, which was introduced after the baseline cognitive examination, was not studied. Finally, we did not evaluate receptor selectivity of BB medications, and therefore were not able to determine whether results were modified by mechanism of BB subtype.
We found that BB use was consistently associated with a lower risk of developing cognitive impairment among elderly Japanese American men, with stronger results among those with diabetes, elderly men, and those with higher PP at baseline. To our knowledge, this is the largest prospective study to demonstrate a protective effect of BB use on cognitive function. Our findings warrant further study, including confirmation or refutation in other populations and evaluation of neuropathologic outcomes.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants of the Honolulu-Asia Aging Study for their commitment and cooperation and the Honolulu-Asia Aging Study staff for their assistance.
GLOSSARY
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- BB
β-blockers
- BMI
body mass index
- CASI
Cognitive Abilities Screening Instrument
- CI
confidence interval
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HAAS
Honolulu-Asia Aging Study
- HHP
Honolulu Heart Program
- HR
heart rate
- IRR
incidence rate ratio
- LRT
likelihood ratio test
- PP
pulse pressure
- SBP
systolic blood pressure
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Gelber drafted the manuscript and contributed to the study concept, study design, and the analysis and interpretation of data, and conducted the statistical analysis. Drs. Ross, Petrovitch, Masaki, Launer, and White revised the manuscript for content and contributed to the analysis and interpretation of data. Dr. White also contributed to the study concept and design.
STUDY FUNDING
Supported by a contract (N01-AG-4-2149) and grant (UO1 AG017155, UO1 AG019349) from the National Institute on Aging, the Intramural Research Program, NIA, a contract (N01-HC-05102) from the National Heart, Lung, and Blood Institute, and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. The information contained in this article does not necessarily reflect the position or the policy of the United States Government, and no official endorsement should be inferred.
DISCLOSURE
R. Gelber reports no disclosures. G. Ross receives support from the NIH. H. Petrovitch receives support from the NIH. K. Masaki receives support from the NIH. L. Launer receives support from the NIH. L. White receives support from the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008;148:379–397 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Dementia: A Public Health Priority. Geneva: World Health Organization; 2012 [Google Scholar]
- 3.Freitag MH, Peila R, Masaki K, et al. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke 2006;37:33–37 [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA 1995;274:1846–1851 [PubMed] [Google Scholar]
- 5.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:49–55 [DOI] [PubMed] [Google Scholar]
- 6.White WB, Wolfson L, Wakefield DB, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011;124:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Rhew IC, Shofer JB, et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc 2007;55:1161–1167 [DOI] [PubMed] [Google Scholar]
- 8.Oveisgharan S, Hachinski V. Hypertension, executive dysfunction, and progression to dementia: the Canadian Study of Health and Aging. Arch Neurol 2010;67:187–192 [DOI] [PubMed] [Google Scholar]
- 9.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS: Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:57–62 [DOI] [PubMed] [Google Scholar]
- 10.Wang LY, Larson EB, Sonnen JA, et al. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J Am Geriatr Soc 2009;57:1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launer LJ, Hughes T, Yu B, et al. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: perspective from the Honolulu Heart Program/Honolulu-Asia Aging Study. Hypertension 2010;55:1352–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrieta-Cruz I, Wang J, Pavlides C, Pasinetti GM. Carvedilol reestablishes long-term potentiation in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2010;21:649–654 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Ono K, Dickstein DL, et al. Carvedilol as a potential novel agent for the treatment of Alzheimer’s disease. Neurobiol Aging 2011;32:2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobarro M, Orejana L, Aguirre N, Ramirez MJ. Propranolol restores cognitive deficits and improves amyloid and tau pathologies in a senescence-accelerated mouse model. Neuropharmacology 2013;64:137–144 [DOI] [PubMed] [Google Scholar]
- 15.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998;352:1347–1351 [DOI] [PubMed] [Google Scholar]
- 16.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008;7:683–689 [DOI] [PubMed] [Google Scholar]
- 17.Hajjar I, Catoe H, Sixta S, et al. Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J Gerontol A Biol Sci Med Sci 2005;60:67–73 [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg PB, Mielke MM, Tschanz J, et al. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry 2008;16:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner G, Icks A, Abholz HH, Schroder-Bernhardi D, Rathmann W, Kostev K. Antihypertensive treatment and risk of dementia: a retrospective database study. Int J Clin Pharmacol Ther 2012;50:195–201 [DOI] [PubMed] [Google Scholar]
- 20.Paran E, Anson O, Lowenthal DT. Cognitive function and antihypertensive treatment in the elderly: a 6-year follow-up study. Am J Ther 2010;17:358–364 [DOI] [PubMed] [Google Scholar]
- 21.Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res 2012;9:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol 1975;102:477–480 [DOI] [PubMed] [Google Scholar]
- 23.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia Aging Study. JAMA 1996;276:955–960 [PubMed] [Google Scholar]
- 24.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci 2008;63:529–535 [DOI] [PubMed] [Google Scholar]
- 25.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990;31:545–548 [PubMed] [Google Scholar]
- 26.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994;6:45–58; discussion 62 [DOI] [PubMed] [Google Scholar]
- 27.Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000;54:1265–1272 [DOI] [PubMed] [Google Scholar]
- 28.Khachaturian AS, Zandi PP, Lyketsos CG, et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol 2006;63:686–692 [DOI] [PubMed] [Google Scholar]
- 29.Li NC, Lee A, Whitmer RA, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 2010;340:b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajjar I, Brown L, Mack WJ, Chui H. Impact of angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol 2012:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajjar I, Hart M, Chen YL, et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double-blind randomized clinical trial. Arch Intern Med 2012;172:442–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu-Asia Aging Study. Hypertension 2012;59:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 2011;134:3398–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.