Abstract
Objective:
To investigate the relationship between neurovascular coupling and cognitive function in elderly individuals with vascular risk factors and to determine whether neurovascular coupling could be modified by cocoa consumption.
Methods:
Sixty older people (aged 72.9 ± 5.4 years) were studied in a parallel-arm, double-blind clinical trial of neurovascular coupling and cognition in response to 24 hours and 30 days of cocoa consumption. Cognitive measures included Mini-Mental State Examination and Trail Making Test A and B. Neurovascular coupling was measured from the beat-to-beat blood flow velocity responses in the middle cerebral arteries to the N-Back Task. In a subset of MRI-eligible participants, cerebral white matter structural integrity was also measured.
Results:
Neurovascular coupling was associated with Trails B scores (p = 0.002) and performance on the 2-Back Task. Higher neurovascular coupling was also associated with significantly higher fractional anisotropy in cerebral white matter hyperintensities (p = 0.02). Finally, 30 days of cocoa consumption was associated with increased neurovascular coupling (5.6% ± 7.2% vs −2.4% ± 4.8%; p = 0.001) and improved Trails B times (116 ± 78 seconds vs 167 ± 110 seconds; p = 0.007) in those with impaired neurovascular coupling at baseline.
Conclusion:
There is a strong correlation between neurovascular coupling and cognitive function, and both can be improved by regular cocoa consumption in individuals with baseline impairments. Better neurovascular coupling is also associated with greater white matter structural integrity.
Neuronal activity is linked to oxygen and glucose delivery. An increase in metabolic demand leads to an increase in blood flow. The close functional and spatial relationship between neuronal activity and cerebral blood flow has been termed neurovascular coupling (NVC) or functional hyperemia.1 Impaired NVC has been associated with significant pathology.2
The relationship between NVC and cognition remains unknown, as does the relationship between NVC and structural brain changes that occur with aging and vascular disease. We have shown that intact NVC was associated with preserved gait speed despite the presence of a high burden of cerebral microvascular disease, known to be associated with slowing of gait.3 Given the established role of NVC in a number of animal models of human disease,4 a better understanding of the clinical and brain structural correlates of this physiologic process is needed.
This study was designed to advance our understanding of NVC in older people with vascular disease. Our first goal was to determine whether lower cognitive performance is associated with lower blood flow responses to a cognitive task, i.e., “impaired NVC.” The second goal was to examine whether impaired NVC is associated with cerebral white matter disease. Finally, we wanted to determine whether NVC was modifiable by flavanol-rich cocoa, which has been shown to improve endothelial5,6 and cognitive function.7
METHODS
Protocol.
Sixty people (older than 65 years, mean 72.9 ± 5.4 years) volunteered to participate in this parallel arm, double-blind clinical trial of the cerebral blood flow and NVC responses to cocoa. Subjects were recruited from local advertisements and were screened with a medical history and physical examination. Subjects had hypertension (systolic blood pressure [SBP] >140 mm Hg or diastolic blood pressure [DBP] >90 mm Hg on repeated occasions, or treatment with antihypertensive medication) and/or well-controlled diabetes mellitus type 2.
Subjects were excluded for absent temporal acoustic windows, intracranial stenosis, history of stroke, chest pain or heart attack in the last 6 months, stage-2 high blood pressure not controlled by medication (>160/100 mm Hg), serum creatinine >2 mg/dL, or diagnosis of dementia.
Cerebral blood flow velocity (BFV) was measured before and after a 30-day randomized trial of flavanol-rich cocoa vs a flavanol-poor cocoa drink. Subjects were studied on day 1, day 2, and day 30. On each visit, all subjects had their blood pressure measured by automatic cuff and the median of 3 values was recorded. Next, they underwent a cognitive assessment with Mini-Mental State Examination8 and Trail Making Tests A and B.9 Finally, a complete transcranial Doppler (TCD) study was performed. After baseline studies on day 1, subjects were given cocoa powder in packets to be mixed with water and were instructed to consume 2 cups of cocoa daily (flavanol-rich cocoa 609 mg, and flavanol-poor cocoa 13 mg flavanols/serving) for 30 days beginning after their first study afternoon. During the study period, subjects continued their usual daily medications. To avoid weight gain or worsening of diabetes control, they were individually advised regarding an element of their usual diet to eliminate in place of each cup of cocoa, which contained about 100 kCal. Subjects refrained from eating chocolate other than cocoa during the study, and abstained from caffeine on the 3 study days.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Partners Healthcare Institutional Review Board and was registered with ClinicalTrials.gov (NCT00825695). All participants followed institutional guidelines for consent.
Instrumentation.
Subjects reported to the Cerebrovascular Laboratory at the Brigham and Women's Hospital in the early afternoon, 2 to 4 hours after the last meal and 4 to 6 hours after the last dose of cocoa (24-hour and 30-day studies). Subjects were instrumented for heart rate, beat-to-beat arterial pressure monitoring (Finapres; Ohmeda Monitoring Systems, Englewood, CO), and end-tidal CO2 monitoring (VacuMed CO2 analyzer, Ventura, CA) as previously described.10
TCD ultrasonography (MultiDop x4; DWL–Transcranial Doppler Systems Inc., Sterling VA) was used to measure changes in middle cerebral artery (MCA) BFV at rest, in response to cognitive tasks (NVC) and in response to changes in end-tidal CO2 (cerebral vasoreactivity [VR]), before and after cocoa consumption. The MCA signal was identified according to the criteria of Aaslid et al.11 and recorded at a depth of 50 to 60 mm. A Mueller-Moll probe fixation device was used to stabilize the Doppler probe for the duration of the study. The envelope of the velocity waveform, derived from a fast Fourier analysis of the Doppler frequency signal, was digitized at 500 Hz, displayed simultaneously with the arterial blood pressure and ECG and end-tidal CO2 signals, and stored for later off-line analysis.
Cerebral VR.
Changes in MCA BFV were measured during alterations in end-tidal CO2.12 Cerebral VR is the slope of the relationship between percentage change in MCA BFV and end-tidal CO2.
Neurovascular coupling.
Changes in MCA BFV were measured during the performance of N-Back (1-Back and 2-Back) tasks and normalized to a control task (Identify letter X) as previously reported3 and detailed in appendix e-1 on the Neurology® Web site at www.neurology.org. The mean percentage change for each MCA was calculated as a ratio of the percentage difference between the BFV during the N-Back (BFVNB) and its corresponding Identify X control period (BFVIDX) divided by BFV during Identify X (BFVIDX) and multiplied by 100 using the following formula:
Based on our prior work in individuals with a similar risk profile and age distribution to the participants of this study,3 normal NVC was defined as >5% increase in BFV during the 2-Back Task. We defined any increase in NVC relative to baseline as a positive response to cocoa.
MRI acquisition.
Twenty-four subjects were eligible and agreed to undergo MRI testing (Siemens Trio 3-tesla system; Erlangen, Germany). Full details of MRI acquisition and processing are provided in appendix e-1. MRI measures included volumes of normal and abnormal white matter (white matter hyperintensities [WMH]), and tissue microstructure measurements in normal and abnormal white matter derived from diffusion tensor imaging (DTI) (fractional anisotropy [FA] and mean diffusivity [MD]). Additional exploratory analyses were performed examining white matter microstructure properties in selected anatomical regions of interest in the cerebral white matter.
Statistical analysis.
Data were examined and extreme values were investigated for veracity. Thirty-day Trails B data for 2 subjects were missing because one subject forgot his eyeglasses and could not perform the task and the second did not return at 30 days. Data were summarized as numbers with percentage or means and SDs. Distributions were assessed to determine the most appropriate statistical tests. Patient characteristics and outcomes were compared between groups using Wilcoxon rank sum test, independent sample t test, or χ2 test. Within-subject changes were tested using paired t test or Wilcoxon signed rank test. Because results are not adjusted for multiple statistical testing, a reduced α may be used to determine statistical significance regarding the analyses of white matter structure. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
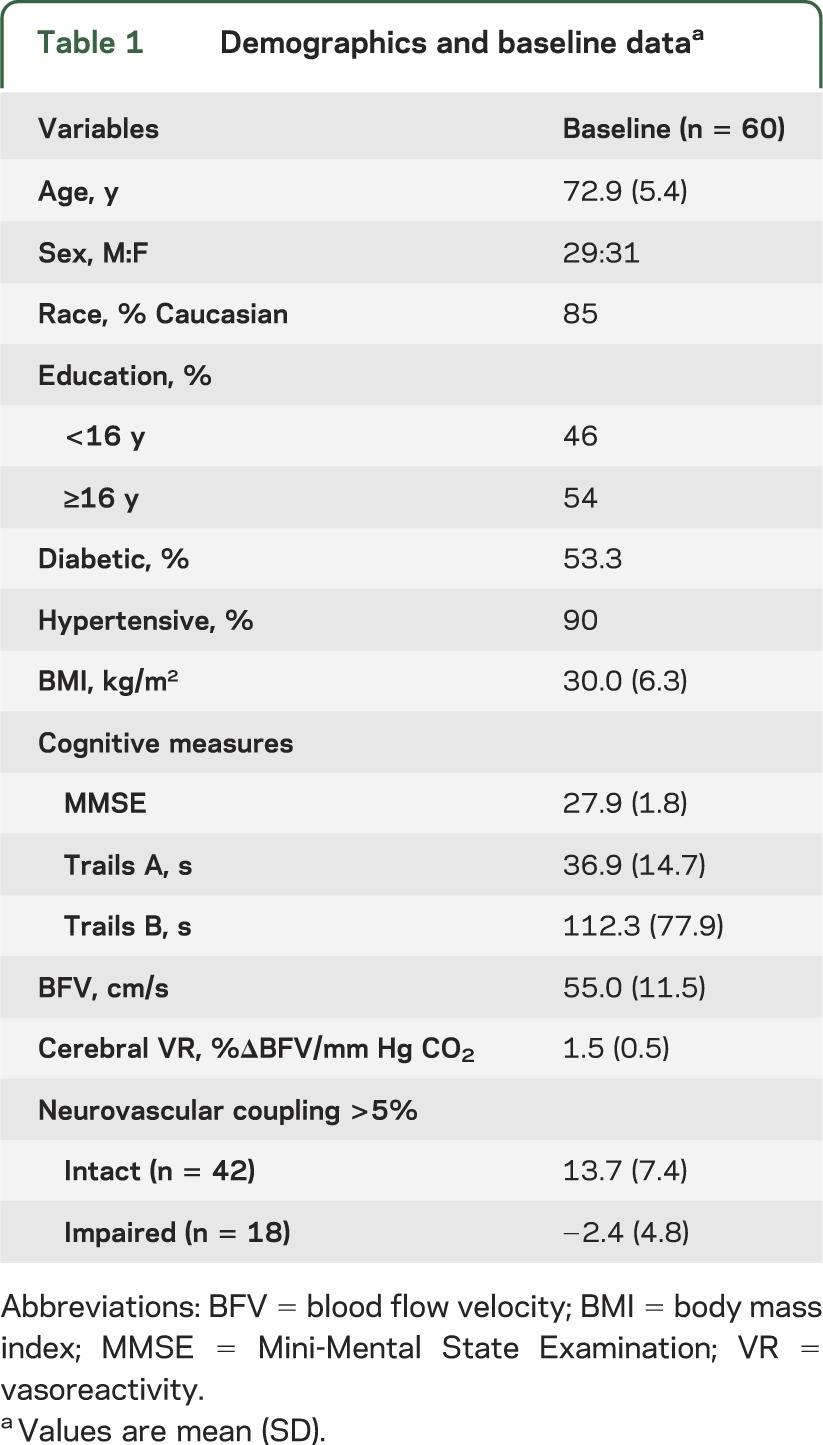
Sixty subjects were evenly split between the sexes; the majority of the subjects were Caucasian (table 1). Slightly more than half of the subjects had a college education. Ninety percent of the subjects were hypertensive, with well-controlled blood pressure as evidenced by normal pressures on study day 1. Half of the subjects had diabetes mellitus type 2, with reasonably good control (mean hemoglobin A1C 7.3%). Three-quarters were overweight or obese (body mass index ≥25 kg/m2). Blood flow responses during the 1-Back Task were not significantly different from the control task. Blood flow responses during the 2-Back Task were significantly higher than responses during the control task (data not shown). Therefore, all of the analyses were performed using blood flow responses to the 2-Back Task relative to the Identify X task condition (NVC during the 2-Back Task condition). NVC during the 2-Back Task was >5% (intact) in about two-thirds of subjects at baseline.
Table 1.
Demographics and baseline dataa
NVC and cognitive function.
Trails B scores were significantly better (lower reaction times) in individuals with intact NVC (89 ± 43 seconds vs 167 ± 110 seconds; p = 0.002). In addition, individuals with intact NVC had significantly better performance on the 2-Back Task (82% ± 19% vs 75% ± 20% corrected; p = 0.02). The relationship between NVC and Trails B score as continuous variables was weaker (p = 0.09) because there was greater heterogeneity among individuals with impaired NVC. There was no significant association between NVC and Mini-Mental State Examination scores (r = 0.10, p = 0.43).
NVC and cerebral white matter.
Higher NVC was associated with overall less white matter macro- and microstructure damage. Table 2 summarizes the volumes of normal white matter and WMH, as well as DTI measures of FA and MD in both normal white matter and WMH. In general, those individuals with intact NVC had a greater volume of normal white matter and smaller volume of WMH. Similarly, individuals with intact NVC had higher FA and lower MD in the normal white matter and WMH. The difference in WMH FA between the 2 groups was statistically significant (p = 0.02). The relationship between NVC and WMH FA remained significant when both measures were analyzed as continuous variables in an association analysis (p = 0.01)
Table 2.
Neurovascular coupling and white matter structurea
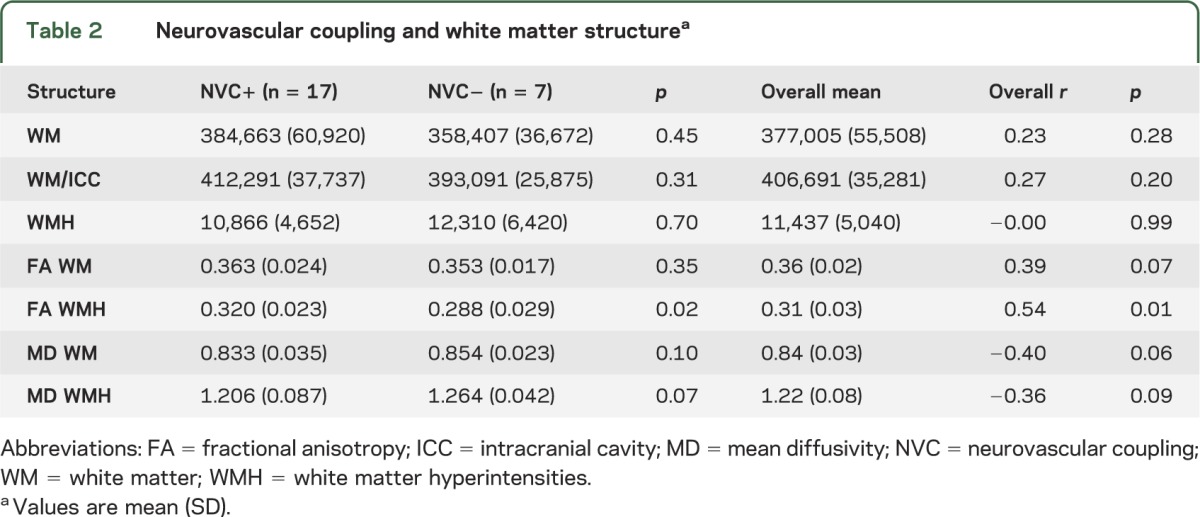
In an exploratory analysis, we also examined the relationship between regional differences in DTI and NVC. The most significant differences were seen in the frontal white matter tracts, where mean and radial diffusivities were significantly lower in those with intact NVC (MD 0.75 ± 0.04 vs 0.78 ± 0.03, p = 0.04; and radial diffusivity 11.92 ± 0.88 vs 12.67 ± 0.73, p = 0.03). In addition, there was significant correlation between FA in the frontal lobes and NVC, with higher anisotropy in those with higher NVC (r = 0.48, p = 0.02). We did not find a significant association between NVC and DTI measures in other lobes of the brain.
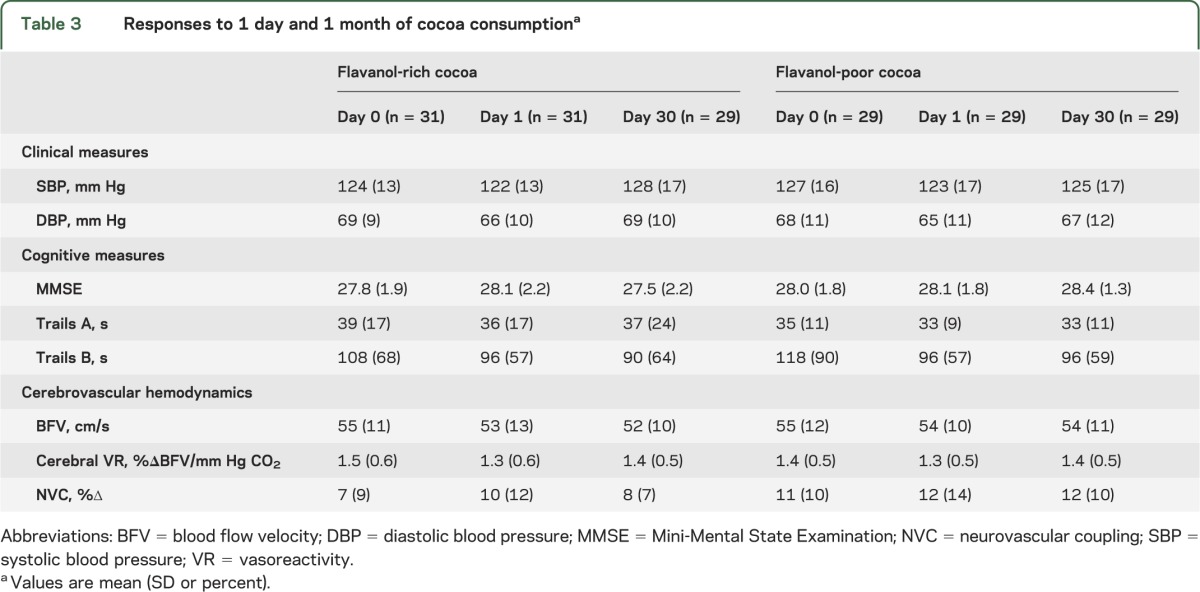
Effects of cocoa on NVC.
Table 3 summarizes blood pressure and cerebral blood flow changes in response to 24 hours and 4 weeks of cocoa consumption. Blood flow and blood pressure changes were not significantly different between the 2 cocoa groups. Similarly, there was no significant difference in change in NVC between flavanol-rich and flavanol-poor groups at 24 hours (2.6 ± 13.2 vs 1.7 ± 12.2; p = 0.79) or at 30 days (0.7 ± 9.2 vs 1.3 ± 10.2; p = 0.8). Therefore, all subsequent analyses were performed with cocoa groups combined. Among all subjects, blood pressure decreased after 1 day of cocoa (ΔSBP: 3.2 ± 13.4 mm Hg, p = 0.07; ΔDBP: 3.0 ± 9.6 mm Hg, p = 0.02). However, after 1 month of cocoa, blood pressures were not significantly different from baseline (p > 0.5). Resting BFV, NVC, and cerebral VR were not significantly different from baseline after 24 hours or 30 days of cocoa consumption among the entire cohort.
Table 3.
Responses to 1 day and 1 month of cocoa consumptiona
In contrast, response to cocoa differed significantly depending on NVC status (figure 1). Cocoa had a significant effect on NVC in those with impaired (<5%) coupling at baseline. Of those with impaired NVC, 89% responded to 30 days of cocoa consumption with increased NVC, compared with only 36% of those with intact NVC (p = 0.0002). In those subjects with impaired baseline coupling, cocoa consumption was associated with 10.6% ± 7.1% (p = 0.0001) and 8.3 ± 7.7% (p < 0.0001) increases in NVC at 24 hours and 30 days, respectively. Cocoa consumption was associated with little or no change in the NVC response at 24 hours (−1.5% ± 12.7%, not significant) or at 30 days (−2.0% ± 8.8%, not significant) when baseline coupling was intact.
Figure 1. Response to cocoa according to baseline neurovascular coupling status.
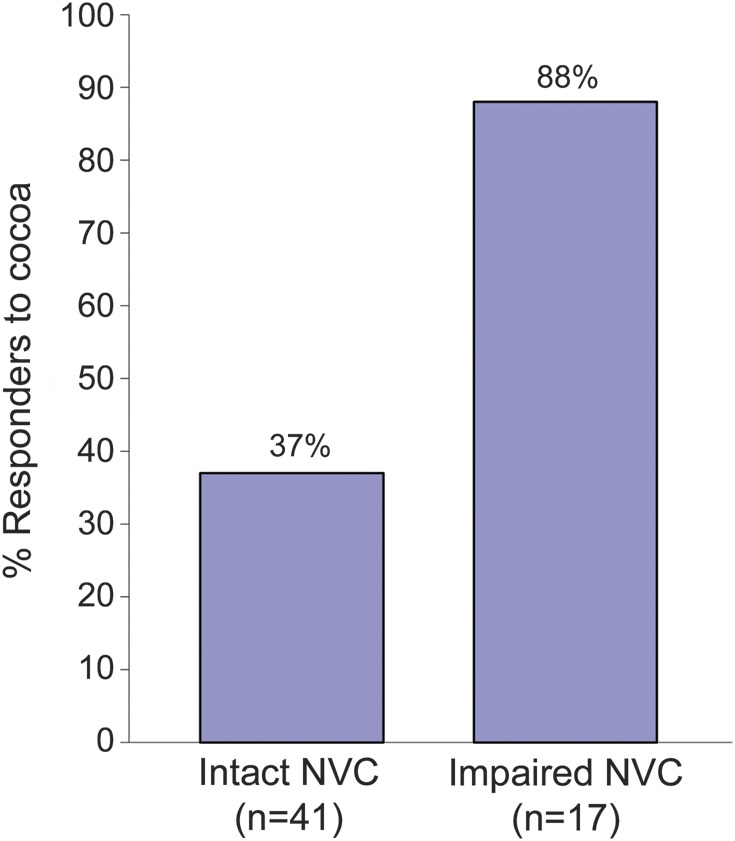
Response to cocoa was defined as an increase in neurovascular coupling (NVC) relative to baseline, and calculated as follows: NVC at 4 weeks − NVC at baseline.
The effect of cocoa consumption on Trails B scores was also significantly dependent on NVC status (figure 2). In those individuals with intact NVC, neither 24 hours nor 30 days of cocoa consumption resulted in any significant change in Trails B scores. However, Trails B performance significantly improved in response to 30 days of cocoa consumption in those with impaired NVC (p = 0.007).
Figure 2. Effect of cocoa consumption on Trails B scores according to baseline neurovascular coupling status.
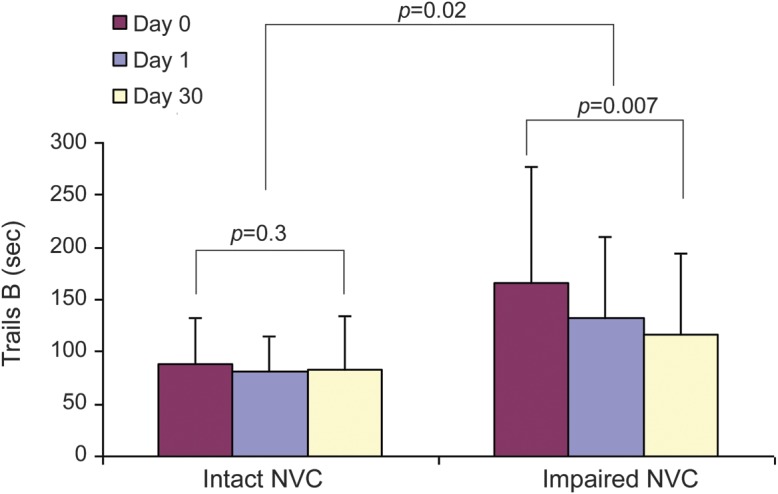
Trails B scores at baseline and after 24 hours and 30 days of cocoa consumption are shown for those with intact and impaired neurovascular coupling (NVC) at baseline.
DISCUSSION
This study shows that NVC is related to cognitive performance and cerebral white matter structural integrity in an elderly cohort with vascular risk factors. Higher NVC was associated with better cognitive performance and with greater macro- and microstructure white matter integrity. Moreover, we show that NVC may be modifiable. Four weeks of cocoa consumption resulted in improved NVC and Trails B scores in those with impaired function at baseline.
Prior studies have demonstrated pathologic changes involving the neurovascular unit in both vascular dementia and Alzheimer disease (AD).13,14 These pathologic changes could result in decoupling of cerebral blood flow from cerebral metabolic demand, and contribute to the resulting brain dysfunction seen in dementing disorders. TCD provides a noninvasive measure of blood flow responses to neuronal activation, hence allowing for the functional assessment of the neurovascular unit. This approach has helped demonstrate that NVC is impaired in patients with AD, and that cholinesterase inhibitors can improve NVC.15 This approach has also been used to differentiate between patients with vascular dementia and AD.16 Findings from the present study significantly advance our knowledge of this physiologic process and demonstrate that NVC is correlated with Trails B scores, a measure of executive function, and with cerebral white matter structural integrity.
The structural correlates of TCD-measured NVC have not been investigated previously. Functional imaging studies have shown that successful cognitive aging is associated with “more extensive brain activation” during task performance.17,18 Using TCD, we have previously shown that cognitively intact older subjects exhibit additional frontal lobe activation beyond that of young subjects in response to cognitive and visual tasks.19 Higher brain activation may enable elderly cognitively intact subjects to compensate for age-related neural changes and achieve an accuracy that equals young subjects. In the present study, we show that individuals with higher NVC also had greater macro- and microstructure white matter integrity. Whether this association means that higher NVC represents increased local activation or rather more spatially distributed activation or both is not clear. Studies with simultaneous PET or fMRI will be helpful to further decipher the structural implications of higher NVC. Moreover, it is important to note that the brain regions implicated in the N-Back Task receive their blood supply from 2 different branches of the circle of Willis, the MCA and the anterior cerebral artery (ACA). Whether blood flow changes in response to the N-Back Task in the MCA territory are reflective of ACA changes, and how these relationships are altered in vascular disease, remain to be determined. We chose the MCA as the initial vessel of investigation because it provides blood flow to a significant portion of the brain, including many areas involved in executive function, and it is a much easier vessel to monitor during clinical studies. Our aim was to identify an initial physiologic marker associated with cognitive function and potentially amenable to intervention. Future studies should include ACA and posterior cerebral artery measures as well because the visual cortex is also activated during the N-Back Task.
The structural importance of the neurovascular unit and the physiologic significance of NVC in motor and cognitive function all point to the value of this entity as a therapeutic target. Our study shows that NVC is modifiable and can be enhanced with cocoa consumption. This finding is supported by the effect of cholinesterase inhibitors on NVC in patients with AD.15 The hope that flavonoid-rich foods would prevent cognitive decline has led to a host of hypotheses and clinical trials. The hypotheses range from cholinergic, anti-inflammatory, antioxidant, and β-amyloid–reducing properties of polyphenols, and trials have incorporated foodstuffs ranging from berries to tea and ginko.20 In fact, a recent study showed that dietary consumption of cocoa rich in flavanols was associated with improved cognitive function in patients with mild cognitive impairment.7
Given our prior work establishing the beneficial effects of cocoa flavanols on systemic and cerebral vascular function,5,6,21 we were surprised to observe the equivalence of neurovascular responses between flavanol-rich and flavanol-poor cocoa subgroups in our study. Improvements in NVC were present in both groups. One possible explanation is that the previously reported responses to flavanol-rich cocoa were not entirely driven by flavanols, but another component(s) of cocoa. Alternatively, it is conceivable that the regulation is so exquisitely sensitive to flavanols that even the slight amounts contained in the flavanol-poor drink were sufficient to improve NVC. This is indeed supported by our prior work on cerebral blood flow in healthy volunteers in which we also observed an increase in cerebral BFV in the group that consumed flavanol-poor cocoa.21 In our earlier work on the peripheral circulation, we also observed an increase in peripheral arterial tonometry with flavanol-poor cocoa.6 We did not observe a significant cerebral blood flow response to cocoa in this study. In addition to duration of exposure, the subjects were completely different. Our earlier study included only healthy people, whereas everyone in the current report had hypertension, diabetes mellitus, or both. It is likely that either the dose-response curve or kinetics of cocoa acting on cerebral vasculature is altered in patients with underlying vascular disease. It is clear that future studies in this realm should be dedicated to untangling the likelihood of these various mechanisms.
Our study shows that cocoa consumption resulted in higher NVC and that individuals with higher NVC had better cognitive function and greater cerebral white matter structural integrity. Future studies combining TCD-measured NVC and fMRI and/or PET imaging are needed to better define the structural implications of this TCD variable. TCD-measured NVC may be a valuable therapeutic target as well as an ideal surrogate measure in future clinical trials of cognitive impairment. It is important to emphasize that the findings of this study are limited to an elderly cohort with vascular disease and should not be interpreted as applicable to the physiology of aging in a healthy population.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Sarah LaRose and Andrew Monk for their excellent work in subject recruiting and collecting data for this project.
GLOSSARY
- ACA
anterior cerebral artery
- AD
Alzheimer disease
- BFV
blood flow velocity
- BFVIDX
blood flow velocity during Identify X
- BFVNB
blood flow velocity during N-Back
- DBP
diastolic blood pressure
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MCA
middle cerebral artery
- MD
mean diffusivity
- NVC
neurovascular coupling
- SBP
systolic blood pressure
- TCD
transcranial Doppler
- VR
vasoreactivity
- WMH
white matter hyperintensities
Footnotes
Supplemental data at www.neurology.org
Editorial, page 863
AUTHOR CONTRIBUTIONS
F.A.S.: design, data analysis, and manuscript preparation. S.H.: statistical analysis and manuscript preparation. D.H.S. and D.N.G.: MRI data gathering, analysis, and manuscript preparation. N.D.L.F.: design, funding, data analysis, and manuscript preparation.
STUDY FUNDING
Supported by grants K23-AG030967 (F.A.S.) from the National Institute on Aging, and RO1HL089570 (N.D.L.F.) from the National Heart, Lung, and Blood Institute, Bethesda, MD. Cocoa was provided by Mars Inc.
DISCLOSURE
F. Sorond and S. Hurwitz report no disclosures. D. Salat has received support from Biogen Idec. D. Greve and N. Fisher report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004;5:347–360 [DOI] [PubMed] [Google Scholar]
- 2.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 2006;100:328–335 [DOI] [PubMed] [Google Scholar]
- 3.Sorond F, Kiely DK, Galica A, et al. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol 2011;70:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011;71:782–797 [DOI] [PubMed] [Google Scholar]
- 5.Fisher ND, Hollenberg NK. Aging and vascular responses to flavanol-rich cocoa. J Hypertens 2006;24:1575–1580 [DOI] [PubMed] [Google Scholar]
- 6.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 2003;21:2281–2286 [DOI] [PubMed] [Google Scholar]
- 7.Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012;60:794–801 [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 9.Trailmaking Tests A and B. Washington, DC: War Department, Adjutant General’s Office; 1944 [Google Scholar]
- 10.Sorond FA, Khavari R, Serrador JM, Lipsitz LA. Regional cerebral autoregulation during orthostatic stress: age-related differences. J Gerontol A Biol Sci Med Sci 2005;60:1484–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982;57:769–774 [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Matsumoto M, Handa N, et al. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial Doppler method. J Hypertens 1994;12:191–197 [PubMed] [Google Scholar]
- 13.Zlokovic BV. New therapeutic targets in the neurovascular pathway in Alzheimer's disease. Neurotherapeutics 2008;5:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010;120:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosengarten B, Paulsen S, Burr O, Kaps M. Neurovascular coupling in Alzheimer patients: effect of acetylcholine-esterase inhibitors. Neurobiol Aging 2009;30:1918–1923 [DOI] [PubMed] [Google Scholar]
- 16.Asil T, Uzuner N. Differentiation of vascular dementia and Alzheimer disease: a functional transcranial Doppler ultrasonographic study. J Ultrasound Med 2005;24:1065–1070 [DOI] [PubMed] [Google Scholar]
- 17.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 2002;17:1394–1402 [DOI] [PubMed] [Google Scholar]
- 18.Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev 2006;5:239–254 [DOI] [PubMed] [Google Scholar]
- 19.Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex 2008;44:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albarracin SL, Stab B, Casas Z, et al. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci 2012;15:1–9 [DOI] [PubMed] [Google Scholar]
- 21.Sorond FA, Lipsitz LA, Hollenberg NK, Fisher ND. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr Dis Treat 2008;4:433–440 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


