Abstract
Varicella-zoster virus (VZV) is a ubiquitous, highly neurotropic, exclusively human α-herpesvirus. Primary infection usually results in varicella (chickenpox), after which VZV becomes latent in neurons of cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis. As humans undergo a natural decline in cell-mediated immunity (CMI) to VZV with age, VZV frequently reactivates to produce zoster, characterized by maculopapular or vesicular rash and dermatomal-distribution pain. Pain and rash usually occur within days of each other. Pain is severe and often burning. Colorful descriptions of zoster exist worldwide. In Arabic, Hezam innar 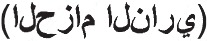 means belt of fire; in Hindi, Baoisayaa daga
means belt of fire; in Hindi, Baoisayaa daga  means big rash; in Norwegian, Helvetesild means Hell's fire (also described as a bell of roses from Hell); and in Spanish, Culebrilla means small snake.1 The most common complication of zoster is postherpetic neuralgia (PHN), operationally defined as pain lasting for more than 90 days after rash. Zoster may be followed by multiple neurologic disorders (meningoencephalitis, myelitis, and vasculopathy, including VZV temporal arteritis) as well as ocular disease (acute or progressive outer retinal necrosis).
means big rash; in Norwegian, Helvetesild means Hell's fire (also described as a bell of roses from Hell); and in Spanish, Culebrilla means small snake.1 The most common complication of zoster is postherpetic neuralgia (PHN), operationally defined as pain lasting for more than 90 days after rash. Zoster may be followed by multiple neurologic disorders (meningoencephalitis, myelitis, and vasculopathy, including VZV temporal arteritis) as well as ocular disease (acute or progressive outer retinal necrosis).
Varicella-zoster virus (VZV) is a ubiquitous, highly neurotropic, exclusively human α-herpesvirus. Primary infection usually results in varicella (chickenpox), after which VZV becomes latent in neurons of cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis. As humans undergo a natural decline in cell-mediated immunity (CMI) to VZV with age, VZV frequently reactivates to produce zoster, characterized by maculopapular or vesicular rash and dermatomal-distribution pain. Pain and rash usually occur within days of each other. Pain is severe and often burning. Colorful descriptions of zoster exist worldwide. In Arabic, Hezam innar 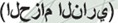 means belt of fire; in Hindi, Baoisayaa daga
means belt of fire; in Hindi, Baoisayaa daga  means big rash; in Norwegian, Helvetesild means Hell's fire (also described as a bell of roses from Hell); and in Spanish, Culebrilla means small snake.1 The most common complication of zoster is postherpetic neuralgia (PHN), operationally defined as pain lasting for more than 90 days after rash. Zoster may be followed by multiple neurologic disorders (meningoencephalitis, myelitis, and vasculopathy, including VZV temporal arteritis) as well as ocular disease (acute or progressive outer retinal necrosis).
means big rash; in Norwegian, Helvetesild means Hell's fire (also described as a bell of roses from Hell); and in Spanish, Culebrilla means small snake.1 The most common complication of zoster is postherpetic neuralgia (PHN), operationally defined as pain lasting for more than 90 days after rash. Zoster may be followed by multiple neurologic disorders (meningoencephalitis, myelitis, and vasculopathy, including VZV temporal arteritis) as well as ocular disease (acute or progressive outer retinal necrosis).
Primary VZV infection produces VZV antibody and VZV-specific T-CMI. Serum of individuals 60–94 years old contains variable antibodies to VZV glycoproteins I–IV and to 3 nonglycosylated proteins; VZV antibodies in some elderly individuals with no history of varicella or zoster indicate subclinical infection. VZV antibodies do not protect against zoster or PHN. Recovery from varicella is associated with VZV-specific T-CMI, detected 1–2 weeks after disappearance of rash, consisting of CD4, CD8 effector, and memory T cells. T-cell immunity to VZV is more important than antibodies. Agammaglobulinemic patients do not produce VZV-specific antibodies but are protected against second episodes of varicella because they mount VZV-specific T-CMI responses. Individuals with T-CMI deficiency disorders or who are immunosuppressed have more zoster and more severe disease than normal hosts. In human stem cell recipients who received inactivated VZV vaccine, protection correlated with VZV-specific T-CMI but not with anti-VZV antibody.
Nearly all children develop varicella and become latently infected. Attenuated VZV in varicella vaccine also becomes latent after childhood vaccination. Thus, zoster remains a global health issue, awaiting the development of a vaccine that produces permanent immunity and prevents virus reactivation. VZV-specific T-CMI maintains VZV in a latent state in ganglia. Immunity is boosted by subclinical reactivation of virus or environmental exposure. Zoster increases with age as VZV-specific T-CMI declines, evident even during the first 3 years after varicella, when VZV-specific memory CD4 T cells decrease. After mid-life, antigenic stimulation provided by re-exposure and asymptomatic reactivation are not sufficient to maintain VZV-specific T-cell immunity. Comparison of CMI response to VZV antigen in vitro in young adults and individuals over age 60 years revealed 5-fold fewer CD4 cells producing interferon-γ or interleukin-4 and -5, as well as fewer CD4 early effectors and CD8 effector memory cells in older age groups. Recognition of the essential role of CMI to VZV for protection against and recovery from varicella and zoster led to studies designed to boost CMI to VZV by immunization of elderly adults.
Any epidemiologic overview of zoster must highlight important caveats. Data from health care systems are limited to medically attended cases and may miss those that are milder or less able to access the medical system. Hospital-based data are particularly prone to artifacts. The prevalence of zoster risk factors within selected study populations can cause substantial differences in observed zoster incidence. Studies using varied methods and conducted in different populations in the United States, Canada, South America, Europe, Asia, and Australia revealed a median zoster incidence of 4–4.5 per 1,000 person-years.e1–e9 The age-adjusted incidence of zoster is increasing.2,3 In Olmsted County, Minnesota, zoster rates increased 28% over 5 years, from 3.2 (95% confidence interval [CI] 2.9–3.5) per 1,000 person-years in 1996–1997 to 4.1 (95% CI 3.8–4.4) in 2000–2001, an increase not explained by changes in age distribution or the prevalence of immunocompromised persons.4 Similar increases over variable time periods have been seen in most, but not all, analyses conducted in the United States, Canada, Europe, Asia, and Australia, but the reasons for these increases are unknown.
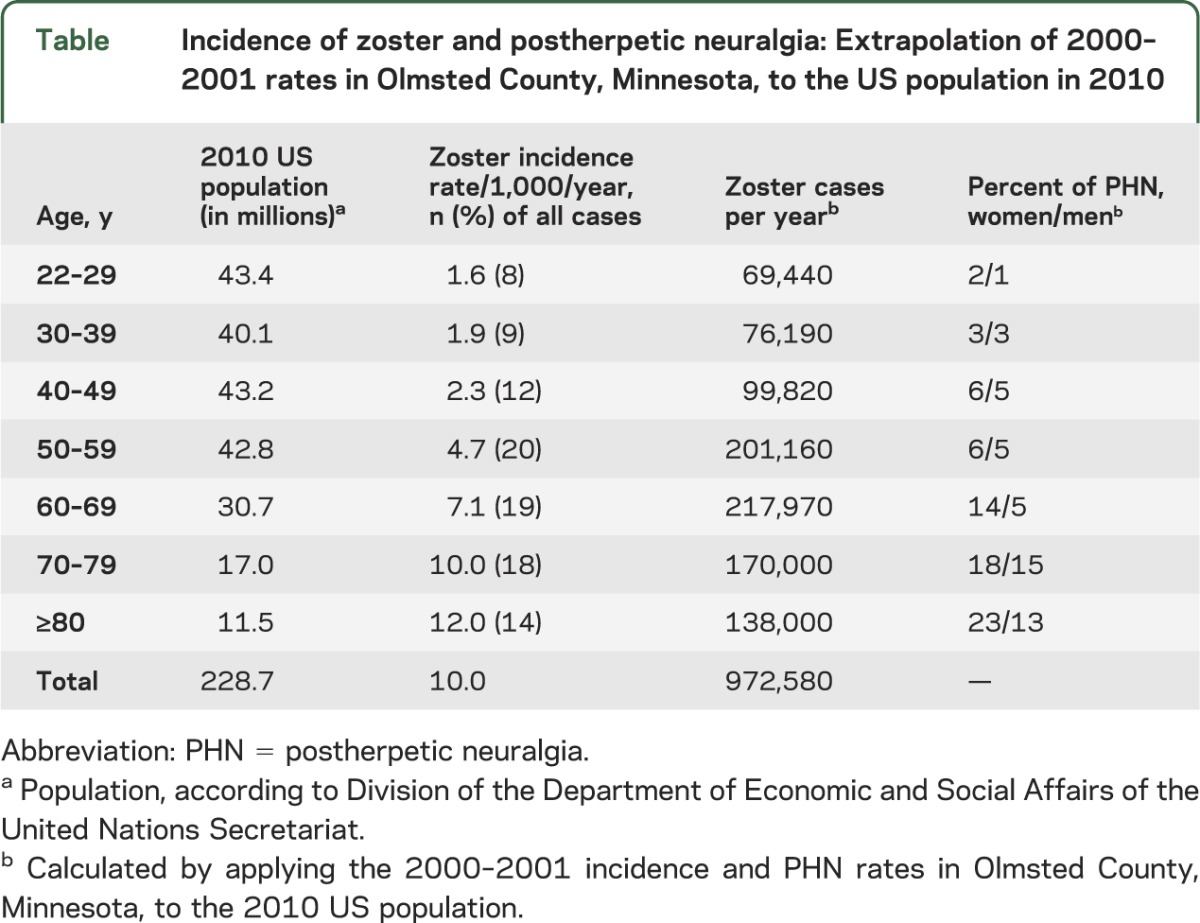
All studies show a striking increase of zoster with age, particularly after age 50 years (figure). The mean age at onset of zoster among adults (age 22 years and older) is 59.4 years, with 68% of cases occurring in those 50 years and older. Age-adjusted rates are higher in women than in men (3.9 vs 3.2 per 1,000 person-years, respectively; p < 0.0001). Based upon these age-specific rates, ∼1 million new episodes of zoster have been estimated to occur in the United States yearly (table), with a lifetime risk of ∼30%.
Figure. Age-specific zoster incidence rates around the world.
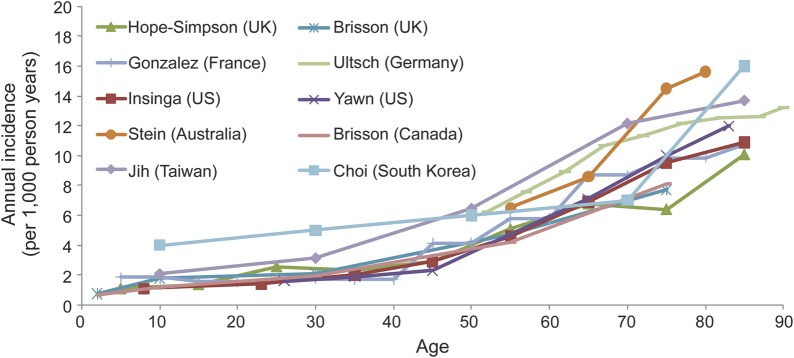
Compiled by E. Brenitz, MD, PhD, Merck and Co., for ICID, Bangkok, Thailand, June 2012. See also references e1–e9 on the Neurology® Web site at www.neurology.org.
Table.
Incidence of zoster and postherpetic neuralgia: Extrapolation of 2000–2001 rates in Olmsted County, Minnesota, to the US population in 2010
In the United States, ∼8% of zoster episodes occur in immunocompromised patients2 (e.g., organ transplant recipients, patients treated with cytotoxic drugs or irradiation, and those with HIV/AIDS). In patients with HIV/AIDS, the risk of zoster varies with stage of HIV infection and treatment, but rates are typically increased many-fold, and episodes can be recurrent and protracted. In regions most affected by the HIV/AIDS pandemic, zoster in young adults is often a marker of HIV infection. Given that globally ∼34 million people are infected with HIV, zoster rates are clearly affected, although data relating to the impact of the HIV/AIDS pandemic on zoster incidence are not available.
PHN also increases with age. Among persons older than 50 years and 80 years, the incidence of PHN in zoster patients is 18% and 33%, respectively. Overall, 80% of all PHN occurs among persons 50 years and older (table).
About 3% of patients with zoster are hospitalized.2,5 Few studies provide valid data relating to zoster mortality, with only ∼0.25 per million population in the United States and Europe, mostly among the elderly. Reported rates of recurrent zoster vary from 3% to 5% lifetime to 6.2% over 8 years,2 and are clearly associated with decreased immune competency. For individuals with HIV, the recurrence rate can be as high as 13% to 26%. Zoster mortality is presumably elevated in countries severely affected by the HIV/AIDS pandemic.
Varicella vaccine is used in a growing number of countries. Attenuated vaccine VZV becomes latent and can reactivate to cause zoster, although risk of vaccine-associated zoster is a fraction of the risk of zoster after natural infection. Over time, countries having varicella vaccination programs may experience less zoster. However, the low incidence of varicella in countries with vaccination programs has reduced opportunities for exposure to varicella and for boosting of VZV-specific immunity. Thus, in those countries, vaccination might lead to increased rates of zoster. Age-specific increases in zoster incidence have already been noted in a number of countries (see above), but similar increases have occurred in the presence and the absence of varicella vaccination programs. While immunization programs continue to monitor the impact of varicella vaccination on age-adjusted zoster incidence, the increasing trends remain unexplained.
In a number of countries, a vaccine (Zostavax) to prevent zoster is recommended for older adults. Vaccine efficacy is 51% for prevention of zoster and 65% for prevention of PHN lasting 90 days or more. However, vaccine has not yet affected the epidemiology of zoster, because uptake has been low, reaching 14.4% in the United States 4 years after licensure (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6104a2.htm). Barriers to uptake in the United States have included high cost, requirement for freezer storage, complex insurance reimbursement, and vaccine supply failures.
Supplementary Material
Footnotes
Supplemental data at www.neurology.org
STUDY FUNDING
Supported in part by NIH grants AG006127 and AG032958 to D.G. and a grant from CDC to B.P.Y.
DISCLOSURE
D. Gilden receives research support from NIH research grants AG006127, AG03258, and NS007321. Dr. Gilden serves as Senior Associate Editor for the Journal of Neurovirology and on the editorial boards of In Vivo, Journal of Virology, Scientific American Medicine, Virus Genes, and Neurology®. Dr. Gilden has consulted for TEVA and Epiphany Laboratories and has received payment for educational lectures from Merck Laboratories. B. Yawn reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Paek E, Johnson R. Public awareness and knowledge of herpes zoster: results of a global survey. Gerontology 2010;56:20–31 [DOI] [PubMed] [Google Scholar]
- 2.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341–1349 [DOI] [PubMed] [Google Scholar]
- 3.Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older, in the U.S., 2008. Am J Prev Med 2011;40:e1–e6 [DOI] [PubMed] [Google Scholar]
- 4.Naveen KN, Tophakane RS, Hanumanthayya K, Pv B, Pai VV. A study of HIV seropositivity with various clinical manifestation of herpes zoster among patients from Karnataka, India. Dermatol Online J 2011;17:3. [PubMed] [Google Scholar]
- 5.Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: a self- limited disease with severe impact. Neurology 1995;45(suppl 8):S52–S53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


