Abstract
Objective:
Small-cell lung carcinoma (SCLC) and limbic encephalitis are recognized γ-aminobutyric acid-B receptor (GABABR) autoantibody accompaniments. We sought to determine in a diagnostic serology laboratory the frequency and accompaniments (neurologic, oncologic, and serologic) of GABABR–immunoglobulin G (IgG).
Methods:
We tested stored serum and CSF specimens from 3 patient groups for GABABR-IgG by indirect immunofluorescence on mouse brain tissue and transfected HEK293 cells. Group 1 included 3,989 patients tested for GABABR-IgG in service evaluation for suspected autoimmune encephalopathy. Group 2 included 49 patients with an unclassified CNS synaptic IgG detected (antedating descriptions of GABABR autoantibody). Group 3 included 384 patients in whom ≥1 SCLC-predictive autoantibodies had been detected.
Results:
GABABR-specific IgG was detected in 17 patients (serum, 14; CSF, 11). N-type calcium channel antibody coexisted with GABABR-IgG in all seropositive patients of groups 1 and 2. In group 1, 7 of 3,989 patients were positive (0.2%). All had limbic encephalitis; 5 had SCLC. Four patients received immunotherapy and improved neurologically. In group 2, 5 of 49 patients were positive (10%). Three had limbic encephalitis, 1 had rapidly progressive encephalomyelopathy, and 1 had cerebellar ataxia. Two patients had SCLC and 1 had multiple myeloma. In group 3, 5 of 384 patients were positive (1.3%); titers were low (detected only by transfected cell assay). The neurologic presentations were diverse and attributable to coexisting T-cell-mediated autoimmunity (indicated by CRMP-5 IgG [2], ANNA-1 [2], and ANNA-3 [2]), rather than to GABABR-IgG.
Conclusion:
GABABR autoantibody is a marker of an uncommon but treatable paraneoplastic neurologic disorder, usually occurring in the setting of limbic encephalitis and SCLC.
Autoantibodies specific for the CNS inhibitory γ-aminobutyric acid-B receptor (GABABR, B1 and B2 subunits) have been reported in patients with paraneoplastic limbic encephalitis (LE). Small-cell lung carcinoma (SCLC) and other neuroendocrine neoplasms1,2 have been reported as oncologic accompaniments. The neuronal N-type voltage-gated calcium channel antibody, another paraneoplastic autoantibody SCLC marker, is commonly reported as a serologic accompaniment.1 Paraneoplastic neurologic disorders associated with autoantibodies targeting neural plasma membrane antigens (e.g., GABABR) tend to improve with early cancer treatment and immunotherapy.1 In contrast, disorders associated with autoantibodies specific for neural intracellular antigens (i.e., nuclear and cytoplasmic) are less responsive to these treatments.
To date, most patients reported with GABABR antibody have been ascertained through evaluation of patients with LE.1,2 Autoimmune serologic evaluation in a clinical service laboratory, involving patients with diverse neurologic presentations, allows broader ascertainment of clinical associations. Here, we report the detection frequency of GABABR antibody and associated neurologic, oncologic, and serologic findings.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Mayo Clinic Institutional Review Board.
Patients.
Archival sera and CSF selected for GABABR antibody testing were from the following.
Group 1.
Group 1 was evaluated to determine the frequency in clinical laboratory practice of GABABR–immunoglobulin G (IgG) detection among patients with suspected autoimmune encephalopathy. This group consisted of 3,989 patients, for whom GABABR-IgG testing was performed as a component of service evaluation for hippocampal synaptic autoantibodies (July 2010–December 2012). For 3,026 patients, serum was tested, for 1,665, CSF was tested; paired serum and CSF samples were tested for 1,332 patients.
Group 2.
Group 2 consisted of 49 patients, in whom tissue-based immunofluorescence (performed 1991–2010, prior to GABABR antibody's discovery1) revealed an unclassified CNS synaptic autoantibody suggestive of GABABR-IgG. Archival laboratory records identified these patients through the described pattern of IgG binding to cerebellum, midbrain, and myenteric plexus. There were 46 serum specimens and 7 CSF specimens (paired in 4 cases).
Group 3.
Group 3 included 384 Mayo Clinic patients in whom service serologic evaluation (January 1986–May 2010) had revealed one or more paraneoplastic neuronal or glial nuclear or cytoplasmic autoantibodies predictive of SCLC (a common accompaniment of GABABR-IgG): ANNA-1; collapsin response-mediator protein 5 (CRMP-5) IgG; Purkinje cell cytoplasmic antibody type 2; amphiphysin IgG; ANNA-2, ANNA-3; antiglial/neuronal nuclear antibody, type 1 (SOX-1 antibody). Paired CSF was available for 54 cases.
Serologic testing.
GABABR-IgG was sought by indirect immunofluorescence on 1) a composite substrate of mouse tissues, consisting of hippocampus, cerebral cortex, cerebellum, basal ganglia, thalamus, kidney, and gut; and 2) HEK293 cells transfected with the GABAB cDNA (EUROIMMUN, Lubeck, Germany). Patients 1 and 2 were tested additionally courtesy of Dr. J. Dalmau. Other testing was performed as previously described.3
RESULTS
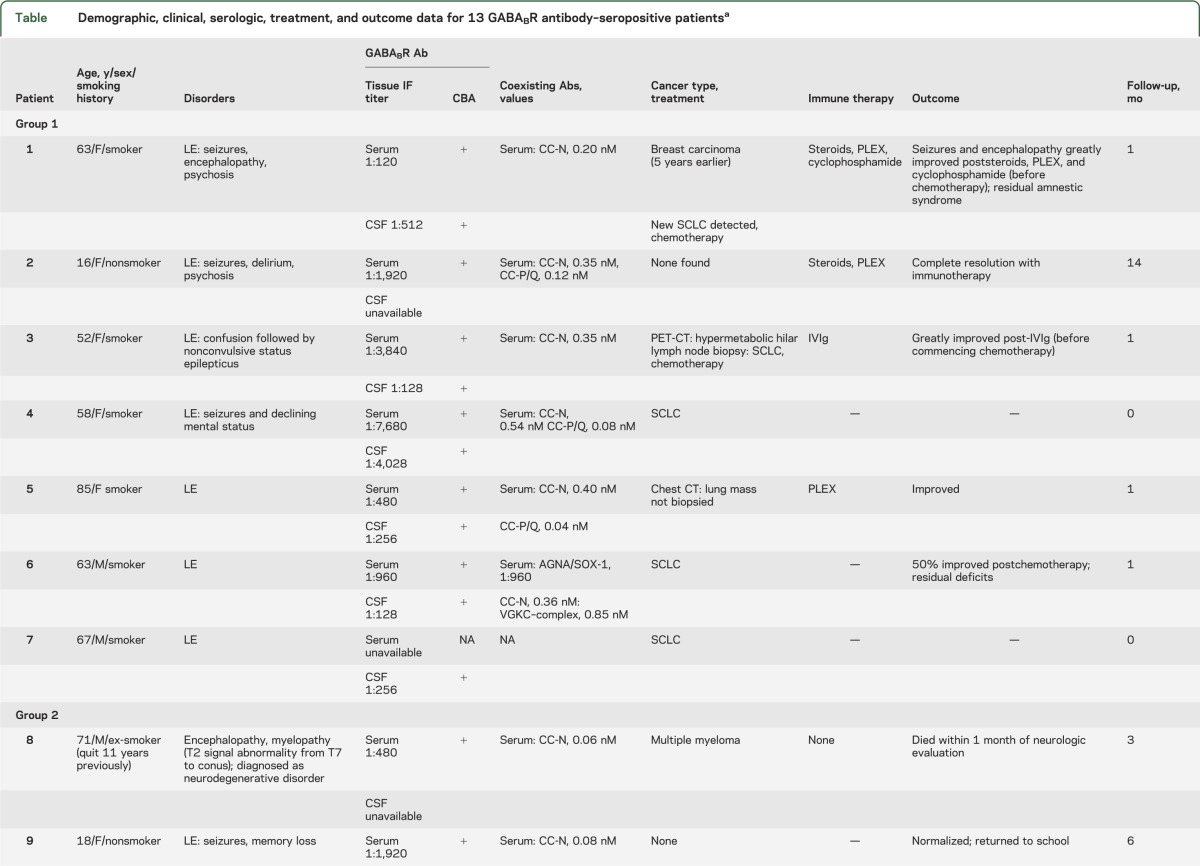
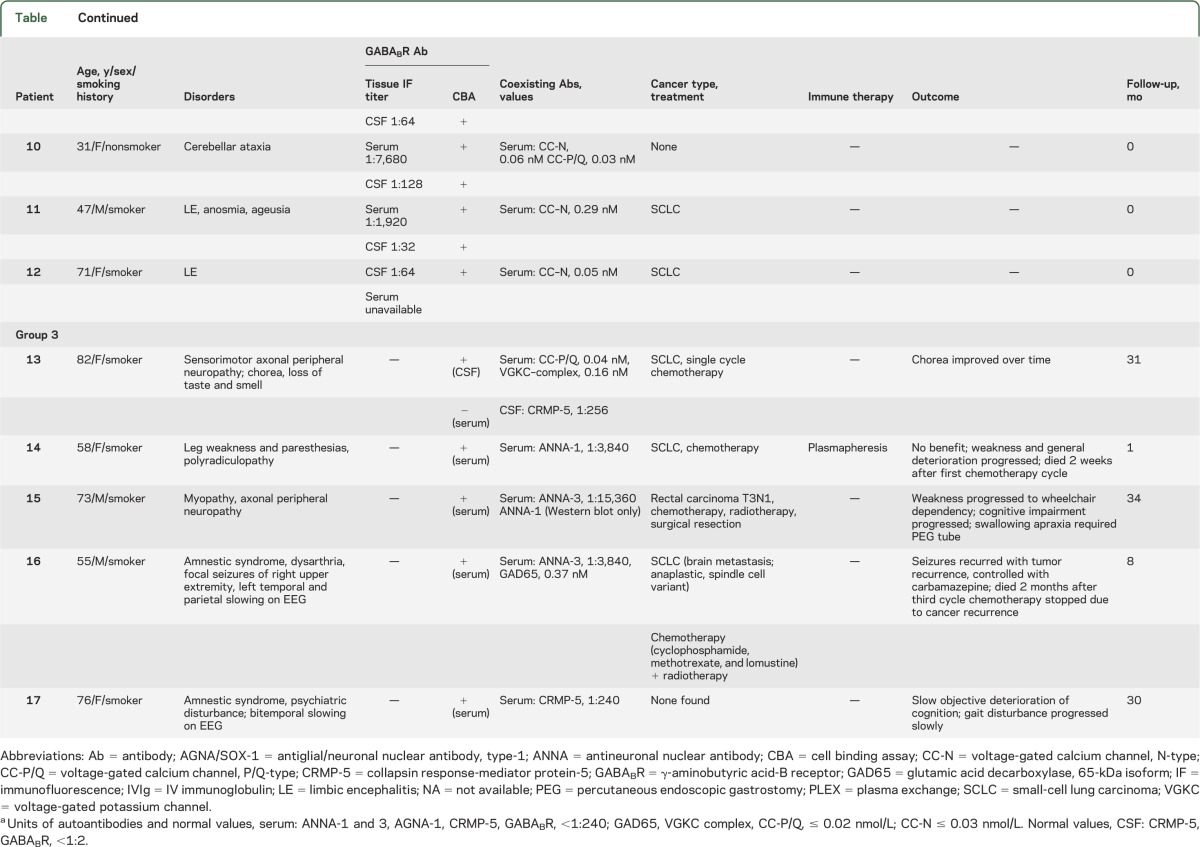
We detected GABABR antibody in 17 patients (table); 11 were women; median symptom onset age was 63 years (range, 16–85).
Table.
Demographic, clinical, serologic, treatment, and outcome data for 13 GABABR antibody–seropositive patientsa
Group 1.
Seven patients of 3,989 (0.2%) were positive (patients 1–7) in 12 total specimens (serum and CSF, 5 cases; serum or CSF, 2 cases with only 1 specimen available). For each specimen, GABABR-IgG was identified by tissue-based assay and confirmed by transfected cell assay. All had LE. Except for one 16-year-old girl, all had SCLC. All 5 patients with available information improved neurologically after receiving immunotherapy or oncologic therapy.
Group 2.
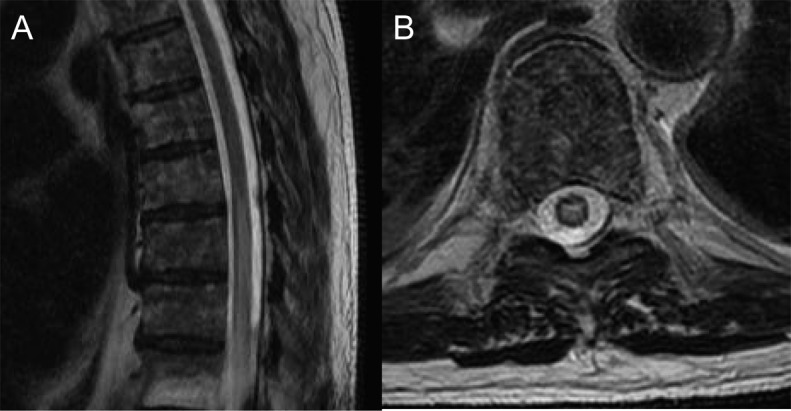
Five patients of 49 (10%) were positive (patients 8–12) in 8 total specimens (serum and CSF, 3 cases; serum or CSF, 2 cases with only 1 specimen available). The staining pattern, scored initially as “unclassified CNS synapse-binding IgG,” was consistent with GABABR antibody (figure 1). GABABR specificity was confirmed by cell binding assay in all 8 specimens. Neurologic diagnoses were LE (3 cases; 2 with SCLC), cerebellar ataxia (1 case, no cancer found), and encephalopathy plus longitudinally extensive myelopathy (1 case, patient 8, who was NMO-IgG seronegative, had multiple myeloma, received no immunotherapy, and died within 1 month of serologic testing [figure 2]).
Figure 1. Synaptic staining pattern of γ-aminobutyric acid-B receptor–immunoglobulin G compared with NMDA-R-IgG staining pattern (tissue immunofluorescence).
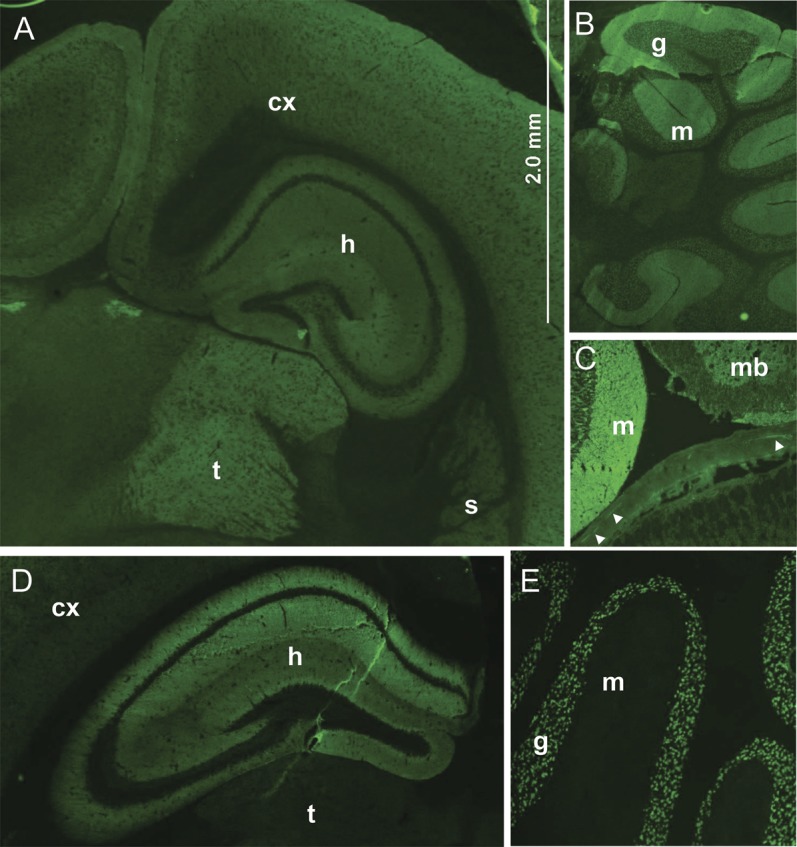
The γ-aminobutyric acid-B receptor (GABABR)–immunoglobulin G (IgG) binding in the cerebrum (A) is most prominent in the thalamus (t), and is also seen in hippocampus (h), cerebral cortex (cx), and striatum (s). GABABR-IgG also binds to cerebellum (B, both molecular [m] and granular [g] layers), midbrain (mb, panel C), and myenteric neurons (arrowheads). In contrast, NMDA-R-IgG binding is most prominent in the hippocampus (h, panel D) and cerebellar granular layer (g, panel E); myenteric neurons are not stained (not shown).
Figure 2. MRI of thoracic spine of patient 8 demonstrates a longitudinally extensive T2 signal abnormality in the thoracic cord (sagittal image, A) that had central cord predominance (axial image, B).
Group 3.
Five patients of 384 (1.3%) were positive (patients 13–17); serum, 4 cases (CSF not available); CSF, 1 case (serum not available). In all cases titers were low (not detected by tissue-based immunofluorescence, but by transfected cell assay in serum diluted 1:10 or in CSF diluted 1:2). The neurologic diagnoses were localized in 3 patients to the CNS (cognitive/behavioral disturbance, 2; seizures, 1; and dyskinesias, 1) and in 3 patients to the peripheral nervous system. SCLC was diagnosed in 3. Coexisting paraneoplastic autoantibodies included CRMP-5 IgG, 2; ANNA-1, 2; ANNA-3, 2. In all cases except patient 1, neurologic deterioration progressed despite treatment.
Of the 17 patients in whom we detected GABABR-IgG, one or more coexisting cation channel antibodies were detected in 13: N-type calcium channel, 11; P/Q-type calcium channel, 5; voltage-gated potassium channel complex, 2 (both negative for LGi1-IgG and CASPR2-IgG specificities). One patient had coexisting GAD65 antibody. Abnormalities detected by routine CSF testing (results available for only 4 patients, groups 2 and 3) included elevated white cell count, 2 cases (12 and 17; [normal <5]); elevated protein, 2 cases (50 and 75 [normal <35 mg/dL]).
DISCUSSION
GABABR antibody is encountered uncommonly in serologic evaluation for evidence of paraneoplastic neurologic autoimmunity (0.2% of patients tested, compared to 0.36% for ANNA-13), but is of clinical significance when detected. Consistent with previous reports, patients in whom GABABR antibody is detected in high titer (i.e., on substrates of hippocampal tissue as well as transfected cells) usually present with isolated LE in the context of SCLC. These patients respond well to immunotherapy. The generally uniform clinical presentations and favorable outcomes in patients presenting with isolated LE (group 1) contrasted with group 3 patients in whom low titers of GABABR-IgG (detected only by transfected cell binding assay) coexisted with high titers of paraneoplastic neuronal nuclear and cytoplasmic antibodies (ANNA-1, ANNA-3, and CRMP-5 IgG).1,2 The diversity of central and peripheral nervous system disorders in this group, and their poor responsiveness to immunotherapy, are characteristic of the neurologic accompaniments of paraneoplastic neural autoantibodies specific for intracellular antigens.3 These serologic profiles are representative of a multifaceted tumor immune response targeting plasma membrane and intracellular onconeural antigens. Lancaster et al.1 likewise reported finding a low level of GABABR-IgG in some patients with nonencephalitic paraneoplastic neurologic disorders.
Seropositivity in group 2 patients was informative for rarer neurologic presentations of GABABR autoantibody (encephalomyelopathy and cerebellar ataxia) because those patients were not selectively tested on the basis of an encephalopathic presentation. Cerebellar ataxia and brainstem encephalitis have been reported previously as rare neurologic accompaniments of GABABR-IgG.4,5 The longitudinally extensive myelitis in patient 8 (who had multiple myeloma and coexisting encephalopathy) was characteristic of a paraneoplastic myelopathy.6 Other rare oncologic associations of GABABR-IgG reported to date include esophageal carcinoma and malignant melanoma.4,5
No cancer was detected in the 2 seropositive girls (aged 16 and 18 years). Cancer detection rates of 1%–7% have been reported for pediatric patient cohorts with autoimmune neurologic disorders accompanied by other neuronal plasma membrane-specific IgG antibodies (voltage-gated potassium channel complex and NMDA receptor).7–9
The common coexistence of neuronal voltage-gated calcium channels antibodies (N-type > P/Q-type) with GABABR antibodies suggests that tumor-derived GABABR receptors and calcium channels might associate in a complex and serve as coimmunogens. Most presynaptic GABAB receptors are inhibitory of these calcium channels, and do so via receptor-coupled Gβγ subunits of inhibitory G (Gi/o) proteins that prevent cAMP production.10
Although not commonly encountered, testing for GABABR antibody is justifiable in patients presenting with an unexplained neurologic disorder and risk for lung cancer. Seropositivity will direct the cancer search to small-cell carcinoma and, in the absence of autoantibodies specific for intracellular antigens, predicts responsiveness to early-initiated immunotherapy. The benefit of early diagnosis is likely greatest for patients presenting with new-onset seizures, cognitive decline, or psychiatric symptoms.
ACKNOWLEDGMENT
Josep Dalmau, MD, PhD, University of Barcelona, for confirming GABABR antibody seropositivity in patients 1 and 2; Amy Moses, Vickie Mewhorter, Jade Zbacnik, and Debby Cheung (Mayo Clinic, Neuroimmunology Laboratory), for technical support.
GLOSSARY
- CRMP-5
collapsin response-mediator protein 5
- GABABR
γ-aminobutyric acid-B receptor
- IgG
immunoglobulin G
- LE
limbic encephalitis
- SCLC
small-cell lung carcinoma
AUTHOR CONTRIBUTIONS
Dr. Jeffery: acquisition of data, analysis and interpretation of data, drafting of manuscript. Dr. Lennon: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript. Dr. Pittock: study concept and design, acquisition of data, critical revision of manuscript. Dr. Gregory: acquisition of data, critical revision of manuscript. Dr. Britton: study concept and design, critical revision of manuscript. Dr. McKeon: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
O. Jeffery reports no disclosures. V. Lennon receives royalties for technology relating to AQP4 antibodies for diagnosis of NMO, is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker, and receives research support from the National Institutes of Health (NS065829). S. Pittock is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker and receives research support from the National Institutes of Health (NS0658291), the Guthy-Jackson Charitable Foundation, and Alexion Pharmaceuticals Inc. J. Gregory and J. Britton report no disclosures. A. McKeon receives research support from the Guthy Jackson Charitable Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology 2011;76:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol 2004;56:715–719 [DOI] [PubMed] [Google Scholar]
- 4.Mundiyanapurath S, Jarius S, Probst C, Stocker W, Wildemann B, Bosel J. GABA-B-receptor antibodies in paraneoplastic brainstem encephalitis. J Neuroimmunol 2013;259:88–91 [DOI] [PubMed] [Google Scholar]
- 5.Jarius S, Steinmeyer F, Knobel A, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol 2013;256:94–96 [DOI] [PubMed] [Google Scholar]
- 6.Flanagan EP, McKeon A, Lennon VA, et al. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology 2011;76:2089–2095 [DOI] [PubMed] [Google Scholar]
- 7.Dhamija R, Renaud DL, Pittock SJ, et al. Neuronal voltage-gated potassium channel complex autoimmunity in children. Pediatr Neurol 2011;44:275–281 [DOI] [PubMed] [Google Scholar]
- 8.Titulaer M, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-N-Methyl-D-Aspartate (NMDA) receptor encephalitis: a cohort study. Lancet Neurol 2013;12:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hacohen Y, Wright S, Waters P, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry 2013;84:748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benarroch EE. GABAB receptors: structure, functions, and clinical implications. Neurology 2012;78:578–584 [DOI] [PubMed] [Google Scholar]


