SUMMARY
Background
Renal failure occurs in 5–18% of sickle cell disease (SCD) patients and is associated with early mortality. At risk SCD patients cannot be identified prior to the appearance of proteinuria and the pathobiology is not well understood. The MYH9 and APOL1 genes have been associated with risk for focal segmental glomerulosclerosis and end-stage renal disease in African Americans.
Methods
We genotyped 26 SNPs in MYH9 and 2 SNPs in APOL1 in 521 unrelated adult (18–83 years) SCD patients screened for proteinuria. Using logistic regression, SNPs were evaluated for association with proteinuria.
Results
Eight MYH9 SNPs and one APOL1 SNP were nominally associated with proteinuria. Six SNPs remained significant after multiple testing correction (p < 0.0025), and a risk haplotype was associated with proteinuria (p=0.001). Using multiple regression, association with APOL1 diminished in the presence of MYH9 SNPs. Glomerular filtration rate was negatively correlated with proteinuria (p < 0.0001), and was nominally associated with MYH9 and APOL1 in age-adjusted analyses.
Conclusion
Our data provide insight into the pathobiology of renal dysfunction in SCD, suggesting that MYH9 is more strongly associated than APOL1. These data also provide the opportunity for early identification of patients at risk and new therapeutics.
Keywords: sickle cell disease, nephropathy, genetics association, genetic modifier, proteinuria
INTRODUCTION
African Americans have an increased risk for chronic kidney disease (CKD), including HIV-associated nephropathy (Kopp and Winkler 2003), focal segmental glomerulosclerosis (FSGS) (Kitiyakara, et al 2004), and hypertensive nephrosclerosis (Toto 2004). These conditions can all lead to irreversible end-stage renal disease (ESRD). African Americans also have a disproportionate risk for developing ESRD compared with whites. The cumulative lifetime risk for ESRD for a 20 year old African American woman is 7.8%, compared to a 1.8% equivalent risk for white women (Kiberd and Clase 2002). Even after adjusting for socioeconomic and other non-genetic risk factors, the relative risk of developing ESRD is still 1.9 times higher in African Americans when compared to whites of the same age (Powe 2003). This suggests that genetic factors contribute to this disproportionate risk of developing ESRD in African Americans. Recently, using the strategy of mapping by admixture linkage disequilibrium (MALD), Kopp et al. and Kao et al. identified MYH9 as one of the genes underlying the ethnicity-driven health disparity in both FSGS and ESRD respectively (Kao, et al 2008, Kopp, et al 2008). Variation in MYH9 was estimated to account for about 70% of ESRD in non-diabetic African American patients (Bostrom and Freedman 2010). Upon further examination, however, it was suggested that the true association with ESRD was with the apolipoprotein L-1 (APOL1) gene, due to both the stronger statistical association with that gene and the lack of identification of causal functional variants in the MYH9 gene (Genovese, et al 2010a, Genovese, et al 2010b, Tzur, et al 2010). The original association with MYH9 was attributed to both the strong linkage disequilibrium (LD) between MYH9 and APOL1 and the insufficient coverage on genome wide SNP chips for the African diversity.
Sickle cell disease (SCD) comprises a group of disorders in which sickle hemoglobin (HbS) is present in red cells due to either homozygosity for the hemoglobin beta chain allele encoding the HbS form or double heterozygosity for the gene variant encoding HbS and another hemoglobin gene variant encoding a beta chain that interacts with HbS, such as HbC or HbOarab to produce clinical features. SCD nephropathy affects a minority of patients with SCD but is a major risk factor for early mortality (Platt, et al 1994). SCD nephropathy is a clinically well characterized entity that includes papillary necrosis, hyposthenuria, impaired renal acidification, proteinuria, hematuria, supranormal proximal tubular function, and renal failure (Stuart and Nagel 2004). In adults with SCD, the incidence of micro- and macro-albuminuria has been estimated to occur in 68% of patients, while macroalbuminuria alone occurs in 26% of patients (Guasch, et al 2006). Mortality due to chronic renal failure in adult SCD patients is about 10.5% (Platt, et al 1994).
Because of the strong linkage disequilibrium in the region of chromosome 22 which contains both the MYH9 and APOL1 genes, we have genotyped SNPs in both genes in order to determine if variants in either gene may be associated with risk of SCD nephrophathy.
METHODS
Data set
Five hundred twenty-one unrelated patients were enrolled as part of a multicenter study of genetic associations with clinical outcomes in SCD. Participating institutions were Duke University, University of North Carolina at Chapel Hill, East Carolina University and Emory University Sickle Cell Centers. Patients enrolled were between ages 18–83 years. Diagnoses included Hb SS (n=452), Hb Sβ0 thalassemia (n=18), Hb Sβ+ thalassemia (n=17), and Hb SC (n=34). Mean and median ages were 33.9 and 32.0 years, respectively. Almost all patients (498, or 98.81%) identified themselves as non-Hispanic African-Americans, 2 (0.40%) as Hispanic Blacks, 1 (0.20%) as a non-Hispanic Caucasian, and 3 (0.60%) as Hispanic Caucasians. Two hundred thirty subjects (44.15%) were male.
All patients provided informed consent and were enrolled for the genetic study according to Institutional Review Board-approved procedures. Blood samples were obtained by routine phlebotomy at steady state. All patients were also evaluated for proteinuria by a standard urine dipstick analysis. Individuals were categorized as having proteinuria if the dipstick analysis results were 1+ through 4+, and as not having proteinuria if the dipstick analysis demonstrated no protein or trace amounts. Because of the historical nature of the data set, it was not possible to obtain a more accurate measure of proteinuria such as collection of 24 hour urine samples. Despite there being more accurate methods of detecting proteinuria, the sensitivity and specificity of dipstick to detect microalbuminuria has been estimated to be 70% and 83%, respectively (Sarafidis, et al 2008). The prevalence of proteinuria in our data set was 26.9%, which is consistent with the 20–30% previously reported in SCD (Pham, et al 2000). Our genetic analysis used the occurrence of proteinuria as our dependent variable, as we did not have complete information regarding SCD nephropathy in our data set and proteinuria is a key component of the nephropathy disease process.
Genotyping
Twenty-six haplotype tagging SNPs in MYH9 were selected using LDSelect v1.0 (Carlson, et al 2004), based on data from the Yoruban population in the HapMap project (www.hapmap.org). In order to minimize redundancy among SNPs in high LD, we selected a single SNP to represent each haplotype block, as defined by r2 > 0.64. SNPs were also required to have a minor allele frequency > 0.05 so that there would be reasonable power to detect associations in our data set. Subsequent to our examining the MYH9 gene, it was reported that the true association with ESRD in African Americans was due to the APOL1 gene (Genovese, et al 2010a, Genovese, et al 2010b, Tzur, et al 2010). Thus, we also typed two SNPs (rs73889319 and rs71785313) in APOL1 that had been identified as being most strongly associated with focal segmental glomerulorsclerosis (Genovese, et al 2010a).
DNA was extracted from study participant blood samples by standard procedures and deposited in the DNA Bank and Tissue Repository of the Center for Human Genetics at Duke University. SNP genotyping was performed using Taqman Predesigned Genotyping Assays (Applied Biosystems, Foster City, CA). The polymerase chain reaction (PCR) was carried out on an ABI 9700 dual 384-well Geneamp PCR system (Applied Biosystems). Each well contained a total of 3ng genomic DNA. Analysis of genotype was done with an ABI Prism 7900 Sequence Detection System (Applied Biosystems).
Quality control measures applied for all genotyping assays included the genotyping of a series of blinded duplicate samples and Centre d’Etude du Polymorphism Humain (CEPH) controls. To pass quality control, all duplicate samples in an assay had to match 100%. Further, we required that 95% of the genotypes for each assay could be called with certainty to be considered for statistical analysis. The sample size for each SNP varied slightly due to differences caused by missing genotype data.
Statistical Analysis
The 26 SNPs in the MYH9 gene and 2 SNPs in the APOL1 gene were assessed for Hardy-Weinberg Equilibrium (HWE) using exact tests implemented in the Genetic Data Analysis program (Zaykin, et al 1995) prior to analysis for association with proteinuria. None of the SNPs deviated significantly from HWE, and thus all 28 SNPs were subsequently analyzed. Pairwise linkage disequilibrium measures (r2) between markers were calculated using the Graphical Overview of Linkage Disequilibrium (GOLD) software (University of Michigan, MI).
We constructed contingency tables and tests of association for both genotypes and alleles by occurrence of proteinuria using PROC FREQ (SAS System v9.1, Cary, NC). Logistic regression was performed to determine the effect of genotype on proteinuria and the effect of alleles on proteinuria using PROC LOGISTIC (SAS System v9.1). Generalized linear models were used to assess the effect of genotype on glomerular filtration rate using PROC GLM (SAS System v9.1). Haplotype associations were explored using functions from the haplo.stats program implemented in R, which accounts for linkage phase ambiguity in unrelated subjects (Lake, et al 2003, Schaid, et al 2002). Haplotype frequencies were estimated using the haplo.em function, and regression analysis to test for association between haplotypes and proteinuria was generated using the haplo.glm function.
We corrected for multiple testing using the method described by Li and Ji (Li and Ji 2005), which constructs an effective number of tests (Meff) based on the LD structure of the SNPs. We then applied the Bonferroni method to generate the corrected threshold for significance testing using the effective number of tests. The corrected significance level for association testing in our analysis was (0.05/Meff = 0.0025).
RESULTS
Of 521 SCD patients studied, 140 had proteinuria, while 381 did not have proteinuria. On average, subjects with proteinuria were 6 years older than subjects without proteinuria (p < 0.0001), indicating that older patients were more likely to have proteinuria, as has been noted in other studies (Guasch, et al 2006). In our data set, the odds of having proteinuria increased by 1.04 (4.2%) for every one year increase in age (starting at age 18). Because of this strong association between age and proteinuria, models examining the effect of genotype on proteinuria were adjusted for age.
Analysis of our data set using the genotype test resulted in identification of 9 SNPs that were nominally associated with proteinuria (p < 0.05); 6 of these SNPs met the corrected multiple comparison threshold (p < 0.0025) (Fig. 1). One SNP was in the APOL1 gene (rs73885319, p = 0.0022) and the remaining five SNPs were in the MYH9 gene (rs11912763, p = 0.0003; rs16996648, p = 0.0025; rs1557529, p = 0.0019; rs8141189, p = 0. 001; rs16996672, p = 0.0001; Table 1). The APOL1 SNP which met the multiple comparison threshold, is a missense mutation (S342G) which is in strong LD with another missense mutation (I384M) that was not typed in this data set. Those two SNPs have been previously referred to as the G1 tag that was associated with FSGS (Genovese, et al 2010a). Among the MYH9 SNPs which met the multiple comparison threshold, rs5750248 and rs11912763 were identified previously as representing the S-1 and F-1 risk haplotypes, respectively, that were very strongly associated with kidney disease among African Americans and Hispanics (Behar, et al 2010, Nelson, et al 2010). Despite our attempt to reduce LD in the MYH9 gene by selecting haplotype tagging SNPs from the Yoruba population, there was some evidence of LD in our own data set. One of the most significant SNPs, rs11912763, was in high LD (r2 =0.72) with rs16996648 and in moderate LD (r2 =0.57) with rs5756152. These correlations are reflected in the correlation among the association results, whereby both rs11912763 and rs16996648 were significantly associated with proteinuria, and rs5756152 approached significance.
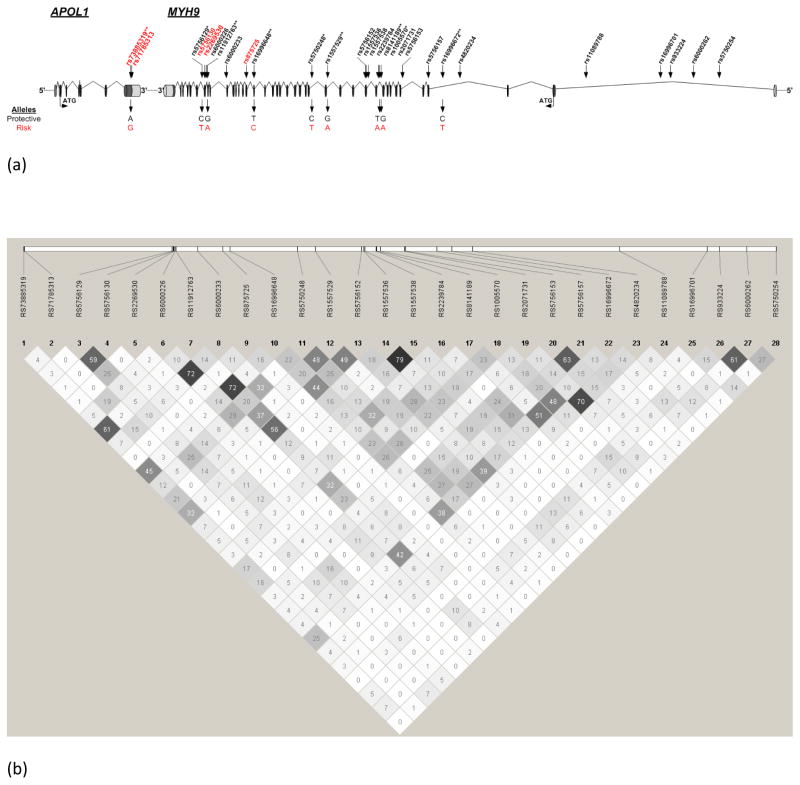
Figure 1.
(a) Structure of the APOL1 and MYH9 genes. Genotyped SNPs are shown in different colors: black – non-coding; red – coding. SNPs defining the MYH9 risk haplotype, along with their risk alleles, are indicated below the gene structure diagram. * = Nominally significant at p<0.05; ** = Significant at threshold adjusted for multiple testing of p<0.0025.
(b) LD map for the 28 SNPs typed shown. Darker squares represent stronger LD.
Table 1.
Significant APOL1 and MYH9 SNPs
| Gene | SNP | Genotype | Frequency of proteinuria | Genotype comparison | Odds Ratio | p-value for comparison | Global p-value |
|---|---|---|---|---|---|---|---|
| APOL1 | rs73885319† | AA | 0.220 | AA-GG | 0.273 | 0.0006 | 0.0022 |
| AG | 0.312 | AG-GG | 0.378 | 0.0145 | |||
| GG | 0.457 | AA-AG | 0.722 | 0.153 | |||
| MYH9 | rs5756129 | CC | 0.294 | CC-TT | 0.815 | 0.714 | 0.0247 |
| CT | 0.187 | CT-TT | 0.511 | 0.007 | |||
| TT | 0.309 | CC-CT | 1.597 | 0.422 | |||
| MYH9 | rs11912763† | AA | 0.5 | AA-GG | 4.604 | 0.0001 | 0.0003 |
| AG | 0.315 | AG-GG | 1.585 | 0.045 | |||
| GG | 0.221 | AA-AG | 2.905 | 0.011 | |||
| MYH9 | rs16996648† | CC | 0.435 | CC-TT | 3.116 | 0.0009 | 0.0025 |
| CT | 0.304 | CT-TT | 1.528 | 0.057 | |||
| TT | 0.218 | CC-CT | 2.039 | 0.042 | |||
| MYH9 | rs5750248 | CC | 0.167 | CC-TT | 0.359 | 0.002 | 0.0042 |
| CT | 0.255 | CT-TT | 0.61 | 0.032 | |||
| TT | 0.344 | CC-CT | 0.589 | 0.088 | |||
| MYH9 | rs1557529† | AA | 0.4 | AA-GG | 2.889 | 0.0004 | 0.0019 |
| AG | 0.276 | AG-GG | 1.49 | 0.092 | |||
| GG | 0.205 | AA-AG | 1.939 | 0.0184 | |||
| MYH9 | rs8141189† | AA | 0.444 | AA-TT | 3.351 | 0.0005 | 0.001 |
| AT | 0.309 | AT-TT | 1.698 | 0.018 | |||
| TT | 0.208 | AA-AT | 1.973 | 0.054 | |||
| MYH9 | rs1005570 | AA | 0.354 | AA-GG | 2.611 | 0.001 | 0.0032 |
| AG | 0.301 | AG-GG | 1.926 | 0.008 | |||
| GG | 0.184 | AA-AG | 1.356 | 0.25 | |||
| MYH9 | rs16996672† | CC | 0.198 | CC-TT | 0.237 | <0.001 | 0.0001 |
| CT | 0.308 | CT-TT | 0.438 | 0.021 | |||
| TT | 0.476 | CC-CT | 0.541 | 0.006 |
Significant at the multiple testing level (p=0.0025)
Among the six SNPs that met the multiple testing correction for significant association with the occurrence of proteinuria, the frequency of proteinuria among the individuals who were homozygous for the risk genotype ranged from 40% to 50%, as compared to the frequency of proteinuria among the individuals who did not have that genotype which ranged from 20% to 30%. Odds ratios for specific genotype comparisons are shown in Table 1.
In order to reduce the dimensionality of the SNPs in the MYH9 gene, we performed haplotype association analysis, using the 8 SNPs in the MYH9 gene that were nominally significant. This analysis revealed that the risk haplotype (including the risk allele for all 8 SNPs) was the second most prevalent haplotype for MYH9 in our study population. About 15% of the subjects in our data set had this haplotype, which was also significantly associated with proteinuria (p = 0.001), such that those with the risk haplotype were about 2.7 times as likely to have proteinuria as those with the most frequent haplotype; which had a prevalence of 20%. Those with the risk haplotype were also 1.6 times more likely to have proteinuria as those with rarer haplotypes, which were grouped together for the purpose of analysis. Analysis by haplotype was also adjusted for age, since age was significantly associated with proteinuria (p = 0.00001).
Given reports that SCD patients with a more severe form of the condition (Hb SS and Hb Sβ0) have a higher prevalence of proteinuria than other SCD forms (Guasch et al, 2006), we decided to perform a second analysis of only these individuals and repeat the tests for association. Using the genotype test, the results were similar. Eight SNPs were nominally significant (p < 0.05), with 4 reaching the corrected multiple comparison threshold (p < 0.0025; rs73885319(APOL1), rs11912763 (MYH9), rs8141189(MYH9) and rs16996672(MYH9)). Hb SS and Hb Sβ0 individuals make up the majority (90.38%) of our data set, which likely explains the similarity to the results in the overall data set.
Because of the known relationship between proteinuria and glomerular filtration rate (GFR), we also examined this relationship in our data set and found that GFR was inversely correlated with proteinuria (r = −0.25, p < 0.0001), such that for every one unit decrease in GFR, the odds of having proteinuria increases (OR=1.03). Further, we examined whether or not the APOL1 and MYH9 SNPs were associated with GFR, but we did not detect any associations in unadjusted analyses. However, when we controlled for age, we observed nominal associations with rs73885319 (APOL1, p = 0.01), rs11912763 (MYH9, p = 0.01), rs4820234 (MYH9, p = 0.05), and rs933224 (MYH9, p = 0.004). When we performed haplotype analysis for the MYH9 gene (as defined above) and controlled for age, we did not detect a significant association with GFR, although there was a trend (p=0.08).
Given that the previous work in non-SCD populations were providing stronger evidence for association between ESRD and APOL1 rather than MYH9 (Genovese, et al 2010a, Genovese, et al 2010b, Tzur, et al 2010), we decided to do multiple regression analyses to determine if we could sort out this question in our own data set. Thus, we repeated the analyses where we predicted proteinuria as a function of each of the eight nominally significant MYH9 SNPs (Table 1), while controlling both for age and rs73885319 (the APOL1 SNP significant with multiple testing correction in our data set). Unlike in the previous reports (Genovese, et al 2010a, Genovese, et al 2010b, Tzur, et al 2010), in our data set, we observed that three of the MYH9 SNPs remained nominally significant predictors of proteinuria, even when rs73885319 was a co-variate. In these multiple regression analyses, the most significant association in the MYH9 gene remained with rs16996672 (p = 0.007). Furthermore, in these analyses, the evidence for association with APOL1 rs73885319 dropped, such that it only remained nominally significant (p = 0.01) for the analysis with a single MYH9 marker, rs5756129. In all other analyses that included any of the other seven MYH9 SNPs (Table 1), the evidence for association between rs73885319 disappeared (data not shown). Thus, in the SCD population, it appears that the evidence for association is stronger for the MYH9 gene than it is for the APOL1 gene.
DISCUSSION
Nephropathy is a major risk factor for early mortality in SCD (Platt, et al 1994). Early detection of those at risk for this outcome and understanding the mechanism of disease are both required in order to develop therapy that can reduce morbidity and mortality. Thus identification of SNPs in MYH9 and APOL1 that are significantly associated with proteinuria is quite intriguing. Although originally the association with ESRD had been detected with the MYH9 gene in non-SCD populations (Kao, et al 2008, Kopp, et al 2008), subsequent work has suggested that this association is likely due to a genetic “hitchhiking” effect with the APOL1 gene, due to the strong LD between these two genes on chromosome 22 and the selective pressures believed to have perpetuated certain genetic variants of APOL1 (Genovese, et al 2010a, Genovese, et al 2010b, Tzur, et al 2010). Our analysis in a SCD study population provides stronger evidence for association with the MYH9 gene, in contrast to these studies. The discrepancies between our results and the previous ones highlight the challenges faced when attempting to identify genes associated with complex disease phenotypes. Until variants are identified which clearly contribute in some functional manner to the disease phenotype, it is difficult to discern which SNP or variant, or in this case even which gene, is the causal culprit.
APOL1 is associated with resistance to Trypanosoma brucei, a common African parasite, and it is believed that this role in host immunity has contributed to the selective pressures on that gene and the surrounding region. Despite this intriguing population genetics story, it remains to be seen how APOL1 directly contributes to kidney disease. Based on our current analyses, MYH9 is a stronger candidate for association with SCD nephropathy and although causal variants and mechanisms remain to be elucidated, MYH9 is a stronger biological candidate, as well. MYH9 encodes the heavy chain of non-muscle myosin IIA and in combination with actin, talin, α-actin-4, and vinculin, make up the cytoskeleton of the podocyte - the cells lining the visceral surface of Bowman’s capsule in the kidney- foot process (Drenckhahn and Franke 1988). This cytoskeleton is joined to the slit membrane-“filtration pore” proteins by adaptor proteins such as zonula occludens-1 (ZO-1), and the proper functioning of the slit membrane, and the ability to keep larger molecules like proteins from being filtered depends on cytoskeletal integrity. Therefore, our identification of the MYH9 gene as significantly associated with proteinuria supports previous observations suggesting that development of proteinuria in SCD may involve the podocyte cytoskeleton. Aslan et al. showed that nitration of three tyrosine residues (Tyr91, Tyr198, Tyr240) of actin in the podocyte cytoskeleton modified actin assembly, causing alterations in cytoskeletal polymerization (Aslan, et al 2003). This nitration is due to increased expression of inducible nitric-oxide synthase (NOS2) caused by chronic ischemia-reperfusion injury in SCD (Aslan, et al 2003, Vinas, et al 2007). The alteration in cytoskeletal polymerization may cause a redistribution of the tight junction protein ZO-1 and could lead to proteinuria (Macconi, et al 2000). However, once podocyte damage has occurred, other mechanisms, possibly involving angiotensin II (Durvasula, et al 2004) and TGF-β (Schiffer, et al 2001), could contribute to further damage and lead to apoptosis of podocytes. Thus, there seems to be a continuum of glomerular damage, as have been suggested by Guasch et al (Guasch, et al 1997).
Although our analysis showed a strong association between MHY9 and APOL1 (rs73885319) with proteinuria, the association with GFR was not as strong. Previous studies have suggested that GFR is not a good indicator of early glomerular damage, and simple measurement of GFR may fail to indicate significant early loss of glomerular function (Guasch et al, 1996). However, both micro- and macroalbuminuria have been found to be quite sensitive markers of glomerular function (Guasch, et al 1996). Thus, our finding that MYH9 and APOL1 genotype were not as strongly associated with GFR is consistent with previous data. We therefore hypothesize that the MYH9 gene is likely to be associated with early damage to the glomerulus, while polymorphisms of other genes, such as bone morphogenetic protein receptor 1B (BMPR1B), found to be associated with GFR in SCD by Nolan and colleagues (Nolan, et al 2007), may be implicated in later stages of kidney damage. Although we tested for association with the two most significant BMPR1B SNPs from the Nolan findings (rs2240036 and rs4145993), neither were associated with proteinuria or GFR in age-adjusted analyses, suggesting that this gene may not be vital in the development of early glomerular damage but may contribute more generally to renal damage in SCD. We did observe nominal evidence for association with a different BMPR1B SNP (rs1434536) and proteinuria in our data set (p=0.004, age-adjusted). To further investigate a possible connection between BMPR1B and MYH9, we performed regression analyses including BMPR1B SNPs (rs1434536, rs2240036 and rs4145993) and the MYH9 haplotype in the models and controlled for age. In all these analyses, MYH9 risk haplotype remained a significant predictor of proteinuria and was only borderline associated with GFR. None of the BMPR1B SNPs were associated with proteinuria or GFR when the MYH9 haplotype was included in the model, suggesting that MYH9 is likely the more important contributor to these processes, at least in our data set.
Early intervention in patients who are likely to develop SCD nephropathy is very important, because by 15 years of age, enough damage may have been done to the kidneys to ensure progression to irreversible hyposthenuria (Saborio and Scheinman 1999) and eventual renal failure. Thus, identification of a risk haplotype can potentially become part of a screening tool to identify SCD patients who are at higher than average risk of developing SCD nephropathy. The parameters GFR and albuminuria have been found to be independent predictors of progression to ESRD and, when combined, show a better predictive value than they do individually (Hallan, et al 2009). This suggests that both the MYH9 and BMPR1B genes may interact to cause kidney damage which progresses to ESRD. It is tempting to hypothesize that these genes may be potentially useful in the creation of a prognostic tool to identify SCD nephropathy patients who are at risk of progressing to ESRD, so that appropriate prevention strategies can be implemented. As yet, however, we do not know what therapies are likely to be efficacious. Specific therapies aimed at reducing damage to podocyte cytoskeletal structures could potentially become new therapies for SCD nephropathy. Angiotensin converting enzyme (ACE) inhibitors, already used in a small study of SCD patients, have been found to bring about proper distribution of ZO-1 proteins and reduce proteinuria (Falk, et al 1992, Macconi, et al 2000). Other potential therapeutic agents might include antagonists of TGF-β and agents that block maladaptive nitration of podocyte cytoskeleton proteins such as actin.
Kopp et al. (Kopp, et al 2008) identified 9 SNPs that were strongly associated with focal segmental glomerulosclerosis, and also found a very prevalent risk haplotype in their data set. Of the SNPs we genotyped, five coincided with those genotyped by Kopp et al., and two among these were significant in both data sets, despite the fact that our dataset and analyses differ in several critical respects from those of the 2008 report. These differences include patient population, the type of renal dysfunction studied, and how multiple testing adjustments were made. The study by Kopp and colleagues was a case/control study in which cases were drawn from a population of European Americans and African Americans who had biopsy confirmed idiopathic or HIV associated focal segmental glomerulosclerosis, while controls were drawn from the same ethnic groups and had either normal or supernormal renal function. Although Kopp et al. applied the Bonferroni correction for multiple testing, the LD pattern among the SNPs they genotyped was not considered in their analysis; consequently, the SNPs making up the risk haplotype were mostly in the same LD bin, and therefore mostly redundant. In contrast, we applied a fairly stringent threshold for multiple testing, taking into consideration the LD pattern of the SNPs examined in order to determine an effective number of tests before applying the Bonferroni correction, and we thereby found that several SNP associations withstood this stringent correction threshold.
Perhaps the largest limitation of our study was the use of a crude measure of proteinuria (dipstick analysis). However, it is important to note that our approach would have potentially misclassified individuals with microalbuminuria as not having proteinuria and this would have weakened, not strengthened, any associations we have currently presented. Thus, despite there being more precise measures of kidney function, we do not believe that our phenotyping method resulted in a false positive finding. However, this will need to be discerned through prospective studies that have the ability to use more precise measures of kidney function. For example, future investigations should consider measuring microalbuminuria as part of screening for early renal dysfunction, since microalbuminuria has been shown to characterize the early stages of SCD nephropathy in both children and adults (Becton, et al 2010, Guasch, et al 2006). Another future avenue for investigation is to determine whether or not MYH9 variation can help explain the increased prevalence of ESRD among African Americans with sickle cell trait (Derebail, et al 2010)
In summary, our study brings us closer to understanding the complex gene/gene and gene/environment interactions that affect clinical outcomes of SCD. Further studies with independent data sets are now needed to confirm this association, to identify more of the genes involved, and to test new therapeutic approaches to prevent or treat SCD nephropathy.
Acknowledgments
Funding: This work was funded in part by RO1 HL079915 from NHLBI, NIH. ECO received support from R90/T90 HG004150.
This work was funded in part by RO1 HL079915 from the National Heart, Lung and Blood Institute, USA. ECO received support from R90/T90 HG004150.
AAK designed the research study, contributed essential reagents, and wrote the paper; ECO performed the research and wrote the paper; MEG analysed the data and wrote the paper; KS performed the research and wrote the paper; LMD performed the research and contributed essential reagents; JCJ performed the research; EPO and JRE designed the study and contributed essential reagents; MJT designed the research study, contributed essential reagents, and wrote the paper.
Footnotes
Disclosures: None.
References
- Aslan M, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- Becton LJ, et al. Prevalence and clinical correlates of microalbuminuria in children with sickle cell disease. Pediatr Nephrol. 2010;25:1505–1511. doi: 10.1007/s00467-010-1536-8. [DOI] [PubMed] [Google Scholar]
- Behar DM, et al. African ancestry allelic variation at the MYH9 gene contributes to increased susceptibility to non-diabetic end-stage kidney disease in Hispanic Americans. Hum Mol Genet. 2010;19:1816–1827. doi: 10.1093/hmg/ddq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clin J Am Soc Nephrol. 2010;5:1107–1113. doi: 10.2215/CJN.08721209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CS, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. American Journal of Human Genetics. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derebail VK, et al. High prevalence of sickle cell trait in African Americans with ESRD. J Am Soc Nephrol. 2010;21:413–417. doi: 10.1681/ASN.2009070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–682. [PubMed] [Google Scholar]
- Durvasula RV, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Falk RJ, et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326:910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010a;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, et al. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int. 2010b;78:698–704. doi: 10.1038/ki.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch A, et al. Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int. 1996;49:786–791. doi: 10.1038/ki.1996.109. [DOI] [PubMed] [Google Scholar]
- Guasch A, et al. Sickle cell anemia causes a distinct pattern of glomerular dysfunction. Kidney Int. 1997;51:826–833. doi: 10.1038/ki.1997.116. [DOI] [PubMed] [Google Scholar]
- Guasch A, et al. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17:2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- Hallan SI, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WH, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nature Genetics. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. J Am Soc Nephrol. 2002;13:1635–1644. doi: 10.1097/01.asn.0000014251.87778.01. [DOI] [PubMed] [Google Scholar]
- Kitiyakara C, et al. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–825. [PubMed] [Google Scholar]
- Kopp JB, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nature Genetics. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JB, Winkler C. HIV-associated nephropathy in African Americans. Kidney Int Suppl. 2003:S43–S49. doi: 10.1046/j.1523-1755.63.s83.10.x. [DOI] [PubMed] [Google Scholar]
- Lake SL, et al. Estimation and tests of haplotype-environment interaction when linkage phase is ambiguous. Hum Hered. 2003;55:56–65. doi: 10.1159/000071811. [DOI] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Macconi D, et al. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000;11:477–489. doi: 10.1681/ASN.V113477. [DOI] [PubMed] [Google Scholar]
- Nelson GW, et al. Dense mapping of MYH9 localizes the strongest kidney disease associations to the region of introns 13 to 15. Hum Mol Genet. 2010;19:1805–1815. doi: 10.1093/hmg/ddq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan VG, et al. Estimated glomerular filtration rate in sickle cell anemia is associated with polymorphisms of bone morphogenetic protein receptor 1B. American Journal of Hematology. 2007;82:179–184. doi: 10.1002/ajh.20800. [DOI] [PubMed] [Google Scholar]
- Pham PT, et al. Renal abnormalities in sickle cell disease. Kidney Int. 2000;57:1–8. doi: 10.1046/j.1523-1755.2000.00806.x. [DOI] [PubMed] [Google Scholar]
- Platt OS, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Powe NR. To have and have not: Health and health care disparities in chronic kidney disease. Kidney Int. 2003;64:763–772. doi: 10.1046/j.1523-1755.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Saborio P, Scheinman JI. Sickle cell nephropathy. J Am Soc Nephrol. 1999;10:187–192. doi: 10.1681/ASN.V101187. [DOI] [PubMed] [Google Scholar]
- Sarafidis PA, et al. A comparative evaluation of various methods for microalbuminuria screening. Am J Nephrol. 2008;28:324–329. doi: 10.1159/000111825. [DOI] [PubMed] [Google Scholar]
- Schaid DJ, et al. Score tests for association between traits and haplotypes when linkage phase is ambiguous. American Journal of Human Genetics. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- Toto RD. Proteinuria and hypertensive nephrosclerosis in African Americans. Kidney Int Suppl. 2004:S102–S104. doi: 10.1111/j.1523-1755.2004.09224.x. [DOI] [PubMed] [Google Scholar]
- Tzur S, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas JL, et al. Role of peroxynitrite on cytoskeleton alterations and apoptosis in renal ischemia-reperfusion. Am J Physiol Renal Physiol. 2007;292:F1673–F1680. doi: 10.1152/ajprenal.00356.2006. [DOI] [PubMed] [Google Scholar]
- Zaykin D, et al. Exact tests for association between alleles at arbitrary numbers of loci. Genetica. 1995;96:169–178. doi: 10.1007/BF01441162. [DOI] [PubMed] [Google Scholar]