Abstract
Until recently, the transport of folates into cells and across epithelia has been interpreted primarily within the context of two transporters with high affinity and specificity for folates, the reduced folate carrier and the folate receptors. However, there were discrepancies between the properties of these transporters and characteristics of folate transport in many tissues, most notably the intestinal absorption of folates, in terms of pH dependency and substrate specificity. With the recent cloning of the proton-coupled folate transporter (PCFT) and the demonstration that this transporter is mutated in hereditary folate malabsorption, an autosomal recessive disorder, the molecular basis for this low-pH transport activity is now understood. This review focuses on the properties of PCFT and briefly addresses the two other folate-specific transporters along with other facilitative and ATP-binding cassette (ABC) transporters with folate transport activities. The role of these transporters in the vectorial transport of folates across epithelia is considered.
Keywords: proton-coupled folate transporter (PCFT) (SLC46A1), reduced folate carrier (RFC) (SLC19A1), hereditary folate malabsorption (HFM), cerebral folate deficiency (CFD), intestinal folate absorption, heme carrier protein-1 (HCP1)
INTRODUCTION
Historically, the world of folate transport research over the past five decades has been pursued from two different avenues that engaged different communities of researchers. One group approached this area of investigation from a nutritional perspective—intestinal and renal transport mechanisms as they relate to folate physiology and folate deficiency disorders, engaging the gastroenterology and hematology communities along with epidemiologists who study the role of more subtle perturbations of folate sufficiency in human health. The other group of researchers was from the oncology community and focused on antifolate transport from a pharmacological perspective. Until very recently, the emphasis was on transport mediated by the reduced folate carrier (RFC) and, to a lesser extent, the folate receptors—the only known folate-specific transporters. Clearly, RFC was recognized as the mechanism by which folates are delivered to cells from the systemic circulation, and there was compelling evidence that RFC-mediated transport of methotrexate (MTX), the major antifolate in clinical use, was a critical determinant of the activity of this agent and an important element in resistance. On the other hand, the characteristics of folate intestinal absorption and transport into a variety of normal tissues had features (i.e., optimal pH and substrate specificity) that clearly distinguished this process from transport mediated by RFC. Yet, the prevailing paradigm attributed this transport to RFC without any significant effort to explore the possibility that there might be another route. In the end, it was studies on the mechanism of transport of a new-generation antifolate, pemetrexed, that provided definitive evidence for a second carrier-mediated folate transport pathway, and this led to the cloning of the proton-coupled folate transporter (PCFT), bringing together, at long last, these diverse communities of researchers. The identification of loss-of-function PCFT mutations in human subjects with hereditary folate malabsorption (HFM) provided an explanation for the molecular basis for this disorder and established this transporter’s critical role in intestinal folate absorption. This review focuses on PCFT, the major new element in folate physiology that plays a major role in folate homeostasis and nutrition in man. RFC, the subject of recent reviews (81, 155), is considered briefly along with folate receptor (FR)-mediated endocytosis, which is emerging in the pharmacological arena as a major new vehicle for the targeted selective delivery of cytotoxics and other agents in the treatment of a variety of malignant and, potentially, other diseases—also the subject of recent reviews (35, 69, 145). Other facilitative carriers in the superfamily of solute carriers (SLC)21 and SLC22 families are considered along with ATP-binding cassette (ABC) exporters with folate transport activities. Finally, the location of these transporters in cells is considered within the context of their roles in the vectorial transport of folates across epithelia.
OVERVIEW: THE FOLATE TRANSPORTERS
The Reduced Folate Carrier (SLC19A1)
The RFC (SLC19A1) is the major transporter that delivers folates to systemic tissues at their ambient neutral pH. The human RFC gene is located on chromosome 21q22.3; five exons encode a protein consisting of 591 amino acids. There is one glycosylation site (N58) that is not required for function (143). The transporter is ubiquitously expressed in human and mouse tissues (72, 141). RFC is an organic anion antiporter that utilizes the high transmembrane organic phosphate gradient to achieve uphill folate transport into cells (152, 155). Influx mediated by this carrier is optimum at pH 7.4; as the pH is decreased, activity is diminished so that at usual constituent levels of expression, there is little activity below pH 6.5 (151). However, with high-level expression, activity can be detected at low pH, but there is little change in its affinities for folate substrates; the decrease in function is due to a decrease in Vmax (136). The influx Km for MTX and the reduced folates is ~2–7 μM; its affinity for other antifolates can be at least an order of magnitude greater (35). RFC’s very low affinity for folic acid (1/100th that for MTX) and its very high affinity for PT523 and related antifolates (>tenfold that of MTX) distinguish it from PCFT, which has the opposite specificity for these compounds and a low pH optimum (136). There are two other members of the SLC19 family (–A2 and –A3) that, despite their 40% homology, transport thiamine and not folates (31, 99). However, their thiamine phosphate derivates formed within cells are substrates for RFC (150, 152, 155). The topological structure of RFC has been determined by the substituted cysteine accessibility method (SCAM) and epitope tagging confirming that 12 transmembrane domains (TMDs) were divided in half by a large intracellular loop with N- and C- termini directed into the cytoplasm (13, 30, 73). Human RFC exists as a homo-oligomer; however, each monomer functions as an independent transport unit and is fully active (46, 47). The RFC regulatory region and multiple 5′ upstream transcripts in a variety of tissues have been described in detail (72, 155). RFC is required for embryonic development. Inactivation of both alleles is embryonic lethal in the mouse, although with sufficient folate supplementation during gestation, live births are possible. However, surviving pups die within two weeks due to failure of all hematopoietic tissues (159). The expression of folate transporters is sensitive to the availability of dietary folates. When mice and rats are subjected to a low-folate diet, total RFC mRNA expression and protein are increased in the small intestine (72, 107); the specific RFC transcripts that are associated with these regulatory changes in intestine and kidney have been characterized (72, 81, 155).
High-Affinity Folate Receptors
Three folate receptors (FRs) are expressed at the cell surface, anchored in the cell membrane by a glycosylphosphoinositol (GPI) domain (28). FRα is expressed on the membrane of epithelial tissues, in particular the placenta, the apical brush-border membrane of proximal renal tubular cells, retinal pigment epithelium, and the choroid plexus (56). In general, expression is increased in epithelial cancers (92). FRβ is expressed in hematopoietic tissues (e.g., spleen, thymus, and CD34+ monocytes) and, in particular, in hematopoietic malignancies such as acute and chronic myelogenous leukemia (104, 135). It is also expressed on activated macrophages and tumor-infiltrating macrophages (96, 144). FRδ is expressed at high levels on natural and TGFβ-induced regulatory T cells (146). FRγ is a secreted protein (117). FRα and FRβ have Kb’s for their preferred substrates of 1–10 nM and transport folates via a receptor-mediated endocytosis (56). In this process, folates bind to FRs at the cell membrane, which then invaginate and bud off to form vesicles that circulate in the endosomal compartment, where they acidify. Folates are then released from the receptors and exit the intact vesicle presumably by a process that operates optimally at low pH.
FRβ-null mice have no phenotype; disruption of both FRα alleles is embryonic lethal at about the time of neural tube closure (94). Partial folate supplementation, before and during gestation, results in fetuses with a variety of developmental defects (119). However, with sufficient gestational supplementation, normal pups can be delivered that do not require further folate supplementation. These animals are fertile and have no phenotype aside from low blood folate due to inefficient renal folate retention (see The Kidney and Folate Transport section below) (119). Hence, FRα-essential functions are restricted to embryonic and fetal development and transport across specific epithelia, consistent with its low level of expression in normal tissues except the proximal renal tubule and the choroid plexus (see Folate Transport and the Placenta section below). Despite its high expression in the placenta, FRα is not essential for folate transport across this organ since, as indicated above, FRα−/− mice are fertile and their progeny viable (10, 74).
The Proton-Coupled Folate Transporter
The cloning of PCFT (SLC46A1) emerged from studies directed to a new-generation antifolate, pemetrexed; in particular, studies that examined the role of membrane transport in resistance to this agent (154). The development of pemetrexed and its transport properties are the subject of a recent review (17). It had been long established that when human and murine cancer cell lines are subjected to MTX-selective pressure, resistance develops due to impaired RFC-mediated transport as a result of low expression or loss-of-function mutations (153). However, when these MTX-resistant cell lines were treated with pemetrexed, they retained full sensitivity to this agent (18, 154). When transport as a function of pH was assessed in wild-type Hela cells, there was high activity at low pH, decreasing to about one-fifth the activity over the neutral pH range. In an MTX-resistant Hela cell line (R5), in which there was a genomic deletion of RFC, the low pH activity was fully preserved, with substantial transport activity for pemetrexed retained at pH 7.4 (154). Hence, there was clear evidence for a transport process genetically distinct from RFC, optimal at low pH, but with residual activity at neutral pH. Further studies demonstrated that this low-pH transporter had all the properties of a carrier-mediated process with a high affinity for folic acid, reduced folates, MTX, and the highest affinity for pemetrexed at low pH (136, 154). When the Hela R5 line was subjected to further MTX-selective pressure, a clone, R1, was identified, which was now resistant to pemetrexed and had lost the low-pH transport activity along with activity at neutral pH (149). With the definitive evidence for a second folate transport pathway, these two cell lines were employed to clone this transporter. Candidate proteins were identified using a data-mining approach and the Ensembl database, and with the search parameter of Distant Homology encompassing the three SLC19 family members as query. These proteins were then screened in an attempt to identify a transporter in which the mRNA was highly expressed in Hela R5 cells, with the low-pH activity, and absent in the Hela R1 line that lacked the low-pH activity. Using this strategy a human transporter was identified that met these criteria and had all the functional properties of the low-pH activities characteristic of transport in intestinal cells, normal and tumor tissues, and cell lines. It was named the proton-coupled folate transporter (PCFT) (97). Human PCFT is located on chromosome 17q11.2. It consists of five exons encoding a protein of 459 amino acid residues. Human PCFT shares 91% similarity and 87% identity to the mouse and rat PCFT (98). Two Asn sites (N58, N68) are glycosylated but are not required for protein stability, trafficking, or function (130).
Expression
Human PCFT mRNA is highly expressed in kidney, liver, placenta, and spleen, and to a lesser extent in brain, testis, and lung. In the intestinal tract, it is highly expressed in human duodenum and jejunum (97). This was also documented in endoscopic biopsies of the duodenum with much lower levels of human PCFT expression in terminal ileum and colon biopsies (131). Mouse PCFT mRNA is highly expressed in kidney, liver, and to a lesser, but still appreciable, extent in brain, skin, lung, stomach, and testis tissues (98). Mouse PCFT message is highly expressed in both duodenum and proximal jejunum; it is barely detectable in the distal small intestine and colon (98). Murine PCFT protein is expressed on the apical brush-border membrane of proximal jejunum and duodenum (98, 116, 131). PCFT targets to the apical membrane of polarized MDCK canine kidney cells (123). Caco-2 cells express high levels of PCFT mRNA and have very high levels of low-pH folate transport activity (97). Because Caco-2 cells form electrically tight monolayers in culture, this has been utilized as a model for the assessment of mucosal→serosal vectorial transport (42). Both sh- and si-RNA constructs directed to PCFT were used to abolish ~80% of the constitutive low-pH folate transport activity in Caco-2 cells. Hence, PCFT mediates the majority of transport in these cells (97). Vectorial mucosal (pH 5.5) to serosal (pH 7.4) folic acid and MTX transport has been demonstrated in PCFT-transfected MDCKII cells in monolayer culture (131).
pH dependency and substrate specificity
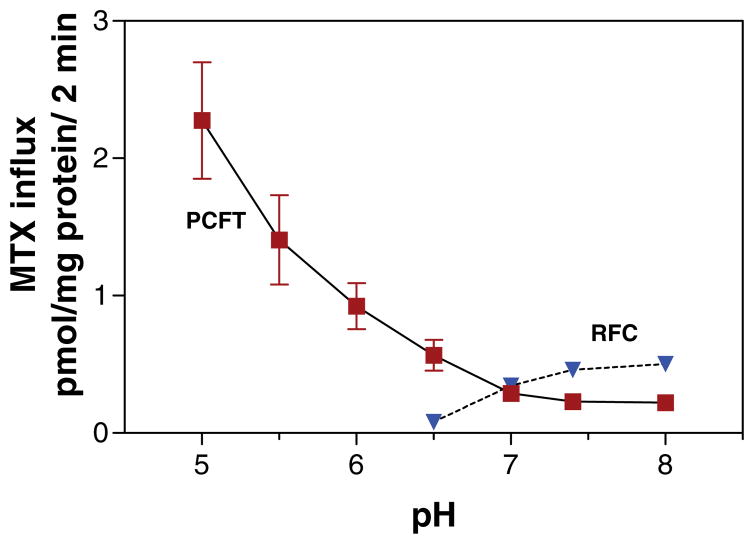
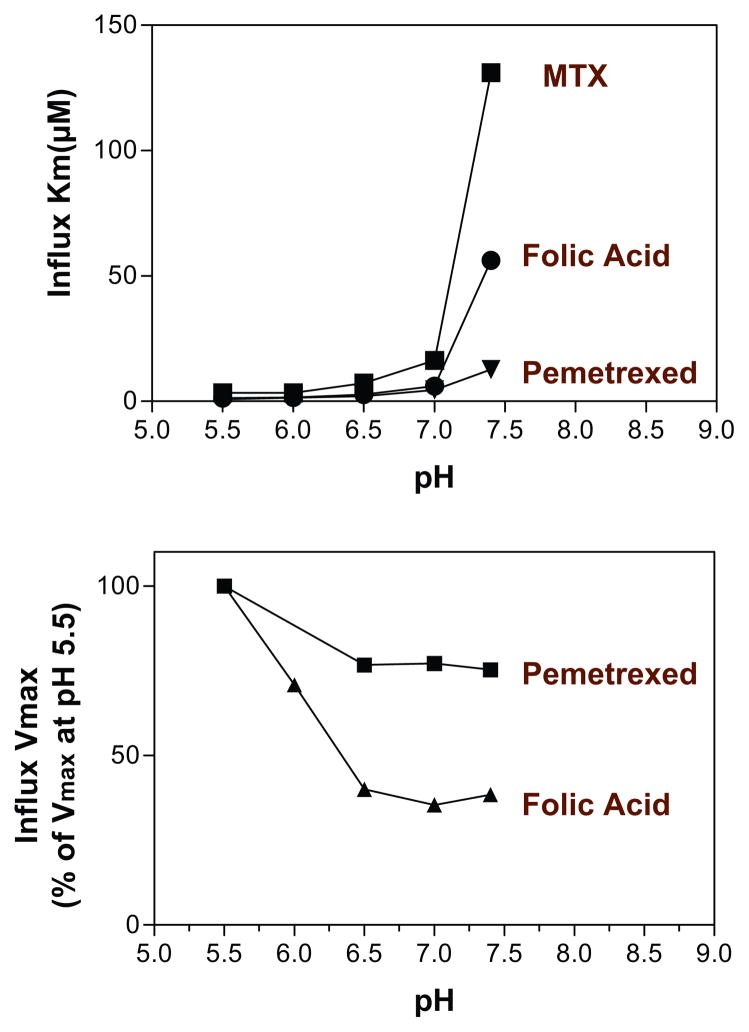
Figure 1 (see color insert) illustrates the pH profile of transport mediated by PCFT and RFC in Hela cells. PCFT-mediated transport markedly decreases as the pH is increased; the RFC-mediated component is optimal at pH 7.4 and negligible below pH 6.5. The top panel of Figure 2 (see color insert) illustrates the kinetic basis for these changes in PCFT-mediated transport for three substrates. The increases in influx Km for folic acid and pemetrexed are modest to pH 7.0; the increase for MTX is greater. However, there are marked increases when the pH is further increased to 7.4 (MTX > folic acid > pemetrexed). The bottom panel of Figure 2 shows the much greater decrease in the influx Vmax for folic acid relative to pemetrexed as the pH is increased to 6.5, following which this parameter is stable as the pH is increased to 7.4. Hence, loss of function with increasing pH is due to an increase in influx Km and a decrease in influx Vmax, the least prominent for pemetrexed, consistent with the preservation of the pharmacological activity of this agent in the absence of RFC (17, 97, 154, 158).
Figure 1.
The pH profile of reduced folate carrier (RFC)- and proton-coupled folate transporter (PCFT)-mediated methotrexate (MTX) influx. These data were obtained from wild-type Hela cells and Hela cells in which RFC was deleted from the genome. From Reference 151.
Figure 2.
How changes in pH affect folate influx kinetics parameters mediated by the proton-coupled folate transporter (PCFT). (Top panel) The pattern of changes in influx Km is compared among folic acid, methotrexate (MTX), and pemetrexed. (Bottom panel) The pattern of changes in influx Vmax for folic acid and pemetrexed. From References 97 and 158.
Human PCFT has a high affinity for folic acid, 5-methyltetrahydrofolate (5-methylTHF), and 5-formyltetrahydrofolate (5-formylTHF) at pH 5.5 (i.e., ~1–5 μM). It highest affinity among folate analogs is for pemetrexed (~0.2–0.8 μM) and lowest for PT523 (>100 μM) (136, 154). MTX polyglutamates are not substrates for PCFT; this is assumed to be relevant to folate polyglutamates (98). A variety of anionic compounds are weak inhibitors of PCFT-mediated folate transport. For instance, 200 μM bromosulfophthalein, para-amino-benzoylglutamate, or 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid inhibit uptake of 10 nM 3H-folic acid in human PCFT-transfected HEK 293 cells (87). Likewise, high concentrations of indomethacin (1 mM) or sulfasalazine (0.2 mM) inhibit 10 nM 3H-folic acid uptake into everted sacs of rat jejunum at pH 5.5 (51). The Ki for sulfasalazine inhibition of folic transport into MDCKII cells is 42 μM; the Ki for pyrimethamine is ~162 μM. Although their inhibitory effects on PCFT-mediated folate transport are clear, it is not known whether any of these structurally unrelated anions are actually transported by this mechanism (51, 87). These observations led to the proposal that ingestion of these and other pharmacologic agents could affect the absorption of folates (51, 131). This might account for the folate deficiency associated with the administration of some of these drugs, for instance, in the treatment of patients with rheumatoid arthritis with sulfasalazine (39, 55). This could also affect the intestinal absorption of MTX that is administered with sulfasalazine in the treatment of rheumatoid arthritis. Substantial inhibition by sulfasalazine of PCFT-mediated intestinal folate or MTX absorption was predicted on the basis of these observations (87, 131).
Electrophysiology
MTX transport is electrogenic. When PCFT cRNA is injected into Xenopus oocytes, current is detected; the lower the pH, the greater the current. The higher the transmembrane voltage gradient, the higher the current. Current as a function of folate substrate concentration follows Michaelis-Menten kinetics, with kinetic constants comparable to those obtained from studies with radiolabeled folates in PCFT-transfected mammalian cells (97, 127). The Xenopus oocyte system provides the opportunity for very accurate assessment of the transport properties of physiological folates and folate analogs, that are not radiolabeled, mediated by PCFT. Currents generated by PCFT (the flow of positive charges) are caused by the cotransport of protons in excess of the folate negative charge. Proton coupling was confirmed by the cellular acidification that accompanies folate transport into oocytes (128).
Transport in the absence of a pH gradient
It is of particular importance that although PCFT-mediated transport is maximum at low pH, transport is also detected at pH 7.4 in human cells and in Xenopus oocytes that express PCFT (97, 127). This accounts for the ability of this transporter to decrease the growth requirement for folic acid and 5-formylTHF and to decrease the IC50 for pemetrexed (a preferred substrate) in cells growing at neutral pH in vitro (158). The transporter operates even when there is no transmembrane pH gradient; under these conditions, transport is based, in part, on the membrane potential. Hence, when the membrane potential is increased, folate-induced currents are increased at pH 7.4 (97, 127). Beyond the membrane potential, transport is also sensitive to the folate gradient. As that gradient increases, transport increases; indeed, at low membrane potentials the current reverses and becomes negative, reflecting the negative charge of the folate molecule and the folate-PCFT complex (97, 127). This transporter also has channel-like activity. At low pH, current can be demonstrated in the absence of folate substrate in Xenopus oocytes that express PCFT (76, 128). Hence, protons alone can flow through the aqueous translocation pathway uncoupled from the flow of folate. Channel-like activities have been observed for other facilitative carriers and membrane-spanning molecules (24).
Topology
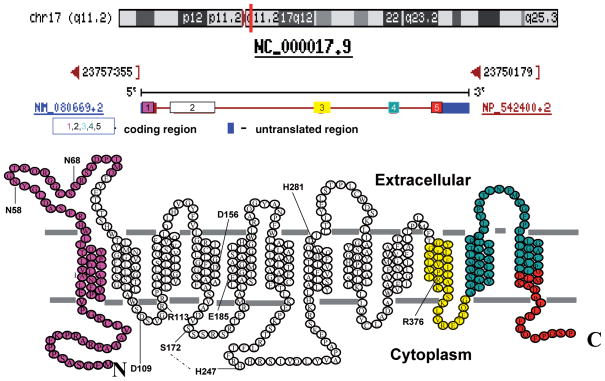
Like RFC, PCFT has a predicted secondary structure typical of members of the superfamily of solute carriers. The Cys-less protein retains nearly complete transport function; hence, Cys residues could be inserted into putative intracellular and extracellular loops in order to employ the substituted cysteine accessibility method (SCAM) to characterize the topological structure of this transporter. When biotin-methanesulfonate treatment, followed by pull-down with streptavidin beads, was used it was possible to discriminate between (a) residues accessible in nonpermeabilized cells (extracellular loops) and (b) residues accessible only in permeabilized cells (intracellular loops) (161). A similar strategy was used to confirm the intracellular location of the N-and C- termini, hemagglutinin-tagged individually and visualized with an antihemagglutinin antibody, only after permeabilization (98, 130). Figure 3 (see color insert) illustrates the predicted PCFT topology, now confirmed by these studies, and indicates the regions of the protein encoded by each exon. It can be seen that exon two encodes eight of the twelve PCFT TMDs. A disulfide linkage exists between Cys66, in the first extracellular loop, and Cys298, in the fourth extracellular loop, that is not required for function (161).
Figure 3.
The genomic location, organization, and topology of the proton-coupled folate transporter (PCFT) and residues that play an important role in function. The top panel is the location of PCFT on chromosome 17, the middle panel is the organization of the PCFT gene, and the lower panel is the confirmed PCFT topology. The colored components illustrate how the five exons code for different regions of the protein. Residues highlighted include Glu185 that is required for proton coupling; His281 that plays a role in proton binding and, allosterically, folate binding; His247 and Ser172 (connected by the interrupted line) that appear to be in proximity in the tertiary structure and play a role controlling substrate access to the folate binding pocket through the aqueous translocation pathway; Asp109 and Arg113 in the first intracellular loop that may be required for carrier oscillation between its inward- and outward-facing conformations; Arg376 that plays a role in folate substrate binding; and Asp156 that is important for protein stability. The topological basis for this structure was reported in References 98, 130, and 161.
Structure-Function
Since the cloning of this transporter, knowledge has emerged on the structural elements required for its function informed by residues (a) mutated in subjects with hereditary folate malabsorption (HFM) and (b) identified by site-directed mutagenesis. The Glu185 residue is critical to proton coupling. Mutations of this residue that result in a profound loss of function at pH 5.5 preserve function at pH 7.4 in the absence of a pH gradient (129). The His281 residue appears to play an important role in proton binding (128): When it is mutated, there is a marked decrease in affinity for folate substrates that is reversed, in part, when the proton concentration is increased. These observations suggest that the His281 residue plays an important role in proton binding, which in turn allosterically affects the folate binding pocket. Mutations at this site decrease carrier affinity for proton(s), which in turn decrease the affinity for folate substrates. Mutations at His281 do not abolish cellular acidification accompanied by folate transport so that this residue is not required for proton coupling (128). Hence, proton binding can be distinguished from proton coupling. The fully conserved His247 residue is located in the large central intracellular loop between the sixth and seventh TMDs. Molecular modeling predicted, and experimental observations were consistent with, an interaction between His247 and Ser172 (located in the second intracellular loop between the fourth and fifth TMDs) that limits access of extracellular folate substrates to the folate binding pocket and is a determinant of the selectivity of folate binding to the carrier. When either of these residues was mutated to Ala, the influx Km was markedly decreased and the selectivity of folate binding was eliminated (128).
Asp109 is absolutely required for function. When mutated, the carrier is inactive, irrespective of charge or polarity of the substituted residue (118). There is only trivial preservation of function with like-charged substitutions at the Arg113 residue (66). Both residues are located in the intracellular loop connecting the second and third transmembrane domains. The data suggest that this region may be required for carrier oscillation between its two conformational states during the transport cycle. This is to be distinguished from a role in either folate or proton binding. This loop encompasses a D109XXGRR114 trafficking domain. When these residues were mutated with multiple Ala substitutions, PCFT trafficking to the apical membrane of polarized MDCK cells was disrupted, with protein trapped in the cytoplasm (123). Despite the apparent role of this region in PCFT trafficking, mutation of the D109 residue did not alter PCFT accessibility at the plasma membrane of Hela cells (118). The R376 residue is an important determinant of substrate binding; mutations at this site produce a protein with altered substrate selectivity properties (76). Finally, the D156 residue is important for proper protein folding and trafficking to the cell membrane (118).
Regulation
Information is beginning to emerge on the regulation of PCFT. PCFT expression is substantially augmented by vitamin D3 in Caco-2 cells and in rat duodenal biopsies in vitro (29). The impact on transport function in Caco-2 cells was modest (a ~twofold increase) but correlated with the increase in PCFT mRNA. A vitamin D receptor response element (VDRE) was identified within positions −1694/−1680 with respect to the transcriptional start site. The cotransfection of the vitamin D receptor and the retinoid X receptor-α in Caco-2 cells, in the presence of vitamin D3, markedly induced the activity of a PCFT promoter luciferase reporter construct. Deletion of the VDRE resulted in only a 50% decrease in the vitamin D3 response, consistent with the presence of at least one other VDRE. Transactivation of the PCFT DR3-like motif by both the vitamin D receptor and its heterodimeric partner, retinoid X receptor-α, was confirmed by electromobility gel shift and chromatin immunoprecipitation assays (29). This unexpected relationship between vitamin D sufficiency and PCFT expression, which could affect intestinal folate absorption and folate homeostasis, is of particular interest in view of the prevalence of vitamin D deficiency worldwide (95).
The minimum transcription regulatory region in Hela cells has been localized between −42 and +96 bases from the transcriptional start site (26, 121). Recently, a role for the nuclear respiratory factor 1 (NRF1) in the regulation of PCFT in Hela cells was reported (36). NRF1 binding sites were indentified in the PCFT promoter. Gel shift assays indicated direct binding between nuclear extracts isolated from Hela cells and PCFT oligonucleotides containing the NRF1 sites. The protein-oligonucleotide complex supershifted to a higher molecular weight complex in the presence of an NRF1-specific antibody. Chromatin immunoprecipitation with the same NRF1 antibody coprecipitated with a PCFT promoter fragment. Overexpression of an NRF1-VP16 construct resulted in a sixfold increase in PCFT reporter activity. An NRF1 siRNA decreased NRF1 transcript and protein along with PCFT transcript levels, although changes in PCFT protein levels and impact on transport function were not reported (36). NRF1 is a key transcription factor involved in the biogenesis of mitochondria and the regulation of mitochondrial genes. Since mitochondria play an essential role in one-carbon reactions, and folates are important to mitochondrial integrity, a case was made for the biological relevance of a role for NRF1 in the regulation of folate sufficiency through an impact on intestinal folate absorption (36). It remains to be seen whether these observations, obtained under in vitro conditions, are relevant to intestinal folate absorption in vivo and can account for the large increase in PCFT expression in the small intestine of folate-deficient mice (98). The extent of methylation of the highly GC-rich PCFT promoter is another potential mechanism of regulation of this transporter. This was recently shown to be associated with the low levels of PCFT expression in two T-cell leukemia cell lines (37). This may be relevant to PCFT expression in lymphoma and leukemia cells, in general, that appear to have low levels of folate transport activity at low pH (41). Hypermethylation of the PCFT promoter was also associated with the loss of PCFT activity in the MTX-resistant Hela R1 cell line (26). In both cases, treatment with a demethylating reagent increased PCFT expression and transport function. Alterations in the methylation of the PCFT promoter could represent a mechanism by which absorption of folates are regulated in the small intestine. Hence, the hypomethylation that can accompany folate deficiency under some conditions (138) could result in activation of PCFT expression and increased intestinal folate absorption. Folate excess would have the opposite effect by turning off transcription of this gene. It will be important to assess, in future studies, the impact of folate deficiency in vivo on methylation and expression of PCFT.
Folate absorption in humans, in vivo, is modulated by conditions and drugs that alter the pH of intestinal fluid. In subjects with pancreatic insufficiency, intestinal folate absorption is increased (105), whereas in subjects treated with antacids or H2 receptor antagonists, absorption is decreased (106). PCFT expression may also change in response to a change in pH at the transport interface. Hence, PCFT mRNA was decreased by ~50% in duodenal biopsies from a small group of subjects treated with proton-pump inhibitors in comparison with untreated controls (131).
A Role for PCFT in the Transport of Heme-Iron?
One year before the identification of PCFT as a high-affinity folate transporter, this carrier was reported to be a low-affinity hemin (heme-Fe) transporter designated as heme carrier protein-1 (116). This was followed by several other reports from the same laboratory (65, 68). A high level of endogenous hemin uptake was noted in Hela cells, with only a modest further increase in PCFT-transfected cells. The hemin uptake Km was in excess of 100 μM, estimated at 125 μM, and there was no evidence for pH sensitivity over a pH range of 6.5–8.5. Uptake of hemin into everted duodenal sacs was noted to be modestly decreased (30%) by an antibody to the C-terminus. However, that region of the carrier is located within the cytoplasm and would not have access to the protein in nonpermeabilized cells. Uptake of hemin in Caco-2 cells was modestly increased (~30%) after transfection of PCFT (68) and was slightly decreased (~20%) when these cells were subsequently treated with PCFT siRNA (65).
PCFT expression in the duodenum was increased in mice exposed to hypoxia for several days; this was accompanied by a small (~37%) increase in duodenal hemin uptake (116). Hemin uptake into mucosal segments from normoxic mice was unaffected by folic acid, but the increase in hemin uptake in duodenal segments from hypoxic mice was slightly decreased (~25%) by folic acid. Although folic acid uptake into everted duodenal sacs was inhibited by hemin, there was no effect of hemin on folic acid uptake in sacs from hypoxic mice in experiments performed at neutral pH (65).
Several other laboratories have reported observations relevant to this issue. Zinc protoporphyrin uptake was not increased in human PCFT-transfected HEK293 cells (87), and although hemin was a very weak inhibitor of folic acid uptake into Xenopus oocytes, neither heme nor hemin currents could be detected in this system (97, 127). Although it is possible that PCFT-mediated hemin transport is not electrogenic, neither heme nor hemin inhibited folic acid–induced currents in PCFT-expressing Xenopus oocytes (127).
Taken together, these observations suggest that PCFT is at most a weak hemin transporter (2). The primacy of PCFT’s role as a folate transporter is compelling, and its physiological role as an intestinal folate transporter is confirmed by its inactivation in HFM (77). A critical issue is whether PCFT-mediated hemin transport contributes in any significant way to the intestinal absorption of iron. This is unlikely to be the case. PCFT−/− children with HFM are not iron deficient (84, 97, 156), and PCFT−/− mice have high levels of blood and liver iron (R. Finnell, personal communication). A careful analysis of intestinal iron absorption and iron homeostasis in PCFT−/− mice should definitively resolve this issue.
ANALYSIS AND IMPLICATIONS OF TRANSMEMBRANE FOLATE GRADIENTS AND FLUXES
The transmembrane gradient of free monoglutamyl folates achieved within the intracellular water is determined by the net impact of several factors. The pKas of the two glutamate residues of folate molecules are 3.5 and 4.5, so that under the physiological conditions in which these transporters operate, folates carry a net negative charge. Since there are high voltage gradients across mammalian cells, the folate chemical gradient at thermodynamic equilibrium will be less than one. For instance, if the membrane potential is −60 mV, and assuming the folate molecule is a bivalent anion, the predicted transmembrane chemical ratio (intracellular to extracelluar concentration) based upon the Nernst equation is 0.3. Any ratio above this would represent uphill transport into the cell. If the intracellular and extracellular concentrations were the same, i.e., the ratio was 1, the electrochemical-potential gradient would be 3. Although transport would not be concentrative, it would nonetheless be uphill and therefore energy dependent.
Both facilitative carriers generate transmembrane folate gradients, but by very different mechanisms. PCFT is a proton symporter that utilizes the transmembrane proton gradient to achieve uphill folate transport into cells. Under conditions in which there is a proton gradient, as in the proximal small intestine, where the pH in the microenvironment is far less than in the cellular compartment, the downhill flow of protons into cells provides the energy for the uphill flow of folates in the same direction (97, 98, 128). On the other hand, RFC is an organic anion antiporter that utilizes the large transmembrane organic phosphate gradient across the cell membrane to achieve uphill transport of folates into the cell. In this case, the downhill flow of organic phosphates out of the cell is coupled to the uphill flow of folates into the cell by the same mechanism (150, 152, 155).
Opposing these concentrative processes are a variety of ABC transporters that are low-affinity, high-capacity pumps in which export of folates is linked directly to the hydrolysis of ATP. When these pumps are blocked with metabolic inhibitors or other transport substrates, efflux of folates is markedly diminished, and the transmembrane folate gradient is markedly increased (5, 33). Analysis of folate efflux properties and kinetics is complicated by the multiplicity of efflux routes dominated by the high-capacity ABC exporters. The RFC-mediated efflux component is small in comparison, and the contribution of PCFT at the neutral pH within the cell should be negligible.
THE INTESTINAL ABSORPTION OF FOLATES AND HEREDITARY FOLATE MALABSORPTION
Transport Across the Apical Brush-Border Membrane of the Proximal Small Intestine
Folates ingested and folates delivered via the bile duct to the duodenum are absorbed in the proximal small intestine in an acid microenvironment (pH 5.8–6.0) achieved by Na+/H+ exchangers (21, 82, 109). Dietary folates, largely 5-methylTHF, are ingested as polyglutamates and are hydrolyzed to the monoglutamate by a γ-glutamyl hydrolase that has optimal activity at low pH (16). This is required for transport across the apical brush-border membrane since MTX polyglutamates are not substrates for PCFT (98). It is assumed that this is also the case for folate polyglutamates. In the early 1970s, it was established that the intestinal absorptive process is mediated by a folate-specific transport mechanism in studies encompassing intestinal segments in vivo and in vitro, everted sacs, and membrane vesicles (78, 115, 155). However, the underlying molecular mechanism was unclear. What was clear was that this process was optimal at low pH, and the influx Km and/or Ki for folic acid, MTX, and reduced folates was ~1–2 μM (79, 115). Shortly after RFC was cloned (27), expression of the identical protein was identified in murine small intestine and was designated as the intestinal folate carrier; intestinal absorption was attributed to transport by this mechanism (63, 108). Indeed, RFC is highly expressed along the entire intestinal apical brush-border membrane in a location consistent with a role in transport at these sites (137). However, despite the discrepancy between the pH dependency of RFC-mediated transport and folate transport in intestinal systems, along with the low affinity of the former and high affinity of the latter for folic acid, the paradigm persisted. Although posttranslational modification of RFC in the intestine was proposed to account for these differences, no such change was identified (20, 63). Ultimately, the cloning of PCFT, a transporter with optimal activity at low pH and high affinity for folic acid, resolved this paradox (97).
The role that PCFT plays in intestinal absorption of folates was established with the observation that there are loss-of-function mutations in this gene in the rare autosomal recessive disorder HFM (97, 156). HFM is characterized by the onset of macrocytic, folate-deficiency anemia and failure to thrive within the first few months of life. This may be accompanied by hypoimmunoglobulinemia associated with infectious complications, most frequently Pneumocystis jiroveci pneumonia. The syndrome is characterized by developmental delays, gait disorders, peripheral neuropathies, and, in the absence of adequate and timely treatment, seizures (34, 77). The pathophysiology of this disorder is attributed to two functional defects: (a) impaired intestinal folate absorption and (b) impaired folate transport across the choroid plexus, manifested by very low levels of folate in the cerebrospinal fluid (CSF) even when the blood folate level is normalized. At the time PCFT was cloned, 11 families had been reported in which at least one member had the clinical syndrome of HFM (34, 54). This laboratory has studied affected subjects from four of these families; all had loss-of-function PCFT mutations involving both alleles (one unpublished) (84, 97, 156). An additional 16 families, with at least one subject with the clinical diagnosis of HFM, have been studied; all have loss-of-function PCFT mutations (nine unpublished) (6, 76, 118, 156). Since the initial reports from this laboratory, three subjects with HFM and PCFT mutations were subsequently reported by other groups (11, 67, 83). Hence, there is now a total of 23 families with confirmed PCFT mutations associated with HFM. This human model of the PCFT-null state establishes that under conditions of physiological folate intake, PCFT is required for intestinal folate absorption and folate sufficiency in man. The fact that subjects who are PCFT null develop severe folate deficiency indicates that RFC does not contribute significantly to folate absorption under these conditions despite its presence in the intestinal epithelium. This is at least in part because this transporter operates very inefficiently in the low-pH microenvironment at the proximal small intestinal absorptive surface.
Ramifications of the Therapeutic Impact of High Oral Folate Loads in the Treatment of Hereditary Folate Malabsorption
Patients with HFM have been treated with oral and/or parenteral folates. The systemic folate deficiency is readily corrected with oral pharmacological doses of 5-formylTHF (leucovorin- racemic) or folic acid. Correction of the central nervous system folate deficiency is more challenging (see section titled Nourishing the Brain in Children with Cerebral Folate Deficiency Due to Hereditary Folate Malabsorption or Loss-of-Function FRα Mutations). When it is studied carefully, the malabsorption of an oral folate load in subjects with HFM is clear (34, 77). Some clinical reports are difficult to interpret because the “folate” administered may not be defined, and the route, dose, scheduling, and folate-containing medications and blood transfusions may not be reported. In any event, at high oral doses of folic acid or 5-formylTHF, normal blood levels are readily achieved; however, the mechanism of intestinal absorption is unclear. As the pH is reduced, there is little change in the affinity of RFC for its folate substrates; the loss of function is due to a decrease in Vmax, a kinetic change that, unlike a high Km, does not allow compensation by increasing the substrate concentration (136). On the other hand, RFC-mediated transport should be possible in more distal segments of the small intestine where RFC is expressed and the pH is within its optimal range. Unanswered, however, is why this would not occur with a physiological folate load that escapes absorption in the proximal jejunum in subjects with HFM. It is possible that RFC located at the apical membrane is not functional or accessible in these tissues, irrespective of pH. Also arguing against a role for RFC is the observation that pharmacological doses of folic acid are absorbed in these subjects despite the fact that the affinity of RFC for this folate is one-hundredth that of 5-formylTHF. The possibility that another member of the SLC superfamily might mediate folate absorption in the absence of PCFT has not been fully explored. Several SLC21A members appear to have substrate-specific low pH optima (40, 71). SLC21A9 (OATP-B) is expressed in small intestine, localized to the apical brush-border membrane (60). This transporter operates most efficiently at low pH for its organic anion substrates (60, 71, 89) and has been assumed to be proton-coupled because when the transmembrane proton gradient is inhibited by FCCP, transport of its substrates is decreased (89). However, its capacity to transport physiological folates or antifolates has not been reported.
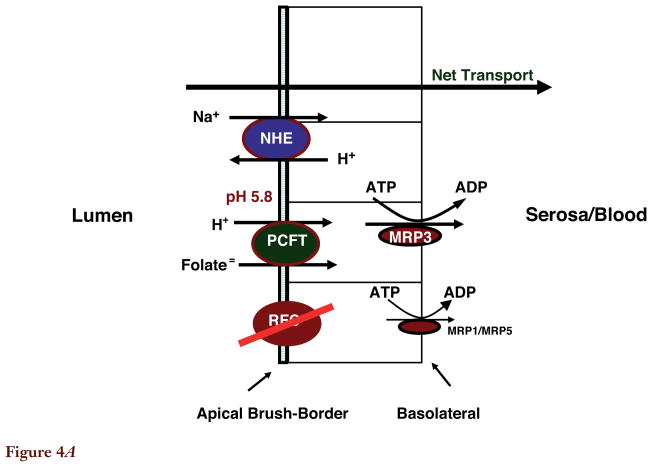
Transport Across the Basolateral Membrane of Proximal Small Intestine
Intestinal folate absorption requires vectorial transport from the lumen to the serosal space across two membranes, the apical brush-border membrane and the basolateral membrane (Figure 4A, see color insert). The former process is now understood. Studies in multidrug resistance-associated protein (MRP)3−/− mice provide some information on the latter process (58, 59). Following an oral folate substrate load in these animals, (a) the maximum folic acid and MTX blood levels and areas under the curve were markedly reduced, but (b) there was no significant decrease in the 5-formylTHF and 5-methylTHF blood levels or areas under the curve, although the absorptive process of the former was significantly slower. The endogenous 5-methylTHF blood levels in mice fed the standard folic acid–containing chow (very high folate content) were the same in MRP3−/− and wild-type mice, and there was no difference in the blood homocysteine levels, a more sensitive indicator of folate deficiency. Interpretation of these studies in everted sacs from these mice is complicated since transport was evaluated at a mucosal pH of 6.8, much higher than the pH at the absorptive surface in vivo. However, the trend is similar: Folic acid (in particular), 5-formylTHF, and (to a much lesser extent) 5-methylTHF transport was decreased across duodenal sacs from Mrp3−/− mice. There was no change in transport of the former two in jejunal sacs. Hence, in mice, MRP3 appears to plays a role in the absorption of oxidized folates primarily in the duodenum. There was no change in MTX blood levels or area under the curve in MRP4−/− mice after an oral MTX load. It is unclear, however, the extent to which these observations are relevant to the absorption of folates across the basolateral membrane of these tissues in humans. MRP1 and MRP5 are also expressed at the basolateral membrane. MRP5 transports MTX mono- and diglutamates with comparable kinetics (23, 142). Their role, if any, in folate absorption in humans in not clear; this has not been evaluated in mice null for these transporters.
Figure 4.
Figure 4A. Folate transport across the enterocyte of the proximal small intestine. The pH at the microenvironment of the apical brush-border is 5.8–6.0. The reduced folate carrier (RFC) is expressed at the apical membrane; however, its pH optimum is 7.4. RFC does not contribute significantly to folate transport across this membrane since it cannot compensate for loss of the proton-coupled folate transporter (PCFT) function in subjects with hereditary folate malabsorption (HFM) on a normal diet. The multidrug resistance-associated protein (MRP)3 plays a role in mediating transport of folates across the basolateral membrane of the proximal small intestine. MRP1 and MRP5 are also expressed at this membrane, but their role in transport of folates has not been demonstrated. Not shown is MRP2/ATP-binding cassette (ABC)G2 along with OATPB (SLC21A9) expressed at the apical membrane. Their impact on folate transport at this site is not clear.
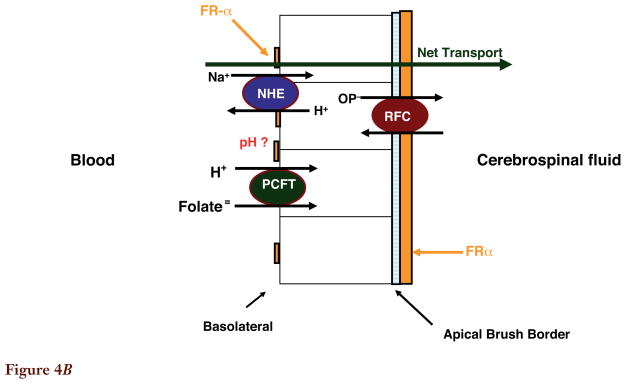
Figure 4B. Folate transport across the choroid plexus. Both the proton-coupled folate transporter (PCFT) and folate receptor α(FRα) are required for transport of folates from blood to cerebrospinal fluid across choroid plexus ependymal cells, based upon studies in human subjects in whom there are loss-of-function mutations in these transporters. However, the bulk of FRα expression, and all reduced folate carrier (RFC) expression, is at the apical brush-border membrane. The pH at the basolateral membrane, where PCFT is expressed, is not known. Not shown are multidrug resistance-associated protein (MRP)1 and MRP4 that are expressed at the basolateral membrane. Their impact on the transport of folates is not clear.
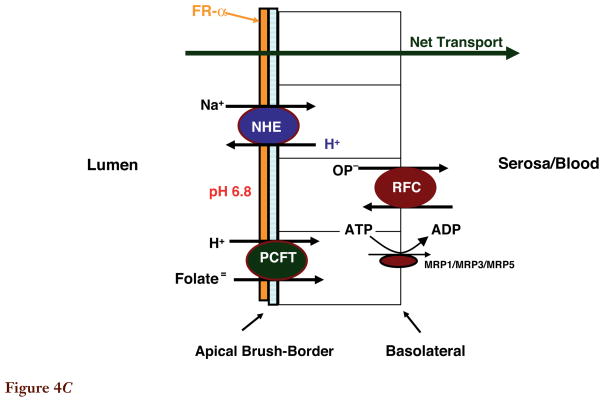
Figure 4C. Folate transport across the proximal renal tubule. Folate receptor α(FRα) is highly expressed at the apical membrane and plays an important role in the reabsorption of filtered folates. The proton-coupled folate transporter (PCFT) is also highly expressed in the kidney and appears to be expressed in the apical membrane based upon the dominant low-pH transport activity in membrane vesicles from this segment of the kidney; however, its role is not clear. Although the reduced folate carrier (RFC) is located at the basolateral membrane, and could contribute to transport into the peritubular compartment, this transporter favors transport into the cells. Not shown are multidrug resistance-associated protein (MRP)2, MRP4, and ATP-binding cassette (ABC)G2 expressed at the apical membrane, and Oat1–4 expressed at the basolateral membrane. Their contributions relative to the folate-specific transporters is not clear, although one or more of the MRPs may account for secretion of methotrexate.
FOLATE TRANSPORT ACROSS THE HEPATIC BASOLATERAL MEMBRANE
Folates absorbed in the proximal small intestine are delivered via the hepatic portal vein to the hepatic sinusoids, where they are transported across the basolateral membrane into hepatocytes. PCFT is highly expressed in the liver (97, 98) at the basolateral membrane (unpublished findings) where, functionally, there is a high level of low-pH MTX and 5-methylTHF transport activity but a very low level of activity at pH 7.4. These observations are based upon studies with hepatic basolateral membrane vesicles, reflecting the dominance of PCFT at this site (44, 45). Na+/H+ exchangers are expressed in the basolateral membrane (3); however, the pH at the membrane microenvironment is not known. Two members of the SLC21 family of solute carriers are expressed at the basolateral membrane and transport MTX: SLC21A6 (OATP-C; LST-1) and SLC21A8 (OATP-C; LST-2) (1, 61). The MTX Km ranges from ~25 to 40 μM, and transport is comparable for the two carriers, although the latter does not transport folate (1). There is no information on the transport of 5-methylTHF by these mechanisms. MRP3 appears to increase efflux of MTX from hepatocytes across the basolateral membrane into the hepatic sinusoids, based upon studies in MRP3−/− mice (58). MRP2 mediates export of MTX across the canalicular membrane into the biliary system to begin the cycle of enterohepatic circulation (80). MRP2 transports 5-methylTHF with a higher affinity (Km = 0.12 mM) (64) than MTX (Km = 0.3 mM) as assessed in rat canalicular membrane vesicles (80). MRP5 is also expressed at the basolateral membrane (142). ABCG2(BCRP) is expressed in the canalicular membrane with a Km for MTX of 0.7–1.3 mM (19, 134). However, unlike MRP2, which transports MTX monoglutamate, ABCG2 transports MTX mono-, di-, and triglutamates and has optimal activity at low pH (12, 19).
FOLATE TRANSPORT ACROSS THE CHOROID PLEXUS
Mechanism
The choroid plexus functions primarily to clear substrates and metabolites from the CSF by export into the blood (112). Its role in folate homeostasis in the central nervous system is, apparently, quite different. Under physiological conditions, in the adult, the folate concentration in the CSF is two- to three-fold greater than in blood, consistent with active transport from blood to CSF across the choroid plexus. As indicated in Figure 4B (see color insert), PCFT is expressed along the basolateral membrane of the choroid plexus in apposition to capillary endothelial cells (157). Na+/H+ exchangers are expressed at this membrane, but there is no information on the pH at the microclimate of the transport interface (111). The location of PCFT is consistent with a role in transporting folates from blood into choroid plexus ependymal cells. FRα is highly expressed at the apical brush-border membrane within the CSF and to a lesser extent at the basolateral membrane (57, 93, 113, 139, 140). Its dominant location is consistent with a role in extracting folates from the CSF. RFC is highly expressed at the apical brush-border membrane, also poised to extract folates from the CSF, although it operates quite well bidirectionally (137). MRP1 and MRP4 are expressed on the basolateral membrane (70, 100). ABC exporters that transport folates have not been identified on the apical membrane.
Beyond consideration of location, two genetic disorders provide compelling evidence of a role for both PCFT and FRα in transporting folates across the choroid plexus. The role of PCFT is clear. Subjects with HFM have a severe transport defect at the choroid plexus, which has been detected at the earliest times after birth that CSF folate levels have been measured. Even when the blood folate level is normalized, CSF folate remains very low, and a normal gradient (CSF/blood) is never achieved. It requires supraphysiological blood folate levels to normalize CSF folate, particularly in infants and children who normally have very high CSF folate levels (see next section) (34, 77). Recently, loss-of-function mutations in FRα have been reported in children with a cerebral folate deficiency disorder that manifests clinically several years after birth (14, 122). In contrast to HFM, intestinal folate absorption in these children is normal, as are their folate blood levels, but CSF folate levels are negligible.
These observations suggest the possibilities that these transporters act in tandem and that both are required to achieve folate transport from blood to CSF. It is possible that PCFT is required for the export of folates from acidified endosomes during receptor-mediated endocytosis. PCFT is coexpressed with FRα in endosomes and has been shown to augment FRα-mediated transport (157). However, it is clear that FRα-mediated endocytosis can occur in some cells that lack PCFT expression or with folate analogs that have a very low affinity for this transporter (25). The enigma here is that children with loss-of-function FRα mutations, unlike children with HFM, present with neurological disorders several years after birth. Complicating the understanding of this discrepancy is the apparent lack of relevance of the FRα−/−mouse. This lesion is embryonic lethal, but normal births are possible when the dam is supplemented with folate during gestation. Other than a decreased folate blood level and an increased renal folate clearance, the adult animals have a normal phenotype (10, 119). However, despite the lack of signs of neurological deficits, these mice could still have low CSF folate levels, a possibility that should be evaluated in the future. This difference between the FRα-null mouse and human phenotype raises the possibility that another transporter is expressed, such as FRβ, that protects children during embryonic and fetal development and early life, as has been suggested (122). In addition, the normal folate blood levels in FRα-null children may provide some CSF folate that contributes to the delay in onset of the clinical manifestations of this disorder.
RFC (137) and PCFT (unpublished findings) are also found at the blood-brain barrier. The presence of the former would limit the areas of the brain subjected to folate deficiency in HFM. It is not likely that PCFT contributes significantly to the transport of folates across this barrier, where the pH should be 7.4 due to the high arterial blood flow. ABCG2 and MRP1–MRP5 are localized to the luminal surface of brain capillary endothelial cells. Their impact on net folate transport into the brain is unclear (32, 88, 148).
Nourishing the Brain in Children with Cerebral Folate Deficiency Due to Hereditary Folate Malabsorption or Loss-of-Function FRα Mutations
A major challenge that is emerging in the treatment of children with HFM is the prevention of seizures that can occur early in the disorder or years beyond infancy (34, 76, 77, 118). The fact that a child is seizure free during the first few years of life does not ensure that treatment is adequate and that seizures will not occur at a later time. What is generally not appreciated is that CSF folate levels in infants are very high, and they remain high during the first 10 to 15 years of life. For instance, the CSF folate levels in the first year are 100 to 150 nM, dropping to ~70 to 90 nM by age 5 and remaining in the 60 to 90 nM range beyond age 10 (90, 91, 133). The endpoint for treatment should be based upon the folate level that is normal for the age of the patient. These considerations are relevant also for children with loss-of-function mutations in FRα. Although both disorders are commonly treated with high-dose oral folates, reported CSF folate levels are frequently below normal for the age of the patient. It may not be feasible to achieve required levels with oral dosing. However, IM administration will achieve physiologically relevant CSF folate concentrations at much lower doses. Folic acid should not be used for the treatment of these disorders. Folic acid is a pharmacological agent that must be reduced to dihydrofolate (very inefficiently) before it can enter the one-carbon cycle. Ultimately, it is converted to the physiological folate, 5-methylTHF. More important, folic acid binds virtually irreversibly to FRα, essentially preventing its use for transport of the physiological folate, 5-methylTHF (56). This effect of folic acid may be particularly harmful to the transport of 5-methylTHF across the choroid plexus.
THE KIDNEY AND FOLATE TRANSPORT
The kidney very efficiently conserves folates filtered at the glomerulus by their essentially complete reabsorption in the proximal tubules (Figure 4C, see color insert). FRα, highly expressed at the apical brush-border membrane, and RFC, expressed at the basolateral membrane, are positioned within the tubule to work in tandem in this process as substrate moves from the apical then basolateral membranes into the peritubular fluid and capillaries (10, 137). However, RFC’s antiporter function favors transport into cells. PCFT is highly expressed in the kidney (97, 98), and while its location within the renal tubules has not as yet been clarified, the high level of low-pH folate transport activity in renal tubule brush-border membrane vesicles makes it likely that PCFT is expressed at this site (8, 9). Whether PCFT might facilitate FRα function or transport folate directly across the proximal tubule membrane is not clear. The acidity in the lumen of the proximal tubule is not sufficiently low (pH 6.8) for PCFT to have much function (53). There is compelling evidence that FRα efficiently extracts all folates from the glomerular filtrate at physiological folate blood levels. It is only at high blood folate levels that the reabsorptive process becomes saturated (10, 38, 114). The role that FRα plays in this process is supported by studies with FRα−/− mice (10). On usual chow, these mice have a low plasma folate level due to increased urinary folate clearance. On a folate-deficient diet there is tenfold higher folate excretion relative to the wild-type animals. Excretion of a 5-methylTHF load is also increased, and renal accumulation is decreased in FR−/− mice (10). Renal clearance of folates in PCFT−/− subjects with HFM has not been studied in detail. The role of the folate transporters in modulating the renal conservation of folates has been considered previously (22) and is the subject of a recent review (155).
Beyond these transporters with high specificity for the folates, several other members of the superfamily of human solute carriers may contribute to the transport of folates in the kidney. However, studies that have addressed this issue have focused on the pharmacological impact of these transporters so that most of the functional data have employed MTX as substrate. SLC21A3 (OATP-A) is a transporter that, for some substrates, functions optimally at low pH but is not proton-coupled. It is expressed in the apical membrane of the distal tubule and has a low affinity for MTX (~450 μM) (7). Three members of the SLC22 family transport MTX (102). SLC22A6 (OAT-1) and SLC22A8 (OAT-3) are expressed on the basolateral membrane of the proximal tubule. The former has a very low affinity for MTX (Km ~ 725 μM at pH 7.4); the affinity of the latter is much higher (Km = 11–18 μM) (15, 102, 126, 132). These are α-ketoglutarate antiporters in which the downhill flow of α-ketoglutarate from cells is linked to the uphill transport of its substrates into the cell (102). Hence, these carriers are positioned to transport their substrates from the blood/serosal fluid into the renal tubule cells in a way that would favor secretion of its substrates into proximal tubule lumen. Although this could contribute to MTX secretion (110), the affinity of these transporters for physiological folates is not known, nor is there evidence for the secretion of natural folates. SLC22A7 (OAT-2) is also expressed at the basolateral membrane of proximal tubule epithelial cells and transports MTX (102, 124). OAT-4 is located in the apical brush-border membrane of the proximal tubule with a Km for MTX of ~18 μM (126).
A number of the ABC transporters are expressed in the kidney and could affect renal folate conservation and antifolate excretion. MRP2, MRP4, and ABCG2 are expressed at the apical brush-border membrane, while MRP1, MRP3, and MRP4 are expressed at the basolateral membrane (23, 103). The former could play a secretory role; the latter would facilitate vectorial transport of folates from proximal tubule fluid to the peritubular fluid and blood.
FOLATE TRANSPORT AND THE PLACENTA
There is full redundancy for all three folate transporters in the placenta (75, 97, 101, 139, 141). There is a high level of low-pH folate transport activity, with a lower level of activity at neutral pH, as assessed in the BeWo trophoblastic cell line. There is vectorial transport driven by a pH gradient when these cells are grown as a monolayer (125, 147). Four ABC transporters, MRP1, MRP2, MRP3 and ABCG2, are expressed at the apical (maternal) membrane of the placenta opposing folate transport to the fetal circulation (23, 103, 120). However, there is evidence that MRP1 and MRP3 are also localized in the basolateral surface of fetal blood vessel endothelial cells (86).
FRα-null mice are fertile. Recently, a woman in her late twenties with HFM, supplemented since shortly after birth with IM 5-formylTHF and with a homozygous stop-codon insertion in the first extracellular PCFT loop between the first and second TMDs (84), had a normal term pregnancy and delivered a normal infant (unpublished findings).
ALTERATIONS IN MEMBRANE TRANSPORTERS IN THE ADAPTATION TO FOLATE DEFICIENCY
Although considerable information exists regarding the regulatory regions of RFC, and some emerging information is available regarding PCFT, it is not yet clear as to what signals regulate expression of these transporters in the intact animal. Folate homeostasis is determined by the amount of folate in the diet, the efficiency of intestinal absorption of dietary folate and folate in the enterohepatic circulation, and folate retention by the kidneys. In the mouse, folate deficiency results in a marked increase in RFC, PCFT, and FRα transcripts in the small intestine and kidney (72, 98). This was documented for RFC protein as well as for message in jejunum along with enhanced transport into jejunal brush-border membrane vesicles at pH 5.5 from folate-deprived rats (107). These adaptive changes should maximize intestinal folate absorption and renal folate conservation.
Several of the ABC exporters are low-affinity, high-capacity folate transporters. These include MRP1–MRP5 and ABCG2 (48, 62). Their effects on folate homeostasis in vivo are mixed and depend upon their location in epithelia along with changes in their expression that occur in response to folate restriction. MRP1 and MRP3 expression is markedly increased in the intestine of folate-starved mice; there was no change in expression of MRP4, and there were no significant changes in expression of these exporters in the kidney (72). MRP1, MRP3, and MRP5 are expressed in the basolateral membrane of the duodenum and jejunum, a location that should facilitate vectorial transport across these epithelia (23, 58, 59). In contrast, MRP2 and ABCG2 are located at the apical membrane of these epithelia opposing intestinal folate reabsorption and reabsorption of folate from the glomerular filtrate (23, 85, 103). However, there is no information on changes in their expression with folate deprivation in vivo.
The impact of expression and activity of the ABC exporters on vectorial transport in the folate-sufficient state and in response to folate restriction, however, must be considered within the context of the contributions of these exporters relative to the folate-specific transporters. For instance, if transport of folates across the basolateral membrane of intestine is very rapid in comparison with influx across the apical membrane, i.e., transport across the apical membrane is rate-limiting, then a further increase, or a modest decrease, in transport activity at the basolateral membrane would have little effect on net transport across this epithelium.
The overexpression of ABC exporters in vitro suppresses cellular folate levels and increases folate growth requirements. This has been observed in cell lines that were selected for resistance to drugs that are substrates for these exporters or after transduction of transporters into cell lines (48). For instance, whereas transfection of MRP1, MRP2, or MRP3 decreased cellular folate content, the folate growth requirement was increased only under conditions in which exposure to folates was brief (43). There are only a few examples in which there were changes in exporter expression in response to folate deprivation in vitro. Folate depletion resulted in a decrease in expression of ABCG2 and redistribution of the protein from the cell membrane to the cytoplasm; MRP1 expression was also decreased (49, 50). In a CEM cell line selected for growth in low levels of 5-formylTHF, RFC expression was markedly increased (52) and, as predicted (160), an exporter (MRP1) expression was markedly decreased (4). The mechanism of adaptation to folate restriction was the subject of a recent review (48).
SUMMARY POINTS.
The proton-coupled folate transporter (PCFT) mediates transport of folate monoglutamates across the apical brush-border membrane of the proximal small intestine.
Loss-of-function PCFT mutations are the molecular basis for the autosomal recessive disorder hereditary folate malabsorption (HFM) resulting in impaired intestinal folate absorption and impaired transport into the central nervous system.
PCFT function is optimal at low pH but operates in the absence of a pH gradient driven by both voltage and folate gradients across the cell membrane.
FRα is required for mouse embryonic development, but with folate supplementation, embryonic and fetal development can be normal. After birth, FRα-null mice do not require folate supplementation, and they are fertile, although their blood folate levels are decreased.
In humans, both FRα and PCFT are required for transport of folates across choroid plexus ependymal cells from blood to the cerebrospinal fluid.
FRα-null humans have no phenotype until several years after birth, when they present with very low levels of cerebrospinal fluid folate, seizures, and other neurological defects—a cerebral folate-deficiency syndrome.
FUTURE ISSUES.
To what extent does PCFT play a role in iron homeostasis; i.e., does this transporter contribute significantly to intestinal hemin absorption?
Does PCFT contribute to the delivery of folates to tissues from the systemic circulation? Are there systemic microenvironments at low pH that favor the activity of this transporter?
What is the basis for the differences in phenotype between FRα-null humans and FRα-null mice? What are the roles of PCFT, FRα, and possibly FRβ in the transport of folates across the choroid plexus of humans?
What are the roles that PCFT and FRα play in the reabsorption of folates in the proximal tubule of the kidney, and are they related? How does the loss of PCFT affect renal conservation of folates?
What is the mechanism of intestinal absorption of pharmacological doses of folates in PCFT-null subjects with HFM?
What is the mechanism(s) by which folates are exported from endosomes and the role of PCFT in those process(es)?
How is PCFT expression regulated during folate deprivation and in other conditions, and is this related to the state of methylation of the PCFT promoter?
Acknowledgments
This work was supported by a grant from the National Institutes of Health CA82621.
Glossary
- RFC
reduced folate carrier
- Folates
refers to folate compounds in general
- MTX
methotrexate
- PCFT
proton-coupled folate transporter
- HFM
hereditary folate malabsorption
- FR
folate receptor
- SLC
superfamily of solute carriers
- TMDs
transmembrane domains
- 5-methylTHF
5-methyltetrahydrofolate
- 5-formylTHF
5-formyltetrahydrofolate
- CSF
cerebrospinal fluid
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–99. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NC. When is a heme transporter not a heme transporter? When it’s a folate transporter. Cell Metab. 2007;5:5–6. doi: 10.1016/j.cmet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Arias IM, Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984;259:5406–8. [PubMed] [Google Scholar]
- 4.Assaraf YG, Rothem L, Hooijberg JH, Stark M, Ifergan I, et al. Loss of multidrug resistance protein 1 expression and folate efflux activity results in a highly concentrative folate transport in human leukemia cells. J Biol Chem. 2003;278:6680–86. doi: 10.1074/jbc.M209186200. [DOI] [PubMed] [Google Scholar]
- 5.Assaraf YG, Sierra EE, Babani S, Goldman ID. Inhibitory effects of prostaglandin A1 on membrane transport of folates mediated by both the reduced folate carrier and ATP-driven exporters. Biochem Pharmacol. 1999;58:1321–27. doi: 10.1016/s0006-2952(99)00227-0. [DOI] [PubMed] [Google Scholar]
- 6.Atabay B, Turker M, Ozer EA, Mahadeo K, Diop-Bove N, Goldman ID. Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatr Hematol Oncol. 2010;27:614–19. doi: 10.3109/08880018.2010.481705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, et al. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther. 2006;318:521–29. doi: 10.1124/jpet.106.104364. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari SD, Fortney T, McMartin KE. Analysis of the pH dependence of folate binding and transport by rat kidney brush border membrane vesicles. Proc Soc Exp Biol Med. 1991;196:451–56. doi: 10.3181/00379727-196-43215. [DOI] [PubMed] [Google Scholar]
- 9.Bhandari SD, Joshi SK, McMartin KE. Folate binding and transport by rat kidney brush-border membrane vesicles. Biochim Biophys Acta. 1988;937:211–18. doi: 10.1016/0005-2736(88)90243-x. [DOI] [PubMed] [Google Scholar]
- 10.Birn H, Spiegelstein O, Christensen EI, Finnell RH. Renal tubular reabsorption of folate mediated by folate binding protein 1. J Am Soc Nephrol. 2005;16:608–15. doi: 10.1681/ASN.2004080711. [DOI] [PubMed] [Google Scholar]
- 11.Borzutzky A, Crompton B, Bergmann AK, Giliani S, Baxi S, et al. Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clin Immunol. 2009;133:287–94. doi: 10.1016/j.clim.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breedveld P, Pluim D, Cipriani G, Dahlhaus F, van Eijndhoven MA, et al. The effect of low pH on breast cancer resistance protein (ABCG2)-mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol. 2007;71:240–49. doi: 10.1124/mol.106.028167. [DOI] [PubMed] [Google Scholar]
- 13.Cao W, Matherly LH. Analysis of the membrane topology for transmembrane domains 7–12 of the human reduced folate carrier by scanning cysteine accessibility methods. Biochem J. 2004;378:201–6. doi: 10.1042/BJ20031288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology. 2009;73:2127–29. doi: 10.1212/WNL.0b013e3181c679df. [DOI] [PubMed] [Google Scholar]
- 15.Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–86. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 16.Chandler CJ, Wang TT, Halsted CH. Pteroylpolyglutamate hydrolase from human jejunal brush borders. Purification and characterization. J Biol Chem. 1986;261:928–33. [PubMed] [Google Scholar]
- 17.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–17. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–49. doi: 10.1158/1535-7163.MCT-05-0243. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–54. [PubMed] [Google Scholar]
- 20.Chiao JH, Roy K, Tolner B, Yang CH, Sirotnak FM. RFC-1 gene expression regulates folate absorption in mouse small intestine. J Biol Chem. 1997;272:11165–70. doi: 10.1074/jbc.272.17.11165. [DOI] [PubMed] [Google Scholar]
- 21.Counillon L, Pouyssegur J. The expanding family of eucaryotic Na(+)/H(+) exchangers. J Biol Chem. 2000;275:1–4. doi: 10.1074/jbc.275.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Damaraju VL, Cass CE, Sawyer MB. Renal conservation of folates role of folate transport proteins. Vitam Horm. 2008;79:185–202. doi: 10.1016/S0083-6729(08)00406-8. [DOI] [PubMed] [Google Scholar]
- 23.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–99. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 24.DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- 25.Deng Y, Zhou X, Kugel DS, Wu J, Cherian C, et al. Synthesis and biological activity of a novel series of 6-substituted thieno[2,3-d]pyrimidine antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors over the reduced folate carrier and proton-coupled folate transporter for cellular entry. J Med Chem. 2009;52:2940–51. doi: 10.1021/jm8011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther. 2009;8:2424–31. doi: 10.1158/1535-7163.MCT-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon KH, Lanpher BC, Chiu J, Kelley K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. J Biol Chem. 1994;269:17–20. [PubMed] [Google Scholar]
- 28.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–84. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Eloranta JJ, Zair ZM, Hiller C, Hausler S, Stieger B, Kullak-Ublick GA. Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol. 2009;76:1062–71. doi: 10.1124/mol.109.055392. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson PL, Flintoff WF. Topological and functional analysis of the human reduced folate carrier by hemagglutinin epitope insertion. J Biol Chem. 1999;274:16269–78. doi: 10.1074/jbc.274.23.16269. [DOI] [PubMed] [Google Scholar]
- 31.Fleming JC, Tartaglini E, Steinkamp MP, Schorderet DF, Cohen N, Neufeld EJ. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet. 1999;22:305–8. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- 32.Franke RM, Gardner ER, Sparreboom A. Pharmacogenetics of drug transporters. Curr Pharm Des. 2010;16:220–30. doi: 10.2174/138161210790112683. [DOI] [PubMed] [Google Scholar]
- 33.Fry DW, White JC, Goldman ID. Effects of 2,4-dinitrophenol and other metabolic inhibitors on the bidirectional carrier fluxes, net transport, and intracellular binding of methotrexate in Ehrlich ascites tumor cells. Cancer Res. 1980;40:3669–73. [PubMed] [Google Scholar]
- 34.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 2002;81:51–68. doi: 10.1097/00005792-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Goldman ID, Chattopadhyay S, Zhao R, Moran RG. The antifolates: evolution, new agents in the clinic, and how targeting delivery via specific membrane transporters is driving development of a next generation of folate analogs. Curr Opin Investig Drugs. 2010;11:1409–23. [PubMed] [Google Scholar]
- 36.Gonen N, Assaraf YG. The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1. J Biol Chem. 2010;285:33602–13. doi: 10.1074/jbc.M110.135640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonen N, Bram EE, Assaraf YG. PCFT promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun. 2008;376:787–92. doi: 10.1016/j.bbrc.2008.09.074. [DOI] [PubMed] [Google Scholar]
- 38.Goresky CA, Watanabe H, Johns DG. The renal excretion of folic acid. J Clin Invest. 1963;42:1841–49. doi: 10.1172/JCI104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haagsma CJ, Blom HJ, van Riel PL, van’t Hof MA, Giesendorf BA, et al. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann Rheum Dis. 1999;58:79–84. doi: 10.1136/ard.58.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 41.Henderson GB, Strauss BP. Characteristics of a novel transport system for folate compounds in wild-type and methotrexate-resistant L1210 cells. Cancer Res. 1990;50:1709–14. [PubMed] [Google Scholar]
- 42.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–49. [PubMed] [Google Scholar]
- 43.Hooijberg JH, Peters GJ, Assaraf YG, Kathmann I, Priest DG, et al. The role of multidrug resistance proteins MRP1, MRP2 and MRP3 in cellular folate homeostasis. Biochem Pharmacol. 2003;65:765–71. doi: 10.1016/s0006-2952(02)01615-5. [DOI] [PubMed] [Google Scholar]
- 44.Horne DW. Na+ and pH dependence of 5-methyltetrahydrofolic acid and methotrexate transport in freshly isolated hepatocytes. Biochim Biophys Acta Bio-Membr. 1990;1023:47–55. doi: 10.1016/0005-2736(90)90008-c. [DOI] [PubMed] [Google Scholar]
- 45.Horne DW, Reed KA. Transport of methotrexate in basolateral membrane vesicles from rat liver. Arch Biochem Biophys. 1992;298:121–28. doi: 10.1016/0003-9861(92)90102-3. [DOI] [PubMed] [Google Scholar]
- 46.Hou Z, Cherian C, Drews J, Wu J, Matherly LH. Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier. J Biol Chem. 2010;285:4732–40. doi: 10.1074/jbc.M109.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou Z, Matherly LH. Oligomeric structure of the human reduced folate carrier: identification of homo-oligomers and dominant-negative effects on carrier expression and function. J Biol Chem. 2009;284:3285–93. doi: 10.1074/jbc.M807206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ifergan I, Assaraf YG. Molecular mechanisms of adaptation to folate deficiency. Vitam Horm. 2008;79:99–143. doi: 10.1016/S0083-6729(08)00404-4. [DOI] [PubMed] [Google Scholar]
- 49.Ifergan I, Jansen G, Assaraf YG. Cytoplasmic confinement of breast cancer resistance protein (BCRP/ABCG2) as a novel mechanism of adaptation to short-term folate deprivation. Mol Pharmacol. 2005;67:1349–59. doi: 10.1124/mol.104.008250. [DOI] [PubMed] [Google Scholar]
- 50.Ifergan I, Shafran A, Jansen G, Hooijberg JH, Scheffer GL, Assaraf YG. Folate deprivation results in the loss of breast cancer resistance protein (BCRP/ABCG2) expression: a role for BCRP in cellular folate homeostasis. J Biol Chem. 2004;279:25527–34. doi: 10.1074/jbc.M401725200. [DOI] [PubMed] [Google Scholar]
- 51.Inoue K, Nakai Y, Ueda S, Kamigaso S, Ohta KY, et al. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am J Physiol Gastrointest Liver Physiol. 2008;294:G660–68. doi: 10.1152/ajpgi.00309.2007. [DOI] [PubMed] [Google Scholar]
- 52.Jansen G, Westerhof GR, Jarmuszewski MJA, Kathmann I, Rijksen G, Schornagel JH. Methotrexate transport in variant human CCRF-CEM leukemia cells with elevated levels of the reduced folate carrier. Selective effect on carrier-mediated transport of physiological concentrations of reduced folates. J Biol Chem. 1990;265:18272–77. [PubMed] [Google Scholar]
- 53.Jaramillo-Juarez F, Aires MM, Malnic G. Urinary and proximal tubule acidification during reduction of renal blood flow in the rat. J Physiol. 1990;421:475–83. doi: 10.1113/jphysiol.1990.sp017956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jebnoun S, Kacem S, Mokrani CH, Chabchoub A, Khrouf N, Zittoun J. A family study of congenital malabsorption of folate. J Inherit Metab Dis. 2001;24:749–50. doi: 10.1023/a:1012905823879. [DOI] [PubMed] [Google Scholar]
- 55.Jensen MK, Ekelund S, Svendsen L. Folate and homocysteine status and haemolysis in patients treated with sulphasalazine for arthritis. Scand J Clin Lab Invest. 1996;56:421–29. doi: 10.3109/00365519609088797. [DOI] [PubMed] [Google Scholar]
- 56.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56:1085–97. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy MD, Jallad KN, Lu J, Low PS, Ben Amotz D. Evaluation of folate conjugate uptake and transport by the choroid plexus of mice. Pharm Res. 2003;20:714–19. doi: 10.1023/a:1023421232689. [DOI] [PubMed] [Google Scholar]
- 58.Kitamura Y, Hirouchi M, Kusuhara H, Schuetz JD, Sugiyama Y. Increasing systemic exposure of methotrexate by active efflux mediated by multidrug resistance-associated protein 3 (Mrp3/Abcc3) J Pharmacol Exp Ther. 2008;327:465–73. doi: 10.1124/jpet.108.140475. [DOI] [PubMed] [Google Scholar]
- 59.Kitamura Y, Kusuhara H, Sugiyama Y. Basolateral efflux mediated by multidrug resistance-associated protein 3 (Mrp3/Abcc3) facilitates intestinal absorption of folates in mouse. Pharm Res. 2010;27:665–72. doi: 10.1007/s11095-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–8. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- 61.Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156–64. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- 62.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 63.Kumar CK, Nguyen TT, Gonzales FB, Said HM. Comparison of intestinal folate carrier clone expressed in IEC-6 cells and in Xenopus oocytes. Am J Physiol. 1998;274:C289–94. doi: 10.1152/ajpcell.1998.274.1.C289. [DOI] [PubMed] [Google Scholar]
- 64.Kusuhara H, Han YH, Shimoda M, Kokue E, Suzuki H, Sugiyama Y. Reduced folate derivatives are endogenous substrates for cMOAT in rats. Am J Physiol. 1998;275:G789–96. doi: 10.1152/ajpgi.1998.275.4.G789. [DOI] [PubMed] [Google Scholar]
- 65.Laftah AH, Latunde-Dada GO, Fakih S, Hider RC, Simpson RJ, McKie AT. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1) Br J Nutr. 2009;101:1150–56. doi: 10.1017/S0007114508066762. [DOI] [PubMed] [Google Scholar]
- 66.Lasry I, Berman B, Glaser F, Jansen G, Assaraf YG. Hereditary folate malabsorption: a positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochem Biophys Res Commun. 2009;386:426–31. doi: 10.1016/j.bbrc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, et al. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008;112:2055–61. doi: 10.1182/blood-2008-04-150276. [DOI] [PubMed] [Google Scholar]
- 68.Latunde-Dada GO, Takeuchi K, Simpson RJ, McKie AT. Haem carrier protein 1 (HCP1): expression and functional studies in cultured cells. FEBS Lett. 2006;580:6865–70. doi: 10.1016/j.febslet.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 69.Leamon CP, Jackman AL. Exploitation of the folate receptor in the management of cancer and inflammatory disease. Vitam Horm. 2008;79:203–33. doi: 10.1016/S0083-6729(08)00407-X. [DOI] [PubMed] [Google Scholar]
- 70.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol. 2009;296:C570–82. doi: 10.1152/ajpcell.00436.2008. [DOI] [PubMed] [Google Scholar]
- 72.Liu M, Ge Y, Cabelof DC, Aboukameel A, Heydari AR, et al. Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. J Biol Chem. 2005;280:5588–97. doi: 10.1074/jbc.M412662200. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Matherly L. Analysis of membrane topology of the human reduced folate carrier protein by hemagglutinin epitope insertion and scanning glycosylation insertion mutagenesis. Biochim Biophys Acta. 2002;1564:333–42. doi: 10.1016/s0005-2736(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 74.Ma DW, Finnell RH, Davidson LA, Callaway ES, Spiegelstein O, et al. Folate transport gene inactivation in mice increases sensitivity to colon carcinogenesis. Cancer Res. 2005;65:887–97. [PMC free article] [PubMed] [Google Scholar]
- 75.Maddox DM, Manlapat A, Roon P, Prasad P, Ganapathy V, Smith SB. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahadeo KM, Diop-Bove N, Shin D, Unal E, Teo J, et al. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol. 2010;299:1153–61. doi: 10.1152/ajpcell.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahadeo KM, Min SH, Diop-Bove N, Kronn D, Goldman ID. Hereditary folate malabsorption. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews [Internet] Seattle: Univ. Washington; 2010. [Google Scholar]
- 78.Mason JB, Rosenberg IH. Intestinal absorption of folate. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven; 1994. pp. 1979–95. [Google Scholar]
- 79.Mason JB, Shoda R, Haskell M, Selhub J, Rosenberg IH. Carrier affinity as a mechanism for the pH-dependence of folate transport in the small intestine. Biochim Biophys Acta Bio-Membr. 1990;1024:331–35. doi: 10.1016/0005-2736(90)90362-r. [DOI] [PubMed] [Google Scholar]
- 80.Masuda M, I’izuka Y, Yamazaki M, Nishigaki R, Kato Y, et al. Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res. 1997;57:3506–10. [PubMed] [Google Scholar]
- 81.Matherly LH, Hou Z. Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam Horm. 2008;79C:145–84. doi: 10.1016/S0083-6729(08)00405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McEwan GT, Lucas ML, Denvir M, Raj M, McColl KE, et al. A combined TDDA-PVC pH and reference electrode for use in the upper small intestine. J Med Eng Technol. 1990;14:16–20. doi: 10.3109/03091909009028758. [DOI] [PubMed] [Google Scholar]
- 83.Meyer E, Kurian MA, Pasha S, Trembath RC, Cole T, Maher ER. A novel PCFT gene mutation (p. Cys66LeufsX99) causing hereditary folate malabsorption. Mol Genet Metab. 2010;99:325–28. doi: 10.1016/j.ymgme.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Min SH, Oh SY, Karp GI, Poncz M, Zhao R, Goldman ID. The clinical course and genetic defect in the PCFT in a 27-year-old woman with hereditary folate malabsorption. J Pediatr. 2008;153:435–37. doi: 10.1016/j.jpeds.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mottino AD, Hoffman T, Jennes L, Vore M. Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J Pharmacol Exp Ther. 2000;293:717–23. [PubMed] [Google Scholar]
- 86.Nagashige M, Ushigome F, Koyabu N, Hirata K, Kawabuchi M, et al. Basal membrane localization of MRP1 in human placental trophoblast. Placenta. 2003;24:951–58. doi: 10.1016/s0143-4004(03)00170-x. [DOI] [PubMed] [Google Scholar]
- 87.Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, et al. Functional characterization of human PCFT/HCP1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther. 2007;322:469–76. doi: 10.1124/jpet.107.122606. [DOI] [PubMed] [Google Scholar]
- 88.Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–60. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 89.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–45. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 90.Opladen T, Ramaekers VT, Heimann G, Blau N. Analysis of 5-methyltetrahydrofolate in serum of healthy children. Mol Genet Metab. 2006;87:61–65. doi: 10.1016/j.ymgme.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Ormazabal A, Garcia-Cazorla A, Perez-Duenas B, Gonzalez V, Fernandez-Alvarez E, et al. Determination of 5-methyltetrahydrofolate in cerebrospinal fluid of paediatric patients: reference values for a paediatric population. Clin Chim Acta. 2006;371:159–62. doi: 10.1016/j.cca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 92.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–93. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 93.Patrick TA, Kranz DM, van Dyke TA, Roy EJ. Folate receptors as potential therapeutic targets in choroid plexus tumors of SV40 transgenic mice. J Neurooncol. 1997;32:111–23. doi: 10.1023/a:1005713115147. [DOI] [PubMed] [Google Scholar]
- 94.Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, et al. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–32. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- 95.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66:S153–64. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 96.Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 97.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–28. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 98.Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, et al. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293:C1669–78. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 99.Rajgopal A, Edmondson A, Goldman ID, Zhao R. SLC19A3 encodes a second thiamine transporter, ThTr2. Biochim Biophys Acta. 2001;1537:175–78. doi: 10.1016/s0925-4439(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 100.Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood–cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96:3900–5. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ratnam M, Marquardt H, Duhring JL, Freisheim JH. Homologous membrane folate binding proteins in human placenta: cloning and sequence of cDNA. Biochemistry. 1989;28:8249–54. doi: 10.1021/bi00446a042. [DOI] [PubMed] [Google Scholar]
- 102.Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res. 2007;24:450–70. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- 103.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, et al. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ross JF, Wang H, Behm FG, Mathew P, Wu M, et al. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85:348–57. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 105.Russell RM, Dhar GJ, Dutta SK, Rosenberg IH. Influence of intraluminal pH on folate absorption: studies in control subjects and in patients with pancreatic insufficiency. J Lab Clin Med. 1979;93:428–36. [PubMed] [Google Scholar]
- 106.Russell RM, Golner BB, Krasinski SD, Sadowski JA, Suter PM, Braun CL. Effect of antacid and H2 receptor antagonists on the intestinal absorption of folic acid. J Lab Clin Med. 1988;112:458–63. [PubMed] [Google Scholar]
- 107.Said HM, Chatterjee N, Haq RU, Subramanian VS, Ortiz A, et al. Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol Cell Physiol. 2000;279:C1889–95. doi: 10.1152/ajpcell.2000.279.6.C1889. [DOI] [PubMed] [Google Scholar]
- 108.Said HM, Nguyen TT, Dyer DL, Cowan KH, Rubin SA. Intestinal folate transport, identification of a cDNA involved in folate transport and the functional expression and distribution of its mRNA. Biochim Biophys Acta Bio-Membr. 1996;1281:164–72. doi: 10.1016/0005-2736(96)00005-3. [DOI] [PubMed] [Google Scholar]
- 109.Said HM, Smith R, Redha R. Studies on the intestinal surface acid microclimate: developmental aspects. Pediatr Res. 1987;22:497–99. doi: 10.1203/00006450-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 110.Sand TE, Jacobsen S. Effect of urine pH and flow on renal clearance of methotrexate. Eur J Clin Pharmacol. 1981;19:453–56. doi: 10.1007/BF00548590. [DOI] [PubMed] [Google Scholar]
- 111.Segal MB. The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell Mol Neurobiol. 2000;20:183–96. doi: 10.1023/A:1007045605751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Segal MB. Transport of nutrients across the choroid plexus. Microsc Res Tech. 2001;52:38–48. doi: 10.1002/1097-0029(20010101)52:1<38::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 113.Selhub J, Franklin WA. The folate-binding protein of rat kidney. Purification, properties, and cellular distribution. J Biol Chem. 1984;259:6601–6. [PubMed] [Google Scholar]
- 114.Selhub J, Nakamura S, Carone FA. Renal folate absorption and the kidney folate binding protein. II Microinfusion studies. Am J Physiol. 1987;252:F757–60. doi: 10.1152/ajprenal.1987.252.4.F757. [DOI] [PubMed] [Google Scholar]
- 115.Selhub J, Rosenberg IH. Folate transport in isolated brush border membrane vesicles from rat intestine. J Biol Chem. 1981;256:4489–93. [PubMed] [Google Scholar]
- 116.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 117.Shen F, Wu M, Ross JF, Miller D, Ratnam M. Folate receptor type gamma is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: protein characterization and cell type specificity. Biochemistry. 1995;34:5660–65. doi: 10.1021/bi00016a042. [DOI] [PubMed] [Google Scholar]
- 118.Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the human proton-coupled folate transporter (PCFT-SLC46A1), a D156Y mutation causing hereditary folate malabsorption. Blood. 2010;116:5162–69. doi: 10.1182/blood-2010-06-291237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spiegelstein O, Mitchell LE, Merriweather MY, Wicker NJ, Zhang Q, et al. Embryonic development of folate binding protein-1 (Folbp1) knockout mice: effects of the chemical form, dose, and timing of maternal folate supplementation. Dev Dyn. 2004;231:221–31. doi: 10.1002/dvdy.20107. [DOI] [PubMed] [Google Scholar]
- 120.St Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1495–503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- 121.Stark M, Gonen N, Assaraf YG. Functional elements in the minimal promoter of the human proton-coupled folate transporter. Biochem Biophys Res Commun. 2009;388:79–85. doi: 10.1016/j.bbrc.2009.07.116. [DOI] [PubMed] [Google Scholar]
- 122.Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, et al. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85:354–63. doi: 10.1016/j.ajhg.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am J Physiol Cell Physiol. 2008;294:C233–40. doi: 10.1152/ajpcell.00468.2007. [DOI] [PubMed] [Google Scholar]
- 124.Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417–22. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi T, Utoguchi N, Takara A, Yamamoto N, Nakanishi T, et al. Carrier-mediated transport of folic acid in BeWo cell monolayers as a model of the human trophoblast. Placenta. 2001;22:863–69. doi: 10.1053/plac.2001.0742. [DOI] [PubMed] [Google Scholar]
- 126.Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002;302:666–71. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 127.Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci. 2007;48:5299–305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem. 2009;284:17846–57. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol. 2009;297:C66–74. doi: 10.1152/ajpcell.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochim Biophys Acta. 2008;1178:1407–14. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB. The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G248–54. doi: 10.1152/ajpgi.00224.2009. [DOI] [PubMed] [Google Scholar]
- 132.Uwai Y, Okuda M, Takami K, Hashimoto Y, Inui K. Functional characterization of the rat multispecific organic anion transporter OAT1 mediating basolateral uptake of anionic drugs in the kidney. FEBS Lett. 1998;438:321–24. doi: 10.1016/s0014-5793(98)01328-3. [DOI] [PubMed] [Google Scholar]
- 133.Verbeek MM, Blom AM, Wevers RA, Lagerwerf AJ, van de GJ, Willemsen MA. Technical and biochemical factors affecting cerebrospinal fluid 5-MTHF, biopterin and neopterin concentrations. Mol Genet Metab. 2008;95:127–32. doi: 10.1016/j.ymgme.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 134.Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63:5538–43. [PubMed] [Google Scholar]
- 135.Wang H, Zheng X, Behm FG, Ratnam M. Differentiation-independent retinoid induction of folate receptor type beta, a potential tumor target in myeloid leukemia. Blood. 2000;96:3529–36. [PubMed] [Google Scholar]
- 136.Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res. 2004;10:6256–64. doi: 10.1158/1078-0432.CCR-04-0645. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 138.Wasson GR, McGlynn AP, McNulty H, O’Reilly SL, McKelvey-Martin VJ, et al. Global DNA and p53 region-specific hypomethylation in human colonic cells is induced by folate depletion and reversed by folate supplementation. J Nutr. 2006;136:2748–53. doi: 10.1093/jn/136.11.2748. [DOI] [PubMed] [Google Scholar]
- 139.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401. [PubMed] [Google Scholar]
- 140.Weitman SD, Weinberg AG, Coney LR, Zurawski VR, Jennings DS, Kamen BA. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res. 1992;52:6708–11. [PubMed] [Google Scholar]
- 141.Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter. Biochem J. 2002;367:629–40. doi: 10.1042/BJ20020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wielinga P, Hooijberg JH, Gunnarsdottir S, Kathmann I, Reid G, et al. The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res. 2005;65:4425–30. doi: 10.1158/0008-5472.CAN-04-2810. [DOI] [PubMed] [Google Scholar]
- 143.Wong SC, Zhang L, Proefke SA, Matherly LH. Effects of the loss of capacity for N-glycosylation on the transport activity and cellular localization of the human reduced folate carrier. Biochim Biophys Acta. 1998;1375:6–12. doi: 10.1016/s0005-2736(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 144.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–46. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 145.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53:6811–24. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 146.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–59. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 147.Yasuda S, Hasui S, Kobayashi M, Itagaki S, Hirano T, Iseki K. The mechanism of carrier-mediated transport of folates in BeWo cells: the involvement of heme carrier protein 1 in placental folate transport. Biosci Biotechnol Biochem. 2008;72:329–34. doi: 10.1271/bbb.70347. [DOI] [PubMed] [Google Scholar]
- 148.Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–87. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- 149.Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin Cancer Res. 2004;10:8735–42. doi: 10.1158/1078-0432.CCR-04-0932. [DOI] [PubMed] [Google Scholar]
- 150.Zhao R, Gao F, Goldman ID. Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. Am J Physiol Cell Physiol. 2002;282:C1512–17. doi: 10.1152/ajpcell.00547.2001. [DOI] [PubMed] [Google Scholar]
- 151.Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res. 2004;10:718–27. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- 152.Zhao R, Gao F, Wang Y, Diaz GA, Gelb BD, Goldman ID. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J Biol Chem. 2000;276:1114–18. doi: 10.1074/jbc.M007919200. [DOI] [PubMed] [Google Scholar]
- 153.Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–57. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- 154.Zhao R, Hanscom M, Chattopadhyay S, Goldman ID. Selective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier: association with the presence of a secondary transport pathway. Cancer Res. 2004;64:3313–19. doi: 10.1158/0008-5472.can-03-3953. [DOI] [PubMed] [Google Scholar]
- 155.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, et al. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110:1147–52. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009;284:4267–74. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities as compared to the reduced folate carrier. Mol Pharmacol. 2008;74:854–62. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao R, Russell RG, Wang Y, Liu L, Gao F, et al. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem. 2001;276:10224–28. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]
- 160.Zhao R, Seither R, Brigle KE, Sharina IG, Wang PJ, Goldman ID. Impact of overexpression of the reduced folate carrier (RFC1), an anion exchanger, on concentrative transport in murine L1210 leukemia cells. J Biol Chem. 1997;272:21207–12. doi: 10.1074/jbc.272.34.21207. [DOI] [PubMed] [Google Scholar]
- 161.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry. 2010;49:2925–31. doi: 10.1021/bi9021439. [DOI] [PMC free article] [PubMed] [Google Scholar]