Abstract
Background: Myths and concerns about the extent and meaning of genetic risk in schizophrenia may contribute to significant stigma and burden for families. Genetic counseling has long been proposed to be a potentially informative and therapeutic intervention for schizophrenia. Surprisingly, however, available data are limited. We evaluated a contemporary genetic counseling protocol for use in a community mental health-care setting by non–genetics professionals. Methods: We used a pre-post study design with longitudinal follow-up to assess the impact of genetic counseling on family members of individuals with schizophrenia, where molecular testing had revealed no known clinically relevant genetic risk variant. We assessed the outcome using multiple measures, including standard items and scales used to evaluate genetic counseling for other complex diseases. Results: Of the 122 family members approached, 78 (63.9%) actively expressed an interest in the study. Participants (n = 52) on average overestimated the risk of familial recurrence at baseline, and demonstrated a significant improvement in this estimate postintervention (P < .0001). This change was associated with an enduring decrease in concern about recurrence (P = .0003). Significant and lasting benefits were observed in other key areas, including increased knowledge (P < .0001) and a decreased sense of stigma (P = .0047). Endorsement of the need for genetic counseling was high (96.1%). Conclusions: These results provide initial evidence of the efficacy of schizophrenia genetic counseling for families, even in the absence of individually relevant genetic test results or professional genetics services. The findings support the integration of contemporary genetic counseling for families into the general management of schizophrenia in the community.
Key words: schizophrenia, genetics, genetic counseling, genetic predisposition to disease, copy number variation, stigma
Introduction
Despite definite progress in understanding the origins of schizophrenia,1 many myths remain concerning etiology and risk factors.2 This may contribute to the considerable stigma and burden of the disease for families.3 There are preliminary data that suggest family members of individuals with schizophrenia may overestimate the familial recurrence risk (ie, the likelihood of the illness “running in the family”).4 As for other complex diseases,5–8 this may result in undue anticipatory anxiety and concern, and could influence reproductive decision making (eg, in unaffected siblings).4 Genetic counseling is the process of communicating information and supporting families around these important issues,9,10 in order to facilitate understanding and adaptation to the medical, psychological, and familial implications of the genetic and nongenetic contributions to a disease.11
Although many have proposed that genetic counseling for schizophrenia may be an informative and therapeutic intervention,9,12–17 a systematic review of the existing literature (Supplementary Methods and table S1) demonstrates that much has been written about the genetic counseling approach but, surprisingly, actual data are limited. There were three studies providing survey data that supported a high rate of hypothetical interest in genetic counseling,16,18,19 but there were no studies documenting the rate of uptake of genetic counseling for schizophrenia when offered and just one pilot study of genetic counseling by a board-certified genetic counselor with outcome data for nine parents (Supplementary Methods).20 Rigorous outcome data are needed to justify any intervention in the current clinical climate, and may also help to resolve key controversies such as the nature of the association between neurobiological explanations for serious mental illness and stigma.21,22
Results of this comprehensive review and our ongoing work to identify rare variants that are clinically relevant to schizophrenia also inspired a re-examination of the traditional approaches to genetic counseling for this illness.1 Providing a candid account of the current state of knowledge and a thoughtful consideration of future prospects is a guiding principle of genetic counseling, even in the absence of individually relevant genetic test results.1 Nongenetic factors, however modest in effect size and intractable to change, and the perceived failures of thousands of research-related candidate gene, linkage, and genome-wide association studies to identify a single major locus for schizophrenia, are routinely discussed. Yet typically there has been little to no discussion of clinically relevant molecular genetic discoveries in schizophrenia (table 1), even in contemporary accounts.23
Table 1.
Core Content Regarding Etiology in the Provision of Genetic Counseling for Schizophrenia (c 2012)
| Classic Findings About Schizophrenia From Epidemiological Studiesa (Including Historic and More Recent Family, Twin, and Adoption Studies) |
| A disease found in all societies with generally stable incidence and high prevalence worldwide |
| High "heritability," with predisposition largely genetically determined |
| Complex patterns of inheritance (ie, not with typical Mendelian patterns) |
| Reduced penetrance and variable expression (twin studies) |
| Positive family history in close relative(s) is a major risk factor, with empiric recurrence risk estimates available |
| Various proposed non-genetic risk factors but none known of medium or greater effect |
| Evidence for neurodevelopmental origins (eg, association with learning difficulties) |
| Prediction of de novo (spontaneous) mutations in the face of decreased reproductive fitness of affected individuals |
| New Findings About Schizophrenia From Neuroscience Studiesa (Including Imaging and Molecular Genetic Studies) |
| Genetic heterogeneity, with no single major locus (common disease—multiple rare variant model) |
| An established clinical genetic subtype (22q11.2 deletion syndrome) affects about one in 100 patients with schizophrenia |
| Additional emerging genetic subtypes of schizophrenia are associated with other large rare copy number variants (CNVs) |
| Reduced penetrance and variable expression of these large rare CNVs |
| Lack of common genetic variants that are clinically relevant |
| Minor effects consistent with the possibility of gene × environment interactions |
| Genome-wide genetic testing for schizophrenia is not routinely used in a clinical setting (c 2012) |
| Evidence for neurodevelopmental origins (eg, premorbid subtle structural brain changes, though not useful diagnostically) |
| Initial evidence for increased burden of de novo rare CNVs and very rare exonic sequence mutations (with promise of more to come) |
aSee Costain et al.1 for key references.
We therefore elected to develop, standardize, and conduct preliminary testing of a contemporary genetic counseling protocol for schizophrenia. We aimed to demonstrate efficacy in a common real-world situation: (1) in a community mental health-care setting, (2) with counseling provided by a non–genetics professional, and (3) in the absence of relevant individual genetic test results. We anticipated that interest in the intervention would be high, and that, at baseline, family members would not have an accurate appreciation of familial recurrence risks. We tested the hypotheses that genetic counseling would (1) improve understanding of the true empiric risk estimate, (2) decrease concern and anxiety related to illness recurrence risk in the family, and (3) increase knowledge about the etiology of schizophrenia, with a potential impact on stigma and blame.
Methods
Participants
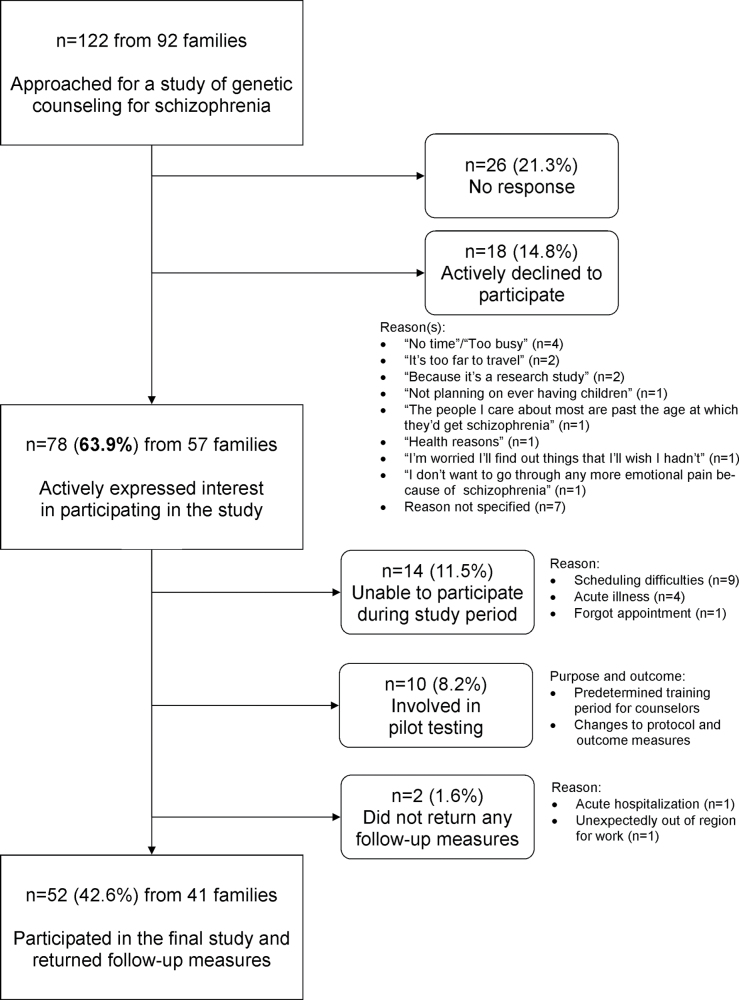
Study participants were family members of unrelated adults (age ≥18 years) with DSM-IV diagnoses of schizophrenia or schizoaffective disorder (collectively termed “schizophrenia”). The process of (1) recruitment, (2) confirmation of diagnoses, (3) acquisition of detailed medical and family histories, (4) clinical screening, and (5) molecular characterization using high-resolution genome-wide microarrays of the source patient population, as part of a genetic case-control study, is described elsewhere.24 The study design allowed us to exclude the minority of individuals found to have karyotypic abnormalities, 22q11.2 deletions, or other recurrent copy number variants (CNVs) clinically relevant to schizophrenia.1,9,13 These individuals and their family members were counseled separately on a clinical basis by a genetics professional. Of the remaining patients, 92 agreed to provide us with contact information for a total 122 of their immediate adult relatives living in the area. We then approached these family members through a personalized letter and up to two telephone calls; a brief explanation of genetic counseling was provided. Where possible, for those who actively declined to participate we noted reason(s) given. After complete description of the study, written informed consent was obtained. No monetary compensation was provided to participants.
Intervention
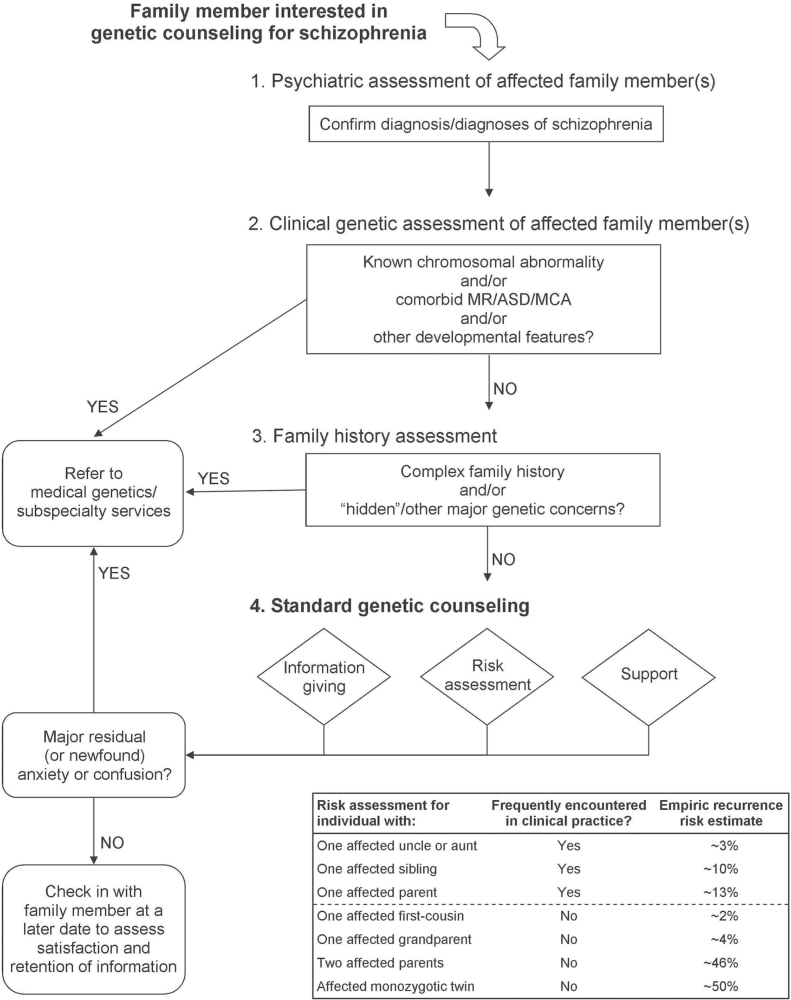
We developed and standardized a genetic counseling protocol for schizophrenia for implementation by mental health-care providers in a community setting (figure 1), based on tested protocols for other complex diseases25 and the existing schizophrenia literature1,9,13,26 (supplementary table S1). Table 1 shows core content about etiology. The intervention (mean 64.3 [SD 14.7] min) was delivered to participants individually except in six cases where pairs of family members were counseled together at their request. Counseling was provided by a graduate student (G.C.) or a research assistant, with oversight and supervision provided by experienced clinicians with expertise in this area (A.S.B., M.J.E.). All aspects of a standard genetic counseling assessment were performed (figure 1).9–11 In particular, participants received the best possible personalized risk assessments in the context of their family histories, as described in detail elsewhere.9,27,28 We also discussed with each participant specific medical and family history factors that may qualitatively affect recurrence risk in their family, but where no rigorous, evidence-based way of quantitatively modifying standard empiric recurrence risk estimates exists at this time (eg, putative environmental exposures).9,27,28 The manual, including sample dialogue and responses to frequently asked questions, is available upon request.
Fig. 1.
Proposed workflow algorithm for genetic counseling for schizophrenia (c 2012). A “complex family history” would include, eg, multiple family members with schizophrenia and/or other psychotic disorders, known consanguinity, or other medical illnesses of concern. Standard genetic counseling involves (1) information giving (“education about inheritance, testing, management, prevention, resources and research”11), (2) risk assessment (“interpretation of family and medical histories to assess the chance of disease occurrence or recurrence”11); and (3) support (“counseling to promote informed choices and adaptation to the risk or condition”11). See Hodgkinson et al.9 for empiric recurrence risk ranges and primary sources. Note that these crude risk estimates must be interpreted with caution, as they represent averages from diverse historical studies and can sometimes be modified based on an individual family’s circumstances (see text). ASD, autism spectrum disorder; MCA, multiple congenital anomalies; MR, mental retardation.
Measures
At baseline, we corroborated the psychiatric family histories previously derived from interviewing the probands and reviewing their lifetime medical records,24 and collected demographic and other clinical information. Each participant completed a battery of self-administered psychological and knowledge-based questionnaires inspired by scales used to evaluate genetic counseling for other complex diseases.25,29 Baseline measures were completed in person; follow-up measures at 1 and 7 weeks postintervention were mailed back using stamped, preaddressed envelopes.
Recurrence Risk Perception and Its Consequences. Following standard practice,8 complementary approaches were used to assess each participant’s perceived lifetime morbid risk for schizophrenia to a specific family member: (1) a visual analog scale; (2) a 5-point (“Very low” to “Very high”) Likert-scale item measuring qualitative risk (ie, description of the risk level); and (3) a 5-point (“Much lower” to “Much higher”) Likert-scale item concerning comparative risk (ie, as compared with the general population). We defined a quantitative risk estimate ≥10% different from the best individualized recurrence risk assessment as inaccurate.5 Concern about genetic risk and illness recurrence was queried using: (1) a visual analog scale (0% = “Not at all concerned” to 100% = “Extremely concerned”), and (2) a modified version of the “Nonintrusiveness” subscale of the Psychological Adaption to Genetic Information Scale (PAGIS),30 which measures freedom from genetic and/or recurrence related uncontrolled spontaneous thoughts and feelings. Opinions concerning statements suggesting people with schizophrenia, or their family members, should not have children were probed using 4-point (“Strongly disagree” to “Strongly agree”) Likert-scale items.
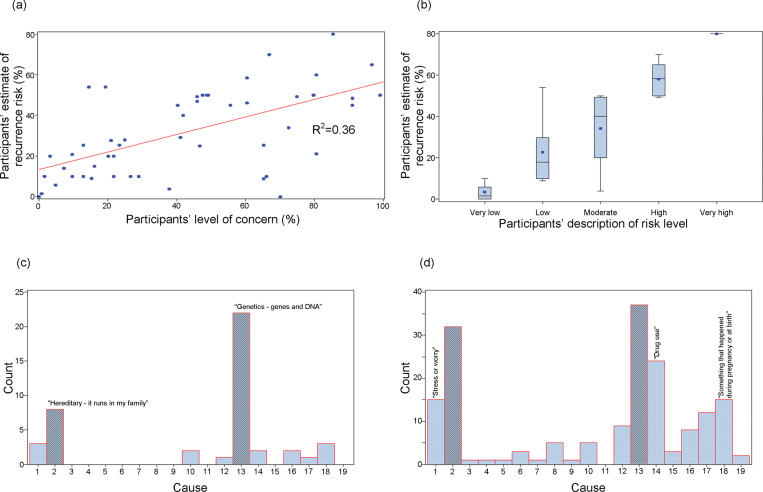
Knowledge and Etiological Attribution. Perceived (subjective) knowledge of schizophrenia etiology was assessed using a visual analog scale (0% = “Don’t understand at all” to 100% = “Understand very clearly”). Participants also completed a questionnaire developed for the study comprising 24 true or false items (supplementary table S3), as a measure of objective knowledge. To contextualize the response profile of the participants, a convenience sample of 22 junior psychiatric residents at a single institution had completed these items as well. This knowledge quiz was found upon testing with the initial group of psychiatric residents to have adequate internal consistency (Cronbach’s α = 0.80). Etiological attribution of schizophrenia in the participant's family was queried in an open-ended fashion, but we included a list of 18 possible factors adapted from the revised Illness Perception Questionnaire (figure 3c).31
Fig. 3.
Participants’ responses to selected items prior to genetic counseling. (a) Estimated risk of recurrence to a specific family member vs concern about familial recurrence. (b) Estimated risk of recurrence by qualitative description of risk. (c) Primary cause of probands’ schizophrenia, as perceived by participants. (d) All possible causes of probands’ schizophrenia, as perceived by participants. See Methods section for item details. Cause coding key: 1 = Stress of worry, 2 = Hereditary - it runs in my family, 3 = A germ or virus, 4 =Diet or eating habits, 5 = Chance or bad luck, 6 = Bad parenting, 7 = Pollution in the environment, 8 = Own behavior, 9 = Negative thinking, 10 = Family problems, 11 = Overwork, 12 = Alcohol, 13 = Genetics - genes and DNA, 14 = Drug use, 15 = Bad life choices, 16 = Childhood trauma, 17 = Head injury, 18 = Something that happened during pregnancy or at birth, 19 = Other.
Stigma, Psychological Symptoms, and Satisfaction. To investigate stigma, we used the Experience of Caregiving Inventory (ECI) stigma subscale.32 This measure is composed of five 5-point (“Never” to “Nearly always”) Likert-type scale items and is known to correlate with psychological functioning in family members of individuals with schizophrenia.33 We included (1) visual analog scales (0% = “Not at all” to 100% = “Completely”) concerning both personal contribution to, and self-blame surrounding, the proband's schizophrenia and (2) two 4-point (“Strongly disagree” to “Strongly agree”) Likert-scale items about whether the proband or anyone else was to blame. The widely validated Brief Symptom Inventory (BSI) was used to measure global psychological functioning.34 The BSI measures nine psychological symptom dimensions based on 5-point (“Not at all” to “Extremely”) Likert-type scale responses to 53 items, and provides a global severity index (GSI). We considered depression and anxiety subscores and the GSI as outcome variables, as in our previous study.25 Satisfaction with genetic counseling was documented using five 5-point (“Strongly disagree” to “Strongly agree”) Likert-scale items (supplementary table S6).
Analyses
We tested for within-participant differences in responses provided at baseline and 1 week postintervention, and to assess the retention of any change at 7 weeks postintervention. Visual inspection and the Cramér-von Mises criterion were used to test the normality of each difference distribution, with subsequent use of the paired Student t test (t) or the non-parametric paired Wilcoxon signed-rank test (S) as appropriate. All participants were treated as independent. Coefficients of determination (R 2) were calculated from single-variable linear regression models. Mean responses of participant and psychiatric residents were compared using the non-parametric Mann-Whitney U test (U). Missing values in follow-up measures, where necessary for a cumulative score, were replaced with baseline responses to bias away from change. However, optimization of item display and questionnaire layout during the pilot testing phase resulted (for the study) in a rate of missing data of <1% in all but one case (knowledge quiz at seven weeks post-intervention = 1.8%). The results of all statistical comparisons are reported in supplementary tables S4–S6. All analyses were two-tailed and performed using SAS version 9.2 (SAS Institute, Inc., Cary, N.C.), with statistical significance defined as P < .05.
Results
Uptake
We approached the 122 known adult family members of those patients affiliated with one of two community mental health centers (figure 2). Seventy-eight (63.9%) actively expressed an interest in the study (figure 2). Those who did and did not express interest differed significantly only by level of education, being higher in those interested (supplementary table S2). The demographics of the 52 family members who participated in the final study and returned outcome measures are shown in table 2.
Fig. 2.
Interest in genetic counseling amongst family members of individuals with schizophrenia. The source patient population was recruited from two community mental health clinics.
Table 2.
Demographic and Clinical Characteristics of Study Participants and the Corresponding Probands With Schizophrenia
| Family Member Participants (n = 52) | Corresponding Probands With Schizophrenia (n = 41) | |||
|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |
| Age at time of intervention (years) | 60.2 | (10.3) | 43.1 | (12.3) |
| Age at onset of psychosis (years)a | — | — | 22.0 | (4.6) |
| Number of liveborn offspring | 2.8 | (1.6) | 0.6 | (1.0) |
| N | (%) | N | (%) | |
| Female sex | 40 | (76.9) | 16 | (39.0) |
| European ancestry | 51 | (98.1) | 40 | (97.6) |
| Family history of psychosisb | c50 | (96.2) | 12 | (29.3) |
| Ever marriedd | 48 | (92.3) | 13 | (31.7) |
| Childless | 7 | (13.5) | 29 | (70.7) |
| Education | ||||
| Did not complete high school | 7 | (13.5) | 16 | (39.0) |
| Completed high school (only) | 13 | (25.0) | 10 | (24.4) |
| Completed some postsecondary education | 32 | (61.5) | 15 | (36.6) |
| Relationship to proband | ||||
| Parent | 31 | (59.6) | — | — |
| Sibling | 15 | (28.8) | — | — |
| Othere | 6 | (11.5) | — | — |
| Primary source of concern about recurrence, in relation to proband | ||||
| Niece/nephew | 28 | (53.8) | — | — |
| Offspringf | 10 | (19.2) | — | — |
| Siblingg | 7 | (13.5) | — | — |
| Third or fourth degree relative | 7 | (13.5) | — | — |
aDefined as age at first treatment for psychosis; none had childhood onset schizophrenia.
bDefined as having one or more first or second degree relative(s) with a psychotic disorder.
cParticipant was a spouse in two cases, with no personal or family history of psychosis.
dIncludes marriage or a common-law arrangement of one year or more.
eSpouse (n = 2), aunt (n = 2), offspring (n = 1), half-sibling (n = 1).
fNone with two parents with schizophrenia.
gIncluding two participants (husband and wife) who were concerned about the risk to the monozygotic twin of their affected son.
Recurrence Risk Perception and Its Consequences
At baseline, the majority of participants were most concerned about the risk to a niece or nephew of an individual with schizophrenia (table 2), where the mean unmodified empiric recurrence risk estimate is about 3%.13 Participants reported high levels of concern about risk (figure 4b), with recurrence risk typically overestimated by nearly an order of magnitude at baseline (figure 4a). Concern and estimated recurrence risk were positively correlated [F(1,51) = 27.9, P < .0001; adjusted R2 = 0.36] (figure 3a). There was also a positive correlation between quantitative and qualitative estimates of risk [F(1,51) = 62.5, P < .0001; adjusted R2 = 0.55] (figure 3b).
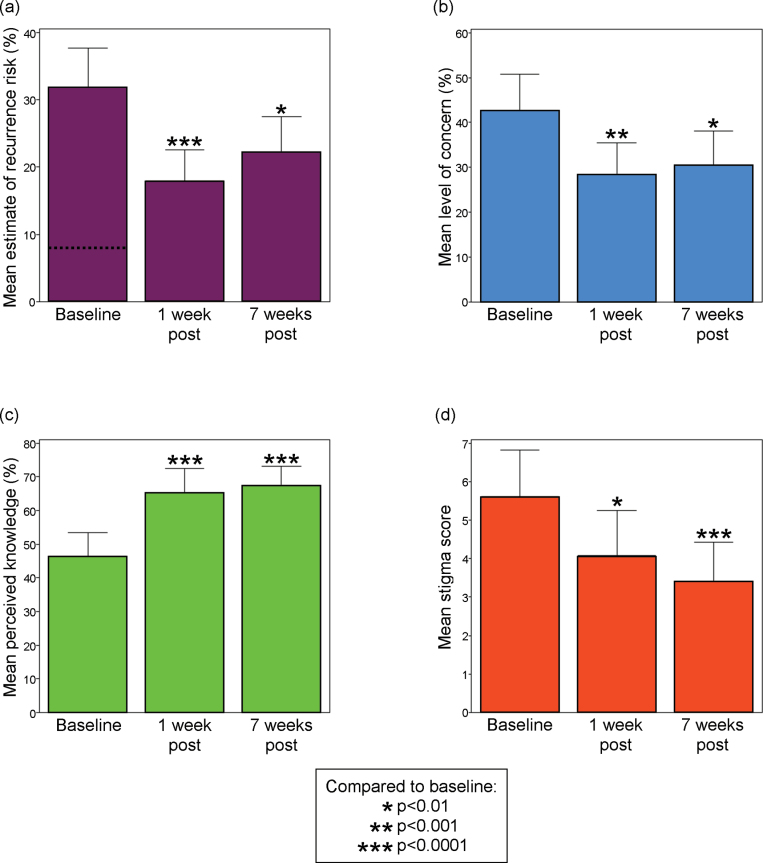
Fig. 4.
Selected significant and lasting benefits of genetic counseling for schizophrenia. (a) Participants’ estimated risk of familial recurrence to a specific family member (dashed line indicates mean personalized recurrence risk assessment). (b) Participants’ concern related to familial recurrence. (c) Participants’ perceived knowledge about the illness. (d) Participants’ sense of stigma. See Methods section for item details. All summary data and test statistics are presented in supplementary table S4. Error bars represent upper 95% confidence intervals.
After the intervention, the mean recurrence risk estimate decreased by nearly half (figure 4a), with a concomitant decrease in mean recurrence-related concern of a third (figure 4b). The proportion of participants who provided an inaccurate quantitative risk estimate decreased from 36 of 52 (35 overestimates) to 22 of 49 (20 overestimates; Fisher’s exact test P = .0267). Risk was also qualitatively perceived to be lower postintervention than at baseline (S = 257, df = 49, P < .0001), although comparative risk perception (ie, as compared with the general population risk) was typically appropriate before counseling and did not significantly change (S = 69.5, df = 49, P = .1240). Participants were less likely after counseling to endorse the statement suggesting that people with a family history of schizophrenia should not have children (S = 51.5, df = 42, P = .0221). The significant differences from baseline at 1 week postintervention remained significant at 7 weeks (supplementary table S4). There was also a significant improvement in the mean Nonintrusiveness subscore (S = 178, df = 50, P = .0145).
Knowledge and Etiological Attribution
Significant and lasting improvements were observed postintervention in mean knowledge quiz score (1 week postintervention: t = 5.8, df = 50, P < .0001; 7 weeks postintervention: S = 364.5, df = 48, P < .0001) and in mean perceived knowledge of schizophrenia etiology (figure 4c). As compared with psychiatric residents, at baseline, participants had a significantly lower mean knowledge quiz score (18.7 vs 21.8; U = 999, df = [22, 52], P < .0001) and a nonsignificantly lower mean level of perceived knowledge (46.5% vs 59.2%; U = 640.5, df = [19,52], P = .0570]. At 1 week postcounseling, the participant mean knowledge quiz score was no longer significantly different from baseline psychiatric resident responses (21.0 vs 21.8; U = 699, df = [22,51], P = .0908) and perceived knowledge shifted to a nonsignificantly greater level than that of the residents (65.0% vs 59.2%; U = 608.5, df = [19,50], P = .0728). Responses by item for the knowledge quiz are displayed in supplementary table S3. Although the primary etiological attribution of schizophrenia was frequently genetic in nature (figure 3c), participants endorsed diverse factors (figure 3d).
Stigma, Psychological Symptoms, and Satisfaction
Consistent with our hypotheses, the mean ECI subscore that measured stigma was significantly lower at 1 week (S = 126.0, df = 51, P = .0047) and lower yet at 7 weeks (S = 311.5, df = 48, P < .0001) postintervention (figure 4d). All levels of blame measured by both Likert and visual analog scale items, although low at baseline, on average decreased further following the intervention (supplementary table S5). Postcounseling, there was no change in the mean BSI depression subscore from baseline (S = 39.5, df = 51, P = .2650). However, the mean BSI GSI score was significantly lower at 1 week postintervention (t = 4.1, df = 50, P = .0002) but not at 7 weeks postintervention (t = 1.8, df = 47, P = .0826). Results appeared to be driven by a significant (S = 136.5, df = 51, P = .0016) and lasting (S = 113, df = 48, P = .0170) decrease in the mean anxiety subscore.
No participant reported having previously been offered “genetic counseling,” but many had gathered information by speaking with the proband’s psychiatrist, participating in workshops or conferences on schizophrenia, and undertaking independent research on the Internet. Satisfaction was high, with the majority of participants endorsing that they “felt better” after the intervention (94.1%), that the intervention was valuable to them (98.0%), and that genetic counseling should be offered to all adults with a family history of schizophrenia (96.1%) (supplementary table S6).
Discussion
To our knowledge, this is the first study that formally demonstrates the feasibility and worth of genetic counseling for schizophrenia in community mental health practice. Although the counseling was not provided by a genetic counselor, we followed fundamental tenets of genetic counseling,9–11 and incorporated genetic epidemiologic and molecular genetic information about schizophrenia into a standardized protocol (table 1). The results provide evidence for the benefits of psychiatric genetic counseling previously proposed in the literature,9,12,14–17 and may help to guide the future translation of new molecular genetic discoveries into clinical practice.
Uptake
We provide initial evidence of a high degree of uptake of genetic counseling for schizophrenia when it is actively offered. The rate was remarkably consistent with previous surveys of theoretical interest in schizophrenia,16,18,19 and preliminary data concerning psychotic disorders more generally.20
Recurrence Risk Perception and Its Consequences
In line with our hypothesis and as for many complex diseases,4,5,7,8,20 perceived familial recurrence risk was usually overestimated, often dramatically. Genetic counseling generally facilitated a better understanding of the true empiric risk estimate, as evidenced by appropriate decreases in the participants’ quantitative and qualitative risk estimates. Nevertheless, the mean quantitative risk estimate of participants postintervention remained higher than any known lifetime risk factor for schizophrenia, apart from having (1) a 22q11.2 deletion, (2) both parents with schizophrenia, or (3) a monozygotic twin with schizophrenia (relevant to only two of the participants’ individual circumstances; table 2).9,13 Continued overestimation of risk postcounseling is common.8 However, the ongoing inability to provide individualized quantitative risk estimates in the context of schizophrenia to the degree possible with, eg, the Gail model in breast cancer,5 and the provision of counseling by a non–genetics professional, may have contributed. Understanding why some individuals show no evidence of (or only temporary) improvement in risk perception remains a promising area for future study.35 Collectively however, these findings indicate that the limitations of existing empirical recurrence risk statistics, and the inability to personalize most risk estimates based on molecular genetic findings, are not major impediments to providing helpful genetic counseling.1,9,27
The genetic counseling intervention generally alleviated anxiety and did not increase distress, and therefore did not appear to cause undue worry or psychological harm. The decrease in concern related to familial recurrence was facilitated in part by an improved understanding of the true risk. The study design did not allow us to discern the contributions of other content and process factors to decreasing anxiety, such as the discussion of the potential benefits of early detection and treatment afforded by a familiarity with the illness.13 Nonetheless, these may also be important. Of note is that only one of the participants in this study was concerned about their own personal risk of developing schizophrenia, and this individual also had greater concern about recurrence risk and measured anxiety than the average participant (data not shown). It is also noteworthy that talking about personal experiences with serious mental illness did not appear to decrease mood.
Etiological Attribution, Knowledge, and Stigma
The etiological attributions of the participants were broadly consistent with a previous report involving relatives of adults with chronic schizophrenia, who may be more likely to endorse biological factors than relatives of first episode patients.36 Future studies could test the hypothesis that family members of individuals earlier in the course of illness may benefit even more from genetic counseling. However, although participants typically embraced genetic etiological attributions of schizophrenia at baseline, the knowledge measure data suggest that necessary contextual information might have been absent. The results supported the likelihood that genetic counseling can help to increase relevant knowledge about schizophrenia and confidence in that knowledge. We speculate that an improved and nuanced understanding of what is known and not known about the origins of schizophrenia may have helped to place current genetic advances in their proper context, dispel misconceptions, and engender a sense of mastery and control. These and other factors may have contributed to the significant decrease in stigma amongst family members of adults with schizophrenia. Possible negative relationships between neurobiological/genetic explanations of schizophrenia and stigma in the general population21 might similarly be addressable, eg, with the provision of public education to dispel common misconceptions about genetics, such as that “genetic” and “inherited” are synonymous terms.
Additional Advantages and Limitations
Detailed molecular and clinical characterization of the patients, inclusive sampling of family members, use of an updated evidence-based manual, and assessments at several time points using multiple standard outcome measures are major strengths of this study. For example, we found previously that universal screening with genome-wide microarrays was necessary to identify the majority of individuals with rare pathogenic CNVs in the source patient population (Bassett AS, Lionel AC, Costain G, et al., under review). Exclusion of their family members, for whom general genetic counseling and recurrence risk assessments as provided in this study would have been inappropriate, was a significant advantage. A natural redundancy in quantitative items provided additional confidence in our results. Where possible, we used measures previously validated and employed in the context of other complex diseases, thereby increasing the generalizability of our findings. All aspects of the study, including the provision of counseling by non–genetics professionals, were designed to facilitate implementation in general psychiatric practice. Notably, the results supported efficacy in the absence of the provision of individual genetic test results.
The novelty of the study is offset to some extent by several natural limitations that could be addressed in subsequent studies. The biases inherent in any nonrandomized, uncontrolled trial are well documented.37 We considered including a comparison group given no, or only written, genetic counseling information. However, given the paucity of available data, we decided to first focus on developing a standardized genetic counseling approach for schizophrenia and measuring its feasibility and preliminary effect. This study represents an essential first step towards more sophisticated study designs; that is, the study validated an initial approach and these preliminary findings are congruent with those of an effective intervention. Future randomized control trials could further test this intervention, both in comparison to other approaches and in order to improve understanding about the role of specific elements of the intervention.
Previous clinically based observations support our interpretations of the primary results.9,12,14–17 Nonetheless, more data are needed to both replicate these findings and to examine specific contextual factors (eg, family member, proband, and counselor characteristics) that may modulate interest and efficacy. Focusing only on one or more specific area(s) of concern or confusion would likely improve efficiency and limit the duration of the intervention; such tailoring was beyond the scope of this study. Extensive subgroup analyses were also not performed, although we note that there were no significant differences by site, counselor, or diagnosis of schizophrenia vs schizoaffective disorder (data not shown). With respect to generalizability, the participants were primarily well-educated mothers of Caucasian adults with chronic schizophrenia (table 2). They were also self-selected and highly motivated, although we excluded 10 family members who expressed interest earliest by involving them in the pilot-testing phase (figure 2) where results were similar (data not shown). This may have contributed to the relatively high level of baseline knowledge and rate of attribution of schizophrenia to genetic causes, and to their receptiveness to genetic counseling. On the other hand, the extent of interest and benefit documented in this study is notable given the age, duration of illness, and site of recruitment (community clinic, rather than research or university center) of the patient sample.
The Role for Genetic Counseling in the Clinic
With genetic research regularly the subject of media attention, those with a family history of any complex disease may increasingly request genetic counseling. There is an acknowledged shortage of qualified genetics professionals, however.5,38 For example, there are currently no medical geneticists or genetic counselors based in either of the two sites from which participants were recruited for this study. Also, many genetic counselors self-report personal discomfort, knowledge deficiencies, and stigma in regard to genetic counseling related to psychiatric illnesses, suggesting the need for further training and experience in psychiatric genetics.39
To decrease burden on medical genetics services, basic genetic counseling for schizophrenia could routinely be performed at point of contact with the family by any capable health-care provider following a clinically based standard protocol (figure 1). Our study is proof-of- principle that this may be a satisfactory approach, and is something most psychiatrists agree is within their scope of practice.40,41 Psychiatrists and other mental health-care providers should be well equipped to manage the psychological processes underlying genetic counseling, and better suited than genetic counselors to confirm psychiatric diagnoses and identify and treat symptomatic family members. There are concerns, however, about genetics training and competency.40,41 These could be addressed with focused continuing education initiatives and attention to clinically-relevant genetic information (table 1 and supplementary table S3), and increased collaboration between psychiatry and medical genetics.1 The fact that the results demonstrated receptiveness to genetic counseling, in addition to the evidence of benefit, may provide an additional impetus for clinicians to engage in genetic counseling with family members who are often caregivers of their patients with schizophrenia, and/or to refer to genetics services where possible and appropriate (figure 1).
Conclusion
The findings provide initial evidence of the efficacy of schizophrenia genetic counseling provided by non–genetics professionals, even where there are no individually relevant genetic test results. In addition to previously proposed benefits,9,12–17 these data suggest that genetic counseling for families could inform part of a multifaceted approach to stigma reduction. The results support the integration of contemporary genetic counseling for families into the general management of schizophrenia. Such informed discussions may ultimately help in “priming” individuals, families, and clinicians for further advances anticipated in the emerging “molecular age” in psychiatry and the potential for return of individually relevant molecular genetic findings in the future.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Acknowledgments
There are no actual or potential conflicts of interest to disclose. The authors thank the families for their participation, and staff at the Saint John Community Mental Health Centre, Saint John Regional Hospital, and Hillsborough Hospital. Kathryn Russell and Chelsea Lowther deserve special thanks. This work was supported by MindCare New Brunswick, Canadian Institutes of Health Research grants (MOP-111238, MOP-53216), a Vanier Canada Graduate Scholarship (G.C.), and a Canada Research Chair in Schizophrenia Genetics and Genomic Disorders (A.S.B.).
References
- 1. Costain G, Bassett AS. Clinical applications of schizophrenia genetics: genetic diagnosis, risk, and counseling in the molecular era. Appl Clin Genet. 2012;5:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bassett AS, Chow EW, Waterworth DM, Brzustowicz L. Genetic insights into schizophrenia. Can J Psychiatry. 2001;46:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Awad AG, Voruganti LN. The burden of schizophrenia on caregivers: a review. Pharmacoeconomics 2008;26:149–162 [DOI] [PubMed] [Google Scholar]
- 4. Austin JC, Smith GN, Honer WG. The genomic era and perceptions of psychotic disorders: genetic risk estimation, associations with reproductive decisions and views about predictive testing. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:926–928 [DOI] [PubMed] [Google Scholar]
- 5. Lerman C, Lustbader E, Rimer B, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Natl Cancer Inst. 1995;87:286–292 [DOI] [PubMed] [Google Scholar]
- 6. Meilleur KG, Littleton-Kearney MT. Interventions to improve patient education regarding multifactorial genetic conditions: a systematic review. Am J Med Genet A. 2009;149A:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peay HL, Hooker GW, Kassem L, Biesecker BB. Family risk and related education and counseling needs: perceptions of adults with bipolar disorder and siblings of adults with bipolar disorder. Am J Med Genet A 2009;149A:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. J Genet Couns. 2009;18:217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodgkinson KA, Murphy J, O’Neill S, Brzustowicz L, Bassett AS. Genetic counselling for schizophrenia in the era of molecular genetics. Can J Psychiatry. 2001;46:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peay HL, Rosen Sheidley B. Principles of genetic counseling. In: Smoller JW, Rosen Sheidley B, Tsuang MT, eds. Psychiatric Genetics: Applications in Clinical Practice. Arlington, VA: American Psychiatric Publishing, Inc.; 2008;27–45 [Google Scholar]
- 11. Resta R, Biesecker BB, Bennett RL, et al. A new definition of genetic counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15:77–83 [DOI] [PubMed] [Google Scholar]
- 12. Austin JC, Honer WG. The genomic era and serious mental illness: a potential application for psychiatric genetic counseling. Psychiatr Serv. 2007;58:254–261 [DOI] [PubMed] [Google Scholar]
- 13. Bassett AS, Chow EWC, Hodgkinson KA. Genetics of schizophrenia and psychotic disorders. In: Smoller JW, Rosen Sheidley B, Tsuang MT, eds. Psychiatric Genetics: Applications in Clinical Practice. Arlington, VA: American Psychiatric Publishing, Inc.; 2008;99–130 [Google Scholar]
- 14. Finn CT, Smoller JW. Genetic counseling in psychiatry. Harv Rev Psychiatry. 2006;14:109–121 [DOI] [PubMed] [Google Scholar]
- 15. Gottesman II, Wolfgram DL. Schizophrenia Genesis: The Origins of Madness. New York, NY: Freeman; 1991. [Google Scholar]
- 16. Schulz PM, Schulz SC, Dibble E, Targum SD, van Kammen DP, Gershon ES. Patient and family attitudes about schizophrenia: implications for genetic counseling. Schizophr Bull. 1982;8:504–513 [DOI] [PubMed] [Google Scholar]
- 17. Tsuang MT. Genetic counseling for psychiatric patients and their families. Am J Psychiatry. 1978;135:1465–1475 [DOI] [PubMed] [Google Scholar]
- 18. DeLisi LE, Bertisch H. A preliminary comparison of the hopes of researchers, clinicians, and families for the future ethical use of genetic findings on schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:110–115 [DOI] [PubMed] [Google Scholar]
- 19. Lyus VL. The importance of genetic counseling for individuals with schizophrenia and their relatives: potential clients’ opinions and experiences. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1014–1021 [DOI] [PubMed] [Google Scholar]
- 20. Austin JC, Honer WG. Psychiatric genetic counselling for parents of individuals affected with psychotic disorders: a pilot study. Early Interv Psychiatry. 2008;2:80–89 [DOI] [PubMed] [Google Scholar]
- 21. Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. “A disease like any other”? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. Am J Psychiatry. 2010;167:1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldman HH. Progress in the elimination of the stigma of mental illness. Am J Psychiatry. 2010;167:1289–1290 [DOI] [PubMed] [Google Scholar]
- 23. Peay HL, Austin JC. How to Talk with Families About Genetics and Psychiatric Illness. New York, NY: W. W. Norton & Company, Inc; 2011. [Google Scholar]
- 24. Bassett AS, Costain G, Fung WL, et al. Clinically detectable copy number variations in a Canadian catchment population of schizophrenia. J Psychiatr Res. 2010;44:1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esplen MJ, Hunter J, Leszcz M, et al. A multicenter study of supportive-expressive group therapy for women with BRCA1/BRCA2 mutations. Cancer. 2004;101:2327–2340 [DOI] [PubMed] [Google Scholar]
- 26. Matheson SL, Shepherd AM, Laurens KR, Carr VJ. A systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophr Res. 2011;133:133–142 [DOI] [PubMed] [Google Scholar]
- 27. Austin JC, Peay HL. Applications and limitations of empiric data in provision of recurrence risks for schizophrenia: a practical review for healthcare professionals providing clinical psychiatric genetics consultations. Clin Genet. 2006;70:177–187 [DOI] [PubMed] [Google Scholar]
- 28. Austin JC, Palmer CG, Rosen-Sheidley B, Veach PM, Gettig E, Peay HL. Psychiatric disorders in clinical genetics II: individualizing recurrence risks. J Genet Couns. 2008;17:18–29 [DOI] [PubMed] [Google Scholar]
- 29. Kasparian NA, Wakefield CE, Meiser B. Assessment of psychosocial outcomes in genetic counseling research: an overview of available measurement scales. J Genet Couns. 2007;16:693–712 [DOI] [PubMed] [Google Scholar]
- 30. Read CY, Perry DJ, Duffy ME. Design and psychometric evaluation of the Psychological Adaptation to Genetic Information Scale. J Nurs Scholarsh. 2005;37:203–208 [DOI] [PubMed] [Google Scholar]
- 31. Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (R-IPQ). Psychol Health. 2002;17:1–16 [Google Scholar]
- 32. Szmukler GI, Burgess P, Herrman H, Benson A, Colusa S, Bloch S. Caring for relatives with serious mental illness: the development of the Experience of Caregiving Inventory. Soc Psychiatry Psychiatr Epidemiol. 1996;31:137–148 [DOI] [PubMed] [Google Scholar]
- 33. Martens L, Addington J. The psychological well-being of family members of individuals with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2001;36:128–133 [DOI] [PubMed] [Google Scholar]
- 34. Derogatis LR. Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual 3rd ed. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 35. Austin JC. Re-conceptualizing risk in genetic counseling: implications for clinical practice. J Genet Couns. 2010;19:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Esterberg ML, Compton MT. Causes of schizophrenia reported by family members of urban African American hospitalized patients with schizophrenia. Compr Psychiatry 2006;47:221–226 [DOI] [PubMed] [Google Scholar]
- 37. Smith GD, Ebrahim S. Data dredging, bias, or confounding. BMJ. 2002;325:1437–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins FS. Preparing health professionals for the genetic revolution. JAMA. 1997;278:1285–1286 [PubMed] [Google Scholar]
- 39. Martin N, Mikhaelian M, Cytrynbaum C, et al. 22q11.2 Deletion Syndrome: attitudes towards disclosing the risk of psychiatric illness [published online ahead of print July 26, 2012]. J Genet Counsel.10.1007/s10897-012-9517-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finn CT, Wilcox MA, Korf BR, et al. Psychiatric genetics: a survey of psychiatrists’ knowledge, opinions, and practice patterns. J Clin Psychiatry. 2005;66:821–830 [DOI] [PubMed] [Google Scholar]
- 41. Hoop JG, Roberts LW, Green Hammond KA, Cox NJ. Psychiatrists’ attitudes, knowledge, and experience regarding genetics: a preliminary study. Genet Med. 2008;12:245–252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.