Abstract
Although urban birth, upbringing, and living are associated with increased risk of nonaffective psychotic disorders, few studies have used appropriate multilevel techniques accounting for spatial dependency in risk to investigate social, economic, or physical determinants of psychosis incidence. We adopted Bayesian hierarchical modeling to investigate the sociospatial distribution of psychosis risk in East London for DSM-IV nonaffective and affective psychotic disorders, ascertained over a 2-year period in the East London first-episode psychosis study. We included individual and environmental data on 427 subjects experiencing first-episode psychosis to estimate the incidence of disorder across 56 neighborhoods, having standardized for age, sex, ethnicity, and socioeconomic status. A Bayesian model that included spatially structured neighborhood-level random effects identified substantial unexplained variation in nonaffective psychosis risk after controlling for individual-level factors. This variation was independently associated with greater levels of neighborhood income inequality (SD increase in inequality: Bayesian relative risks [RR]: 1.25; 95% CI: 1.04–1.49), absolute deprivation (RR: 1.28; 95% CI: 1.08–1.51) and population density (RR: 1.18; 95% CI: 1.00–1.41). Neighborhood ethnic composition effects were associated with incidence of nonaffective psychosis for people of black Caribbean and black African origin. No variation in the spatial distribution of the affective psychoses was identified, consistent with the possibility of differing etiological origins of affective and nonaffective psychoses. Our data suggest that both absolute and relative measures of neighborhood social composition are associated with the incidence of nonaffective psychosis. We suggest these associations are consistent with a role for social stressors in psychosis risk, particularly when people live in more unequal communities.
Key words: psychosis, epidemiology, urban, social environment, inequality, deprivation, Bayesian modeling, schizophrenia
Introduction
Urban birth, upbringing, and living appear to be associated with increased risk of nonaffective psychotic syndromes,1,2 but not affective psychoses.3–5 For nonaffective psychoses, the accumulated evidence with respect to urbanicity meets many of the Bradford-Hill criteria for causation.6 For example, this phenomenon has been frequently replicated,7–17 in studies of different designs,7,10,11,13,14 and persists after control for different patterns of confounders, including family history,7,10,11 parental age at birth,10 substance use,7 age,10–12,14–17 season of birth,10 sex,10,12,15–17 marital status,14 education,12,14 immigration status,12,14 and ethnicity.8,9,16 Risk also follows a dose-response relationship with both degree of urbanicity and time resident in urban environments.11 Risk begins early in life and remains present through adolescence and into adulthood. Finally, familial liability to psychosis is modified after moving to or from more or less urbanized environments,12,18–20 suggesting an interplay between genetic and environmental factors influences psychosis risk.
Despite this, less is understood about the specific environmental factors that underpin these associations. Various factors have been hypothesized, including impaired nutrition,21 infection,22 and social stress.23 Higher risk in urban environments may theoretically be explained by increased transmission of infections associated with increased schizophrenia risk,22 or insufficient exposure to sunlight and vitamin D early in life in densely populated environments.24 In extreme conditions, such as famines, early life dietary malnutrition (particularly of key micronutrients related to normal one-carbon metabolism and brain development) appears to increase adulthood risk of psychotic disorders.25,26 It is unknown whether poor nutrition as a result of living in deprived, urban environments also increases psychosis risk. Furthermore, nutrition- and infection-related hypotheses assume that the critical exposure window is early in life, but the association between schizophrenia and urbanicity persists into adolescence and adulthood.7,16
Social environmental factors, which may operate throughout the life course, have received greater attention as an explanation of schizophrenia risk in urban areas. Incidence of schizophrenia is raised in more deprived communities,27 where social and economic strains are likely to be more severe. Other studies have observed higher rates of schizophrenia in neighborhoods with less social support,13,15,28–31 conceptualized using measures including social cohesion, fragmentation, and residential mobility. Emerging research suggests that some social factors may operate in a relative rather than absolute fashion32–36; eg, psychosis risk among migrants and their descendants appears to be partly conditional upon both the proportion of and the proximity to one’s own ethnic group in the community.34–36 Neighborhood socioeconomic inequality (ie, relative rather than absolute deprivation) may also be associated with schizophrenia risk though no study has yet investigated this in a multilevel, population-based epidemiological study of first-episode psychosis (FEP).
While recent epidemiological studies of psychosis have used multilevel techniques to simultaneously model individual and neighborhood effects,4,13,28,30,35,36 only 1 study4 has additionally accounted for spatial patterning in incidence that might betray important underlying covariation in environmental risk. However, this study did not control for individual-level social class or investigate which neighborhood factors accounted for neighborhood variation in psychosis risk. Using appropriate Bayesian hierarchical spatial models, we therefore sought to investigate whether the incidence of psychotic disorders varied between neighborhoods in East London, after adjustment for individual-level age, sex, ethnicity, and social class. We tested whether such variation was associated with several neighborhood-level environmental factors, including absolute (deprivation, social fragmentation, social cohesion, and population density) and relative (inequality, ethnic density, and ethnic separation) measures.
Methods
Study Design
Data were obtained from the East London first-episode psychosis (ELFEP) study,37,38 a large population-based incidence study conducted in 3 neighboring inner-city, ethnically diverse and socially deprived boroughs of London, UK: City & Hackney, Newham, and Tower Hamlets. Ethical approval was obtained from the local research ethics committee in East London.
Subject Ascertainment
We identified everyone aged 18–64 years resident in our catchment area who made contact with mental health services with a first episode of any probable psychosis, nonpsychotic mania, or bipolar disorder. The study took place over 24 months; December 1996–November 1998 in City & Hackney, December 1998–November 2000 in Newham and Tower Hamlets. Health services were contacted weekly to identify potential subjects. A leakage study was conducted to minimize missed subjects, using the method by Cooper et al.39 Subjects completed several assessments, including the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) and a sociodemographic schedule. Consensus diagnoses were made using the SCAN and all other available information, according to the Diagnostic and Statistical Manual (4th edition) (DSM-IV). Here, we investigated 2 outcomes; nonaffective psychoses (including schizophrenia, schizophreniform disorder, and schizoaffective disorder) (DSM-IV 295.xx, 297.xx, 298.8, 298.9) and the affective psychoses (DSM-IV 296.x4, 296.4, 296.89).
Residential address at first contact was geocoded to their respective statistical ward as the neighborhood unit of analysis. ELFEP included 60 such wards with a mean population size of 6195 (2001 census). We excluded 4 wards that covered London’s main financial district, the City of London, because the resident population was sparse and atypical. No incident subjects were observed in these areas. Subjects without fixed abode, or who could not otherwise be geocoded were excluded.
Variable Generation
Individual-Level Variables.
Age-at-first-contact was categorized into 5 age-bands; 18–24, 25–34, 35–44, 45–54, 55–64 years. Ethnicity was ascribed to 1 of 16 categories in the 2001 census using all available information, including self-ascription, place of birth, and parental place of birth. From this, we created a 10-category variable: white British, non-British white, black Caribbean, black African, mixed white and black Caribbean, other mixed ethnicities, Indian, Pakistani, Bangladeshi, and all other ethnic groups. Social class was based on main occupation, using decision rules provided for the Office for National Statistics’ National Statistics-Socioeconomic Classification (NS-SEC).40 We used a 7-category social class variable: managerial and professional, intermediate occupations, small account and own account workers (self-employed), lower supervisory and technical occupations, semiroutine and routine occupations, never worked and long-term unemployed, and “not classifiable” for other reasons (eg, students or people with inadequately stated occupations).
Neighborhood-Level Variables.
We characterized statistical wards according to several a priori environmental factors collected from routine data sources immediately prior to, or during, the case ascertainment period (table 1). Deprivation was estimated from 2004 English Indices of Deprivation (EID) using the Index of Multiple Deprivation, an aggregated deprivation score composed of 37 indicators obtained from routinely collected national surveys, generally estimated concomitantly with our case ascertainment period.41 Because deprivation scores were not published for wards directly but at the nested “lower super output area” (SOA), we estimated ward-level deprivation as the mean of SOA deprivation in each statistical ward, weighted by SOA population size. Income deprivation was also obtained from the EID (% of people in each SOA classified as income deprived). From this we derived inequality in income deprivation across all SOAs in each ward using the Gini coefficient (G).42 G gave the extent of dispersion in income deprivation (at SOA-level) away from a theoretical distribution where income deprivation took the same value for all SOA in each ward (ie, perfect equality). When G equalled zero, there was perfect equality and when G was 1, perfect inequality.
Table 1.
Overview of Neighborhood Variables in East London and England
| Variable | Percentile | Mean (SD) | Mann-Whitney 2-Sample Testz-Score; P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10th | Median | 90th | ||||||||
| Index of Multiple deprivationa | 11.5; < 0.01 | |||||||||
| East London | 35.6 | 42.5 | 51.5 | 43.8 (6.1) | ||||||
| England | 6.8 | 14.7 | 35.5 | 18.3 (11.9) | ||||||
| Income inequalityb | −6.7; < 0.01 | |||||||||
| East London | 6.3 | 11.5 | 18.7 | 13.2 (6.6) | ||||||
| England | 8.1 | 21.1 | 35.1 | 21.5 (10.3) | ||||||
| Population density (people per hectare) | 11.8; < 0.01 | |||||||||
| East London | 47.8 | 108.6 | 150.0 | 102.8 (37.7) | ||||||
| England | 0.6 | 13.1 | 49.9 | 20.9 (25.0) | ||||||
| Social fragmentation indexc | 9.9; < 0.01 | |||||||||
| East London | 1.9 | 4.0 | 5.6 | 3.9 (1.5) | ||||||
| England | −3.2 | −0.6 | 4.0 | 0.0 (3.1) | ||||||
| Voter turnout (%)d | — | |||||||||
| East London | 26.4 | 33.4 | 39.1 | 32.9 (4.7) | ||||||
| England | — | — | — | 28.2 (−) | ||||||
| Own-group ethnic density (%)e | ||||||||||
| White British | 23.9 | 42.3 | 53.6 | 40.4 (11.8) | — | |||||
| Non-British white | 5.0 | 7.9 | 15.9 | 10.0 (4.6) | — | |||||
| Black Caribbean | 2.5 | 8.0 | 15.1 | 8.3 (4.6) | — | |||||
| Black African | 2.9 | 10.1 | 17.4 | 9.9 (5.5) | — | |||||
| Indian | 1.4 | 2.6 | 17.8 | 6.0 (7.4) | — | |||||
| Pakistani | 0.5 | 1.2 | 11.7 | 3.5 (4.9) | — | |||||
| Bangladeshi | 2.5 | 7.1 | 40.1 | 14.1 (15.3) | — | |||||
| Mixed, white & black Caribbean | 0.5 | 1.2 | 1.7 | 1.2 (0.5) | — | |||||
| Mixed, other | 1.6 | 2.3 | 2.9 | 2.2 (0.5) | — | |||||
| Other ethnicities | 2.7 | 4.3 | 7.3 | 4.7 (2.2) | — | |||||
| Own-group ethnic separation (%)e | ||||||||||
| White British | 14.0 | 21.0 | 30.3 | 21.3 (5.9) | — | |||||
| Non-British white | 11.1 | 18.2 | 27.1 | 18.4 (6.0) | — | |||||
| Black Caribbean | 13.8 | 19.4 | 28.7 | 20.2 (5.1) | — | |||||
| Black African | 17.1 | 25.3 | 32.7 | 24.9 (6.2) | — | |||||
| Indian | 20.8 | 29.6 | 38.1 | 29.5 (7.2) | — | |||||
| Pakistani | 21.5 | 43.8 | 64.3 | 43.1 (16.1) | — | |||||
| Bangladeshi | 23.7 | 34.2 | 44.1 | 33.8 (8.5) | — | |||||
| Mixed, white & black Caribbean | 23.7 | 32.0 | 54.5 | 35.1 (12.1) | — | |||||
| Mixed, other | 20.4 | 25.5 | 31.0 | 25.6 (4.3) | — | |||||
| Other ethnicities | 19.2 | 22.7 | 29.7 | 24.0 (4.5) | — | |||||
aIMD 2004 scores are on a continuous scale where higher scores indicate greater deprivation.
bWithin-neighborhood disparity in % of people classified as income deprived at SOA-level from IMD 2004, estimated by Gini coefficient. Summary statistics for England are approximate, based on 6,685 of 7,932 statistical ward, because scores could not be derived for statistical ward containing only a single SOA.
cHigher scores indicate greater social fragmentation. Derived using the method of Allardyce et al. using aggregated z-scores for % unmarried, % living alone, % living at a different address in previous year, % of privately rented accommodation. Estimated from 2001 census.
dLocal elections only held in 3,072 of 5271 wards in England in 1998. Average turnout = 28.2%.73 No comparison between England & ELFEP was possible as ward-level turnout for England was unavailable.
eEthnic density (% of own ethnic group in neighborhood) & separation scores (% segregation of own ethnic group from others) not provided for England for brevity.
We used the 2001 census to estimate population density (people per hectare), own-group ethnic density, own-group ethnic separation, and social fragmentation. We defined own-group ethnic density in each statistical ward as the size of one’s own ethnic group as a proportion of total neighborhood population. Own-group ethnic separation is a related concept, but it measured the extent to which 1 ethnic group was segregated from other ethnic groups in a given neighborhood (see figure 1). We used the Index of Dissimilarity to measure ethnic separation,43 where scores could vary from 0 (no segregation) to 1 (complete segregation). We used the method of Allardyce et al.15 to construct an index of social fragmentation. For each statistical ward, we estimated the proportion of unmarried persons, people living alone, people living at a different address 1 year ago, and proportion of privately rented accommodation using the 2001 census. We calculated z-scores for each variable, which we summed into an overall index. We obtained voter turnout statistics at 1998 local government elections as a proxy indicator of social cohesion, as previously described.44 All neighborhood variables were standardized to have a mean of zero and SD of one.
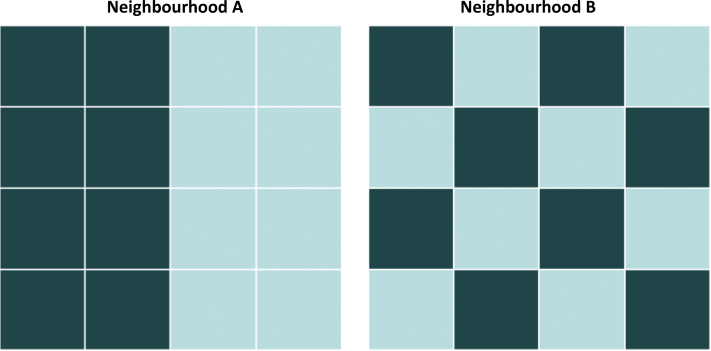
Fig. 1.
Conceptualization of neighborhood-level ethnic density and ethnic separation.
Neighborhoods A and B share the same level of ethnic density because the proportion of people shaded in the darker boxes is the same. However, the residential patterning—or ethnic separation—of this group differs in the 2 neighborhoods. In Neighborhood A there is perfect segregation of each ethnic group (ethnic separation = 1), while in Neighborhood B there is perfect integration of the 2 groups (ethnic separation = 0). For every statistical ward in the East London study region (n = 56), we estimated own-group ethnic density and ethnic separation for the 10 ethnic groups under study.
Statistical Analyses
To identify neighborhood variation in psychosis risk, we first estimated standardized incidence ratios (SIR) for each statistical ward, which gave the ratio of observed to expected subjects in each neighborhood compared with the overall ELFEP study region average, having indirectly standardized for age group, sex, ethnicity, and social class. SIRs greater than one indicate neighborhoods in the study region where risk was elevated, having standardized for individual-level factors.
Bayesian Hierarchical Modeling of Disease Risk.
SIRs are problematic for the small-area study of rare diseases. First, observed counts are assumed to follow a Poisson distribution, but this is often violated in small area studies of rare disorders because sampling variability in the count of cases across neighborhoods exceeds the mean (overdispersion).45 Second, neighborhoods closer together may have more similar SIR than neighborhoods further apart for important etiological reasons. This spatial autocorrelation must also be investigated and modeled.46 We adopted Bayesian hierarchical modeling to simultaneously account for these issues in a multilevel framework. An overview of this approach is provided here, with further information and model specification provided in online supplementary Appendix 1. As in a frequentist multilevel approach, Bayesian hierarchical models allow for the introduction of random effects to determine any variation attributable to the neighborhood level, independent of individual-level factors. However, under a Bayesian approach, all unknown parameters in the model, including random effects, are treated as stochastic, and are allowed to arise from a combination of prior beliefs—specified via prior distributions—and the data itself (see online supplementary Appendix 1).
This framework allowed us to fit several models with different prior assumptions about possible spatial dependency present in neighborhood-level random effects. This approach “smoothed” SIR in each statistical ward (Bayesian relative risks [RR]), by weighting them according to RR in neighboring areas, reducing sampling variability by borrowing information about psychosis risk from neighboring wards. We tested 4 different spatial models, each initially fitted without neighborhood covariates. Model 1 (M1) included an unstructured random effects term, which assumed no spatial autocorrelation and weighted RR in each statistical ward toward the overall study mean. Our second model (M2) included a structured variability term, which assumed RR in neighboring wards were more similar than in those further apart. The random effect was assumed to follow a conditional autoregressive normal prior distribution,47 weighting risk estimates in each statistical ward by those in immediately adjacent wards, specified via an “adjacency matrix” to define all neighborhood connections. Model 3, known as a convolution model, included both these random effect terms.47 Finally, we tested a mixture model (Model 4),48 which extended model 3 to include a third “jump” random effect term to model discontinuities in risk between noncontiguous neighborhoods in the study area.49
Model selection was based on several diagnostics, including mean absolute prediction error (MAPE), mean squared prediction error (MSPE), and the Deviance Information Criterion (DIC). MAPE and MSPE reported the average error between observed and fitted counts from each model. The DIC is a Bayesian equivalent to Aikaike’s Information Criterion, which gives an estimate of model fit penalized for complexity. Lower scores indicated better model fit.50 We inspected standardized residuals from all models to check they were distributed randomly. Final models did not exhibit evidence of nonnormality (data available from authors).
For each psychotic outcome, we added all neighborhood-level covariates, except own-group ethnic density and own-group ethnic separation, to our best-fitting spatial model using a backward-fitting modeling approach to identify our most parsimonious model of psychosis risk. Because values of own-group ethnic density and separation varied by ethnic group, we could not include these in the overall (combined ethnic groups) analyses. Instead, we included these variables in separate analyses for ethnic groups with a sufficient sample size to investigate spatial effects (n > 30).
Implementation of Bayesian Models.
Bayesian models use Monte Carlo Markov Chain (MCMC) simulation to combine the prior distribution with the data, leading to the posterior likelihood. Once all plausible values of the posterior distribution are thought to have been sampled, the model is said to have converged. We assessed convergence using Gelman-Rubin plots. We ran the model for 10 000 iterations after convergence on 2 independent MCMC chains (20 000 total) to provide mean parameter estimates and corresponding 95% credible intervals (95% CI) for all parameters in our models, including RR in each ward and effect sizes for any neighborhood covariates. When neighborhood covariates were added to the model, RRs in each statistical ward represented any residual risk not explained by covariates. We also obtained the probability that RR in a given neighborhood exceeded unity over all MCMC iterations. Probabilities greater than 0.8 are regarded as strong evidence of excess risk.46 Bayesian models were compared with the classical SIR approach (ISTDZE command, Stata version 11)51 and fitted in WinBUGS (version 1.4).52 Maps were constructed in GeoBUGS (version 1.2).52
Results
Subject Sample
We identified 484 FEP subjects over more than 1.6m person-years at-risk as previously reported.37,38 We excluded 20 (4.1%) subjects of no fixed abode, 35 (7.2%) with insufficient postcode data to be geocoded, and 2 (0.4%) later found to be resident outside the catchment area. They did not differ on sociodemographic characteristics (data available from authors), but excluded subjects were more likely to be diagnosed with nonaffective psychosis (86% vs 73%; χ2-test P = 0.04). We included 313 (73%) subjects with nonaffective psychosis and 114 (27%) subjects with affective psychosis in the present analyses.
Neighborhood Variables
East London neighborhoods were characterized by higher levels of deprivation, population density, and social fragmentation although income inequality was lower compared with England as a whole (table 1). Own-group ethnic density and ethnic separation in East London varied according to ethnic group. Aside from the majority white British group, median neighborhood ethnic density was highest for people of black African (10.1%; 10th–90th percentile [10-90PC]: 2.9–17.4) and black Caribbean (8.0%; 10-90PC: 2.5–15.1) origin, and lowest for people of Pakistani (1.2%; 10-90PC: 0.5–11.7) and mixed white and black Caribbean ethnicities (1.2%; 10-90PC: 0.5–1.7). Pakistani (43.8%; 10-90PC: 21.5–64.3) and Bangladeshi (34.2%; 10-90PC: 23.7–44.1) groups had the highest median neighborhood levels of ethnic separation from other groups, with lowest separation (ie, greatest integration) observed for people of black Caribbean (19.4%; 10-90PC: 13.8–28.7) and non-British white ethnicities (18.2%; 10-90PC: 11.1–27.1).
Bayesian Analyses of Nonaffective Psychotic Disorders
Spatial Patterning of Nonaffective Psychosis Risk.
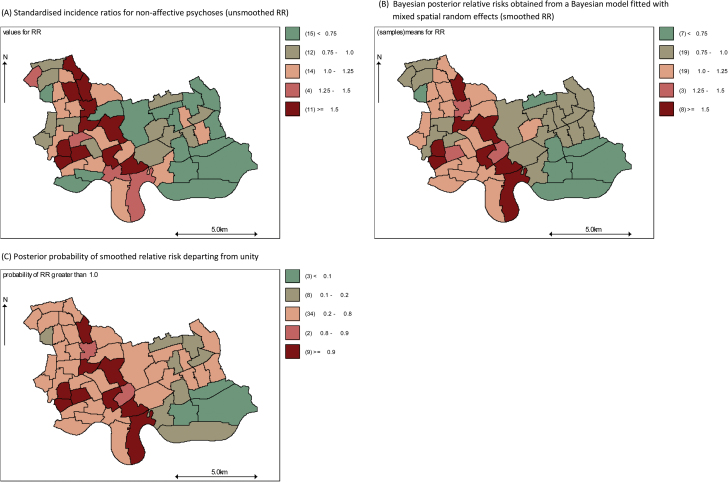
The count of subjects with nonaffective psychosis ranged from 0 (N = 2) to 14 (N = 1) across our neighborhoods and exhibited overdispersion (mean = 5.6, variance = 10.3). SIR varied from 0.00 to 3.24 across the study region (figure 2a). All Bayesian models reduced variance in RR between the 5th and 95th percentile (see table 2). A Bayesian-mixed model including unstructured, structured, and jump random effects (Model 4) fitted the data best according to all model fit diagnostics (see table 2). Having accounted for spatial dependency and overdispersion in the data and individual-level age, sex, ethnicity, and social class, this model indicated significant variation in RR remained at the neighborhood level (figure 2b), with high probabilities of RR greater than unity in several neighborhoods toward the centre and west of the study region (figure 2c). Neighborhoods toward the southeast of the study region had high probabilities of RR less than unity ie, lower risk of nonaffective psychosis.
Fig. 2.
Standardized incidence ratios (A), Bayesian posterior relative risks (B), and probabilities of risk departing from unity (C) from a Bayesian hierarchical mixed model for nonaffective psychotic disorders.
Standardized incidence ratios for age, sex, ethnicity, and social class reveal variation in the risk of nonaffective psychoses across the study region (A). A Bayesian hierarchical model with mixed random effects (unstructured, structured, and jump) provides the best fit to the data given the prior information, resulting in smoothed RR (B). The posterior probability that RR depart from unity in the region are mapped in (C) and reveal evidence of excess rates in central parts of the study region, with high probabilities of significantly lower rates towards the southeast of the study region.
Table 2.
Spatial Variation in Unsmoothed and Smoothed Relative Risks of Psychotic Syndromes After Indirect Standardization
| Outcome | Observed Subjects | Expected Subjects | Unsmoothed SIRa | Smoothed RRb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 (Unstructured) | Model 2 (Structured) | Model 3 (Convolution) | Model 4 (Mixed) | |||||||||||
| Nonaffective psychoses (F20-29) | ||||||||||||||
| Minimum | 0 | 2.90 | 0.00 | 0.46 | 0.54 | 0.48 | 0.44 | |||||||
| 5th percentile | 1 | 3.71 | 0.18 | 0.49 | 0.62 | 0.48 | 0.54 | |||||||
| Median | 5.5 | 5.49 | 0.88 | 0.97 | 0.93 | 0.95 | 1.02 | |||||||
| 95th percentile | 11 | 7.52 | 2.75 | 1.83 | 1.89 | 1.92 | 2.37 | |||||||
| Maximum | 14 | 8.26 | 3.24 | 2.02 | 2.04 | 2.05 | 2.41 | |||||||
| Mean | 5.59 | 5.59 | 1.03 | 1.03 | 1.02 | 1.03 | 1.11 | |||||||
| DIC | — | — | — | 279.54 | 273.40 | 273.88 | 262.62 | |||||||
| MAPE | — | — | — | 2.60 | 2.60 | 2.58 | 2.56 | |||||||
| MSPE | — | — | — | 11.39 | 11.30 | 11.18 | 11.03 | |||||||
| Affective psychoses (F30-33) | ||||||||||||||
| Minimum | 0 | 1.14 | 0.00 | 0.96 | 0.93 | 0.91 | 0.95 | |||||||
| 5th percentile | 0 | 1.41 | 0.00 | 0.97 | 0.94 | 0.91 | 0.95 | |||||||
| Median | 2 | 2.01 | 1.03 | 1.00 | 0.98 | 0.98 | 1.01 | |||||||
| 95th percentile | 4 | 2.65 | 1.94 | 1.03 | 1.10 | 1.11 | 1.10 | |||||||
| Maximum | 5 | 2.78 | 2.42 | 1.05 | 1.11 | 1.16 | 1.16 | |||||||
| Mean | 2.04 | 2.04 | 0.98 | 1.00 | 0.99 | 0.99 | 1.02 | |||||||
| DIC | — | — | — | 182.83 | 182.24 | 183.24 | 182.79 | |||||||
| MAPE | — | — | — | 1.44 | 1.44 | 1.43 | 1.44 | |||||||
| MSPE | — | — | — | 3.45 | 3.42 | 3.41 | 3.43 | |||||||
Notes: DIC, Deviance Information Criterion; MAPE, Mean Absolute Prediction Error; MSPE, Mean Squared Prediction Error.
aClassical (unsmoothed) Standardized Incidence Ratio–standardized for age, sex, ethnicity, and social class.
bBayesian spatial modeling after standardization for age, sex, ethnicity, and social class. Type of random effects at neighborhood-level in parentheses.
Neighborhood-Level Influences on Psychosis Risk.
We added neighborhood covariates to Model 4 using backward-fitting modeling to identify our most parsimonious model. This model suggested that a 1-SD increase in multiple deprivation (RR: 1.28; 95% CI: 1.08, 1.51), income deprivation inequality (RR: 1.25; 95% CI: 1.04, 1.49), and population density (RR: 1.18; 95% CI: 1.00, 1.41) was independently associated with increased incidence of nonaffective psychoses, after standardization for age, sex, ethnicity, and social class (table 3). No other neighborhood variables improved this model. Residual relative risk had been substantially reduced after modeling these neighborhood factors (see online supplemental figure 1), suggesting that these factors largely explained variance in RR for nonaffective disorders across our study region. We found no evidence of an interaction between inequality and deprivation (RR: 1.00; 95% CI: 0.87, 1.15) or of a nonlinear (quadratic) association between deprivation and psychosis (RR: 1.01; 95% CI: 0.87, 1.16).
Table 3.
Association Between Neighborhood-Level Environmental Variables and Incidence of DSM-IV Nonaffective Psychosis
| Variable (z-standardized)a | Control for | |
|---|---|---|
| Individual-Level Factorsb | Full Multivariatec | |
| RR (95% CI) | RR (95% CI) | |
| IMD | 1.20 (1.00, 1.43) | 1.28 (1.08, 1.51) |
| Income inequality | 1.09 (0.89,1.28) | 1.25 (1.04, 1.49) |
| Population density | 1.15 (0.95, 1.39) | 1.18 (1.00, 1.41) |
| SFI | 1.05 (0.87, 1.26) | 1.08 (0.87, 1.34) |
| Voter turnout | 1.21 (0.98, 1.45) | 1.12 (0.91, 1.35) |
Notes: IMD, index of multiple deprivation; SFI, social fragmentation index.
aRelative Risk (RR) report relative change in incidence associated with a 1 SD increase in value of neighborhood variable.
bAfter indirect standardization for individual-level age group, sex, ethnicity, and socioeconomic status.
cAdditional control for neighborhood-level variables included in final model: IMD, income inequality and population density. SFI & voter turnout not retained in final model given nonsignificance.
Own-Group Ethnic Density and Ethnic Separation for Specific Ethnic Groups.
There was sufficient data to study ethnic density and separation effects on nonaffective psychosis in the white British (n = 68; 21.7%), non-British white (n = 38; 12.1%), black Caribbean (n = 55; 17.6%), black African (n = 49; 15.7%), and Bangladeshi (n = 53; 16.9%) groups. For the black Caribbean group, there was evidence that incidence increased in neighborhoods where they were more segregated from people of other ethnic groups (RR: 1.54; 95% CI: 1.12, 2.03). A 1-SD increase in neighborhood-level own-group ethnic density among people of black African ethnicity was significantly associated with reduced risk of psychosis risk (RR: 0.70; 95% CI: 0.48, 0.99), after standardization for individual-level factors. No other statistically significant ethnic density or separation effects were observed (data available from authors).
Bayesian Analyses of Affective Psychotic Disorders
Spatial Patterning of Affective Psychosis Risk and Neighborhood-Level Influences.
The mean number of subjects with affective psychosis per neighborhood was 2.04 with a variance of 1.71. SIR ranged from 0.00 to 2.42 (see online supplemental figure 2a), which was attenuated following Bayesian spatial modeling (table 2). All Bayesian models performed similarly (table 2, affective psychoses). We did not observe significant variation in RR in the study region, after standardization for individual-level factors in any Bayesian model (Model 2: online supplemental figures 2b and 2c). No neighborhood covariates improved the model or were significantly associated with the incidence of affective psychoses (data available from authors).
Discussion
Principal Findings
We identified marked spatial variation in the relative risk of nonaffective psychotic disorder in East London, not explained by individual-level factors including age, sex, ethnicity, and social class. Together with our observation that the incidence of affective psychoses showed no such spatial variation, our data replicate previous studies to have found such differences with respect to the environment.3–5 Our study extends these findings, however, by using appropriate spatial multilevel models to demonstrate that the incidence of nonaffective psychosis was independently associated with increased deprivation, income inequality, and population density. When we studied the influence of socioenvironmental factors in specific ethnic groups, we observed that ethnic separation and ethnic density were associated with nonaffective psychosis risk for people of black Caribbean and black African, respectively.
Strengths and Limitations
Our data set had sufficient power to detect variation in incidence of nonaffective psychoses at the statistical ward level, after standardizing for several individual-level variables. It is possible that our models were underpowered to detect such variation for the affective psychoses, given the smaller overall sample size and smaller counts per neighborhood for this outcome. Nevertheless, point estimates of RR for the affective psychoses were close to zero in each neighborhood after Bayesian modeling, and our findings are consistent with the previous literature.3–5 Furthermore, we were able to identify some variation in RR of nonaffective psychoses for specific ethnic groups where sample sizes were considerably smaller than for the affective psychoses overall.
We used data from a large, well-characterized FEP sample using a robust epidemiological methodology.37,38 Neighborhood-level variables were obtained from nationally collected data sources, reliable surveys, and official voter turnout statistics. Local election results were used to minimize contamination of wider national political factors affecting voting behavior. Derived variables, such as inequality, ethnic separation, and social fragmentation were all estimated using reliable, validated methodologies. Unlike previous studies of ethnic density in the UK that have inspected ethnic density for a single ethnic group,53 or used the same composite measure for all ethnic minority groups,34,35 we estimated own-group ethnic density in several different ethnic groups.
We used environmental measures estimated prior to or during case ascertainment to minimize misclassification of neighborhood exposures. Nonetheless, our results are cross-sectional limiting causal inference. Some of the association between our neighborhood factors and psychosis could have been attributable to downward drift although we believe this is unlikely to fully account for our findings, particularly because the duration of untreated psychosis appears to be unrelated to neighborhood residence at first contact.54 Both social drift and causation may occur simultaneously, either independently of each other (ie, acting on different individuals) or in an interactive fashion (ie, acting on the same individual). Elucidation of such mechanisms requires testing in future studies.
This is the first neighborhood investigation of psychotic disorders to have fully considered spatial and multilevel effects. We used relatively uninformative prior distributions (see online supplementary Appendix 1) in our Bayesian models to allow the data to drive our findings. We included several major individual-level confounders in our analyses. While current social class may partially have reflected downward drift, any such misclassification would have only strengthened its confounding effect. It was only possible to control for up to 4 individual level variables in our analyses because census data could not be stratified more finely without leading to the possible identification of individuals. We were unable to control for individual-level substance misuse or family history of mental illness because these data were not available from denominator sources. Previous studies that included these variables suggest they do not fully confound associations between urbanicity and psychosis risk.7,10 We excluded 7.2% of subjects from our analyses because we could not determine their residential neighborhood at first contact. While these subjects did not differ significantly from the included sample on any individual-level characteristics, they could have biased neighborhood-level findings if they were more likely to come from certain parts of the catchment area than others. We believe this is unlikely to have substantially affected our results however, given the small number of subjects (n = 35; n = 32 for nonaffective psychosis) and given that any limited postal data available on these subjects (n = 22) suggested they lived in districts throughout the entire catchment area (data available from authors).
Although we adopted a spatial multilevel modeling strategy, the ecological fallacy still requires a caveat; we did not measure whether individuals who developed psychosis in certain neighborhoods were, themselves, “exposed” to the degree of deprivation, inequality, or population density observed at the neighborhood level. It is theoretically possible that some variance in incidence attributed to neighborhood-level (or individual-level) factors could have been due to other exposure levels, such as the family or school. Zammit et al.13 observed that neighborhood variation in nonaffective psychoses in Sweden could be mostly explained by school-level social fragmentation, raising the possibility that the exposure level at which socioenvironmental factors shape psychosis risk is conditional upon stage of the life course. We also did not know whether residential neighborhoods constituted the relevant exposure neighborhood for risk; future studies will be required to investigate the role of and time spent in different environments on psychosis risk.
Interpretation of Findings
Our findings suggest that the spatial distribution of nonaffective psychotic disorders is not a compositional artifact of individual-level age, sex, ethnicity, or, importantly, social class. This decreases the possibility that the association with urban living close to onset is solely due to social drift. A recent publication from the Moving To Opportunities study has found moving to less deprived neighborhoods in adulthood increased subjective well-being,55 suggesting that neighborhood environments can shape mental health over the entire life course. In our study variation in psychosis risk in East London was predominantly accounted for by 3 neighborhood factors: population density, deprivation, and income inequality. For some ethnic groups, exact psychosis risk was also related to neighborhood ethnic composition, in line with previous findings.3,34–36,53
Although our study design limits causal inference, our findings are consistent with a role of environmental factors in the onset of nonaffective psychoses.1 If true, we suggest our findings would be most parsimoniously interpreted as providing a role for the social rather than physical (ie, infections, nutrition) neighborhood environment during adulthood. Theoretically, both physical and social antecedents could be hypothesized to link psychosis risk to population density and deprivation. From a physical environment perspective, greater population density may facilitate greater transmission of infections relevant to later psychosis risk, or may present a marker for reduced exposure to sunlight and vitamin D.56 Similarly, exposure to deprivation could involve biological antecedents in psychosis onset by restricting access to adequate nutrition at key periods of brain development over the life course.25,26 However, the critical period of risk for such factors is thought to operate early in life—and most likely in utero—making it harder to attribute exposures such as poor nutrition or infection to associations between adulthood environments and psychosis risk.
While it is possible that residency close to onset may reflect cumulative exposure to biological antecedents over the life course (absence of sunlight and poor nutrition, infection), we suggest any causal association between adulthood environment and psychosis risk is more likely to be due to social stressors. For example, living in more deprived neighborhoods may expose people to a range of stressful experiences, resultant from lower social and economic investment, including higher rates of crime and antisocial behavior, poorer educational, leisure and health facilities, and more physical health problems, all of which may induce stress with further consequences for mental health.57 Individuals who lack sufficient social or financial capital to offset exposure to these stressful events, either by drawing upon social support or by moving to less-deprived neighborhoods,55 may face chronic exposure to such threats over the life course; several studies have observed an association between lower social support and psychosis risk.13,15,28–31 Interestingly, we did not observe a direct association between social fragmentation and nonaffective psychosis in the present study, having considered other neighborhood variables. However, social fragmentation was highly positively correlated with income inequality (correlation: 0.59; P < 0.05), suggesting that inequality could be an underlying driver of neighborhood social cohesiveness.
The association between population density and nonaffective psychosis risk observed in our study, independent of deprivation and inequality, is also open to a number of possible social interpretations. For example, it may reflect reduced opportunity to create meaningful social contacts in highly urban areas where social interaction is more transient. Alternatively, more dense environments may serve to increase paranoid ideation through exposing people susceptible to psychosis to a greater number of unknown individuals. Because humans tend to organize themselves into social hierarchies, another possibility is that more densely populated environments increase the number of levels in such hierarchies, increasing the propensity of exposure to chronic social defeat58 in those individuals further down the hierarchy.
Our observation in regard to inequality is in line with a larger literature that has revealed several associations between inequality and many health and social outcomes, including all-cause mortality59; cause-specific mortality,60 including some cancers, cardiovascular, and respiratory diseases and nonmedical deaths including accidents and suicide; general self-rated health61; educational attainment and crime rates;62 and common mental disorders.63 The association between inequality and psychotic disorders has received less attention to date, but our findings support and extend 2 ecological studies whose results were consistent with our own.32,33 Boydell et al.33 found some evidence that schizophrenia incidence was greater in neighborhoods with higher income inequality, but only where absolute deprivation was already low. While we did not observe such an interaction, East London was universally deprived relative to the remainder of England, and our findings thus support the possibility that income inequality (although low in our study) may have particularly pronounced effects on psychosis risk in poor communities, where differences between those with and without access to resources may be most marked. Future studies are required to investigate the effect of inequality on psychosis risk across broader levels of inequality. Inequality may invoke several pathways to stress, including increased risk of subordination (under a social defeat paradigm), or via marginalization of vulnerable individuals (ie, social isolation). Neighborhood ethnicity composition, which may reflect specific social inequalities for certain ethnic groups, also had distinct effects on nonaffective psychosis risk for black African and black Caribbean groups in our study.
In comparison to absolute measures of neighborhood exposure (population density and deprivation), it is more difficult to attribute relative exposures (inequality, ethnic density) to biological explanations of disease or reverse causation (social drift). First, under a biological paradigm, how malnourished one is relative to one’s neighbor is irrelevant in relation to later psychosis risk; what matters here is absolute nutritional intake in governing brain development at key periods of the life course. Second, while it is plausible that individuals drift into more deprived neighborhoods during the onset of disorder, often unable to hold down employment or afford high-quality accommodation, we can think of fewer compelling reasons why such individuals would also drift into more unequal neighborhoods, unless the 2 were highly positively correlated. In our study, they were not (correlation: -0.32, P = 0.02). We therefore suggest that the most plausible hypothesis to explain relationships between relative environmental exposures and psychosis risk will involve a social stress-orientated paradigm.57
Our findings correspond to previous observations that the incidence of nonaffective psychotic disorder varies with respect to the environment, but affective psychoses do not.3–5 It is possible that the trajectories underlying each disorder differ, despite some shared genetic liability.64 It has been suggested that additional neurodevelopmental insults following interactions with genetic factors65 may lead to cognitive and social impairment and structural brain abnormalities,66–70 which lead some individuals toward schizophrenia rather than affective psychoses. This “second-hit” hypothesis,70 suggests that those with early-life neurodevelopmental impairment are more likely to be propelled into adverse social environments, where exposure to negative life events and a lack of social support increase the risk of permanent, deleterious effects on functional brain processes, resulting in onset of psychotic symptoms.71 A recent finding from social neuroscience supports the possibility that the urban environment can modify brain function in adulthood in response to stress.72 Our findings suggest that inequality, absolute deprivation, and the experience of living in dense, urban environments in adulthood may contribute to psychosis risk. Continued efforts to integrate social neuroscience with social epidemiology should help reveal how and when environmental exposures over the life course have critical effects on brain processes that increase psychosis risk.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
St Bartholomew’s & the Royal London Hospital Special Trustees; East London & the City Mental Health National Health Service Trust R&D; Wellcome Trust (WT085540 to J. B. Kirkbride); National Institute of Health Research Collaboration for Leadership in Applied Health Research and Care; National Institute of Health Research programme grants (RP-PG-0407-10500 to S. Ullrich and J. W. Coid, RP-PG-0606-1335 to P. B. Jones).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study. The funders had no role in any aspect of the conduct of this research or preparation of this manuscript. J. W. Coid takes responsibility for the integrity of this data. All authors had full access to data in this study.
References
- 1. March D, Hatch SL, Morgan C, et al. Psychosis and place. Epidemiol Rev. 2008; 30: 84–100 [DOI] [PubMed] [Google Scholar]
- 2. Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull. 2012; 38: 1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faris REL, Dunham HW. Mental Disorders in Urban Areas. Chicago: University of Chicago Press; 1939. [Google Scholar]
- 4. Kirkbride JB, Fearon P, Morgan C, et al. Neighbourhood variation in the incidence of psychotic disorders in Southeast London. Soc Psychiatry Psychiatr Epidemiol. 2007; 42: 438–445 [DOI] [PubMed] [Google Scholar]
- 5. Pedersen CB, Mortensen PB. Urbanicity during upbringing and bipolar affective disorders in Denmark. Bipolar Disord. 2006; 8: 242–247 [DOI] [PubMed] [Google Scholar]
- 6. Bradford-Hill A. . The environment and disease: association or causation? Proceedings of the Royal Society of Medicine. 1965; 58: 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis G, David A, Andréasson S, Allebeck P. Schizophrenia and city life. Lancet. 1992; 340: 137–140 [DOI] [PubMed] [Google Scholar]
- 8. Marcelis M, Navarro-Mateu F, Murray R, Selten JP, Van Os J. Urbanization and psychosis: a study of 1942–1978 birth cohorts in the Netherlands. Psychol Med. 1998; 28: 871–879 [DOI] [PubMed] [Google Scholar]
- 9. Marcelis M, Takei N, van Os J. Urbanization and risk for schizophrenia: does the effect operate before or around the time of illness onset? Psychol Med. 1999; 29: 1197–1203 [DOI] [PubMed] [Google Scholar]
- 10. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999; 340: 603–608 [DOI] [PubMed] [Google Scholar]
- 11. Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001; 58: 1039–1046 [DOI] [PubMed] [Google Scholar]
- 12. van Os J, Hannssen M, Bak M, Bijl R, Vollebergh W. Do urbanicity and familial liability coparticipate in causing psychosis? Am J Psychiatry. 2003; 160: 477–482 [DOI] [PubMed] [Google Scholar]
- 13. Zammit S, Lewis G, Rasbash J, Dalman C, Gustafsson JE, Allebeck P. Individuals, schools, and neighborhood: a multilevel longitudinal study of variation in incidence of psychotic disorders. Arch Gen Psychiatry. 2010; 67: 914–922 [DOI] [PubMed] [Google Scholar]
- 14. Sundquist K, Frank G, Sundquist J. Urbanisation and incidence of psychosis and depression: follow-up study of 4.4 million women and men in Sweden. Br J Psychiatry. 2004; 184: 293–298 [DOI] [PubMed] [Google Scholar]
- 15. Allardyce J, Gilmour H, Atkinson J, Rapson T, Bishop J, McCreadie RG. Social fragmentation, deprivation and urbanicity: relation to first-admission rates for psychoses. Br J Psychiatry. 2005; 187: 401–406 [DOI] [PubMed] [Google Scholar]
- 16. Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Arch Gen Psychiatry. 2006; 63: 250–258 [DOI] [PubMed] [Google Scholar]
- 17. Allardyce J, Boydell J, Van Os J, et al. Comparison of the incidence of schizophrenia in rural Dumfries and Galloway and urban Camberwell. Br J Psychiatry. 2001; 179: 335–339 [DOI] [PubMed] [Google Scholar]
- 18. Pedersen CB, Mortensen PB. Are the cause(s) responsible for urban-rural differences in schizophrenia risk rooted in families or in individuals? Am J Epidemiol. 2006; 163: 971–978 [DOI] [PubMed] [Google Scholar]
- 19. van Os J, Pedersen CB, Mortensen PB. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. Am J Psychiatry. 2004; 161: 2312–2314 [DOI] [PubMed] [Google Scholar]
- 20. Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr Bull. 2005; 31: 795–799 [DOI] [PubMed] [Google Scholar]
- 21. Harrison G, Fouskakis D, Rasmussen F, Tynelius P, Sipos A, Gunnell D. Association between psychotic disorder and urban place of birth is not mediated by obstetric complications or childhood socio-economic position: a cohort study. Psychol Med. 2003; 33: 723–731 [DOI] [PubMed] [Google Scholar]
- 22. Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011; 93: 23–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allardyce J, Boydell J. Review: the wider social environment and schizophrenia. Schizophr Bull. 2006; 32: 592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGrath JJ, Eyles DW, Pedersen CB, et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry. 2010; 67: 889–894 [DOI] [PubMed] [Google Scholar]
- 25. Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996; 53: 25–31 [DOI] [PubMed] [Google Scholar]
- 26. St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005; 294: 557–562 [DOI] [PubMed] [Google Scholar]
- 27. Croudace TJ, Kayne R, Jones PB, Harrison GL. Non-linear relationship between an index of social deprivation, psychiatric admission prevalence and the incidence of psychosis. Psychol Med. 2000; 30: 177–185 [DOI] [PubMed] [Google Scholar]
- 28. Silver E, Mulvey EP, Swanson JW. Neighborhood structural characteristics and mental disorder: Faris and Dunham revisited. Soc Sci Med. 2002; 55: 1457–1470 [DOI] [PubMed] [Google Scholar]
- 29. Hare EH. Mental illness and social conditions in Bristol. J Ment Sci. 1956; 102: 349–357 [DOI] [PubMed] [Google Scholar]
- 30. van Os J, Driessen G, Gunther N, Delespaul P. Neighbourhood variation in incidence of schizophrenia. Evidence for person-environment interaction. Br J Psychiatry. 2000; 176: 243–248 [DOI] [PubMed] [Google Scholar]
- 31. Giggs JA. Distribution of schizophrenics in Nottingham. T I Brit Geogr. 1973; 59: 5–76 [Google Scholar]
- 32. Burns JK, Esterhuizen T. Poverty, inequality and the treated incidence of first-episode psychosis: an ecological study from South Africa. Soc Psychiatry Psychiatr Epidemiol. 2008; 43: 331–335 [DOI] [PubMed] [Google Scholar]
- 33. Boydell J, van Os J, McKenzie K, Murray RM. The association of inequality with the incidence of schizophrenia–an ecological study. Soc Psychiatry Psychiatr Epidemiol. 2004; 39: 597–599 [DOI] [PubMed] [Google Scholar]
- 34. Boydell J, van Os J, McKenzie K, et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001; 323: 1336–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirkbride JB, Boydell J, Ploubidis GB, et al. Testing the association between the incidence of schizophrenia and social capital in an urban area. Psychol Med. 2008; 38: 1083–1094 [DOI] [PubMed] [Google Scholar]
- 36. Veling W, Susser E, van Os J, Mackenbach JP, Selten JP, Hoek HW. Ethnic density of neighborhoods and incidence of psychotic disorders among immigrants. Am J Psychiatry. 2008; 165: 66–73 [DOI] [PubMed] [Google Scholar]
- 37. Kirkbride JB, Barker D, Cowden F, et al. Psychoses, ethnicity and socio-economic status. Br J Psychiatry. 2008; 193: 18–24 [DOI] [PubMed] [Google Scholar]
- 38. Coid JW, Kirkbride JB, Barker D, et al. Raised incidence rates of all psychoses among migrant groups: findings from the East London first episode psychosis study. Arch Gen Psychiatry. 2008; 65: 1250–1258 [DOI] [PubMed] [Google Scholar]
- 39. Cooper JE, Goodhead D, Craig T, Harris M, Howat J, Korer J. The incidence of schizophrenia in Nottingham. Br J Psychiatry. 1987; 151: 619–626 [DOI] [PubMed] [Google Scholar]
- 40. Office for National Statistics The national statistics socio-economic classification user manual. Office for National Statistcs. 2005; http://www.ons.gov.uk/ons/guide-method/classifications/current-standard-classifications/soc2010/index.html Accessed November 29, 2012 [Google Scholar]
- 41. Noble M, Wright G, Dibben C, et al. Indices of Deprivation 2004. London: ODPM; 2004; http://data.gov.uk/dataset/imd_2004 Accessed November 29, 2012 [Google Scholar]
- 42. Kawachi I, Kennedy BP. The relationship of income inequality to mortality: does the choice of indicator matter? Soc Sci Med. 1997; 45: 1121–1127 [DOI] [PubMed] [Google Scholar]
- 43. Peach C. Does Britain have ghettos? Trans Inst Br Geogr. 1996; 21: 216–235 [Google Scholar]
- 44. Kirkbride JB, Morgan C, Fearon P, Dazzan P, Murray RM, Jones PB. Neighbourhood-level effects on psychoses: re-examining the role of context. Psychol Med. 2007; 37: 1413–1425 [DOI] [PubMed] [Google Scholar]
- 45. Mollie A. Bayesian mapping of disease.. In: Gilks WR, Richardson S, Spiegelhalter DJ, eds. Markov Chain Monte Carlo in Practice. London: Chapman and Hall; 1996; 359–379 [Google Scholar]
- 46. Richardson S, Thomson A, Best N, Elliott P. Interpreting posterior relative risk estimates in disease-mapping studies. Environ Health Perspect. 2004; 112: 1016–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Besag J, York J, Mollie A. Bayesian image restoration, with application in spatial statistics with discussion. Ann Inst Stat Math. 1991; 43: 1–59 [Google Scholar]
- 48. Lawson AB, Browne W, Vidal RCL. Disease Mapping With WinBUGS and MLwiN. Chichester: Wiley & Sons; 2003. [Google Scholar]
- 49. Lawson AB, Clark A. Spatial mixture relative risk models applied to disease mapping. Stat Med. 2002; 21: 359–370 [DOI] [PubMed] [Google Scholar]
- 50. Spiegelhalter DJ, Best N, Carlin BP, van der LA. Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Methodol. 2002; 64: 583–640 [Google Scholar]
- 51. Stata. [computer program]. Version 11.2. College Station, Texas: StataCorp; 2009. [Google Scholar]
- 52. winBUGS. [computer program]. Version 1.4.1. Cambridge: MRC Biostatistics Unit; 2004. [Google Scholar]
- 53. Schofield P, Ashworth M, Jones R. Ethnic isolation and psychosis: re-examining the ethnic density effect. Psychol Med. 2011; 41: 1263–1269 [DOI] [PubMed] [Google Scholar]
- 54. Kirkbride JB, Lunn DJ, Morgan C, et al. Examining evidence for neighbourhood variation in the duration of untreated psychosis. Health Place. 2010; 16: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ludwig J, Duncan GJ, Gennetian LA, et al. Neighborhood effects on the long-term well-being of low-income adults. Science. 2012; 337: 1505–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003; 63: 73–78 [DOI] [PubMed] [Google Scholar]
- 57. Hill TD, Ross CE, Angel RJ. Neighborhood disorder, psychophysiological distress, and health. J Health Soc Behav. 2005; 46: 170–186 [DOI] [PubMed] [Google Scholar]
- 58. Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Br J Psychiatry. 2005; 187: 101–102 [DOI] [PubMed] [Google Scholar]
- 59. Kondo N, Sembajwe G, Kawachi I, et al. Income inequality, mortality, and self rated health: meta-analysis of multilevel studies. BMJ. 2009; 339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lau EW, Schooling CM, Tin KY, Leung GM. Income inequality and cause-specific mortality during economic development. Ann Epidemiol. 2012; 22: 285–294 [DOI] [PubMed] [Google Scholar]
- 61. Zimmerman FJ, Bell JF. Income inequality and physical and mental health: testing associations consistent with proposed causal pathways. J Epidemiol Community Health. 2006; 60: 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilkinson RG, Pickett R. The Spirit Level: Why Equality is Better for Everyone. London: Penguin; 2010. [Google Scholar]
- 63. Weich S, Lewis G, Jenkins SP. Income inequality and the prevalence of common mental disorders in Britain. Br J Psychiatry. 2001; 178: 222–227 [DOI] [PubMed] [Google Scholar]
- 64. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009; 373: 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Os J, Rutten BPF. Gene-environment-wide interaction studies in psychiatry. Am J Psychiatry. 2009; 166: 964–966 [DOI] [PubMed] [Google Scholar]
- 66. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002; 159: 1080–1092 [DOI] [PubMed] [Google Scholar]
- 67. Zanelli J, Reichenberg A, Morgan K, et al. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry. 2010; 167: 78–85 [DOI] [PubMed] [Google Scholar]
- 68. MacCabe JH, Lambe MP, Cnattingius S, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008; 38: 1133–1140 [DOI] [PubMed] [Google Scholar]
- 69. Rietschel M, Georgi A, Schmael C, et al. Premorbid adjustment: a phenotype highlighting a distinction rather than an overlap between schizophrenia and bipolar disorder. Schizophr Res. 2009; 110: 33–39 [DOI] [PubMed] [Google Scholar]
- 70. Demjaha A, MacCabe JH, Murray RM. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr Bulletin. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009; 35: 549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011; 474: 498–501 [DOI] [PubMed] [Google Scholar]
- 73. Office for the Deputy Prime Minister Turnout at Local Elections: Influences on Levels of Voter Registration and Voting. London: Office for the Deputy Prime Minister; 2002. http://webarchive.nationalarchives.gov.uk/+/http://www.dca.gov.uk/elections/elect_odpm_turnout.pdf Accessed November 29, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.