Abstract
This study aimed to characterize the deficit syndrome in drug-naive schizophrenia patients and to examine the relationship between deficit features and primary neurological abnormalities. Drug-naive schizophrenia patients (n = 102) were examined at baseline for demographics, premorbid functioning, duration of untreated illness (DUI), psychopathology, neurological signs, and deficit symptoms, and reassessed at 1-year follow-up. Neurological abnormalities were examined before inception of antipsychotic medication and included four domains of spontaneous movement disorders (SMD) and four domains of neurological soft signs (NSS). Patients fulfilling the deficit syndrome criteria at the two assessments (n = 20) were compared with nondeficit patients (n = 82) across demographic, clinical, and neurological variables. Deficit and nondeficit groups showed similar demographic characteristics and levels of psychotic, disorganization, and depressive symptoms. Compared with nondeficit patients, deficit patients showed poorer premorbid adjustment, higher premorbid deterioration, a lengthier DUI, and much poorer functional outcome. Relative to the nondeficit patients, those with the deficit syndrome showed higher levels of SMD—excepting akathisia—and NSS. This association pattern was also evident for deficit and neurological ratings in the whole sample of schizophrenia patients. Parkinsonism, motor sequencing, and release signs were all independently related to the deficit syndrome. These findings confirm that the deficit/nondeficit categorization is replicable and reliable in first-admission patients and raise the possibility that premorbid deterioration, deficit symptoms, and neurological abnormalities represent a triad of manifestations that share common underlying neurobiological mechanisms. More specifically, the data are consistent with a neurodevelopmental model of deficit symptoms involving basal ganglia dysfunction.
Key words: deficit schizophrenia, drug-naive, neurological signs, outcome, symptom remission, basal ganglia
Introduction
Schizophrenia is a heterogeneous disorder that traditionally has been subtypified according to alternative criteria as a means of reducing clinical and neurobiological complexity.1 While most subtyping systems have reached limited success in this regard, the deficit/nondeficit categorization2 remains one of the most valid attempts to subclassify schizophrenia. Deficit schizophrenia is characterized by primary and enduring negative symptoms, which is believed to have unique etiology, biological substrate, and clinical features distinguishable from the nondeficit form.3–7
Neurological impairment in schizophrenia is now recognized to comprise two domains: spontaneous movement disorders (SMD) and neurological soft signs (NSS), which are thought to be highly informative on the neurobiological substrate of the illness.8 Neurological dysfunction has been a major focus of research in validating the deficit/nondeficit distinction and a number of reports have noted that patients with deficit/negative schizophrenia are more impaired than their nondeficit/nonnegative counterparts regarding extrapyramidal movement disorders9–13 and NSS.3,6,12,14,15 Most of these studies, however, were conducted in chronic and medicated patients. Antipsychotic drugs are a well-known source of deficit symptoms,2,16 extrapyramidal movement disorders,17,18 and NSS,19 although the latter is a more debatable question.20 Thus, antipsychotic treatment is a major factor confounding deficit symptoms and primary neurological abnormalities, such as their relationship.
The best way to address the relationship between the deficit syndrome and primary neurological abnormalities is to examine schizophrenia patients who have never been exposed to antipsychotic medication. To the best of our knowledge, no previous study has examined the relationship between the deficit syndrome and NSS in drug-naive patients, and only two previous studies have examined such an association in relation to SMD. In a retrospective study of never-treated patients in which, both the deficit/nondeficit categorization and the assessment of SMD were made on the basis of chart records, Fenton et al.21 found that spontaneous dyskinesia was more likely to be observed in patients with deficit schizophrenia than in those with the nondeficit form. In a preliminary study from our group,22 we reported that drug-naive patients with deficit schizophrenia (n = 12) showed higher ratings of SMD that psychotic patients without the deficit syndrome (n = 88). These results, however, need to be cautiously interpreted because of the small number of patients with the deficit syndrome, the comparison with nondeficit psychosis rather than with nondeficit schizophrenia and the assessment of deficit features during the acute episode. As a consequence of the methodological limitations of previous studies, the extent to which deficit features are related to primary neurological abnormalities including SMD and NSS continues to be an unresolved question.
In the present study, we examined deficit features and neurological abnormalities in drug-naive schizophrenia patients with two main goals in mind. The first goal was to characterize the deficit syndrome in a population sample of first-admission schizophrenia patients, which included examination of the diagnostic stability of the deficit syndrome over 1-year follow-up, and examination of the differential characteristics of the deficit syndrome regarding demographic, clinical, and outcome variables. The second goal was to examine the prevalence and severity of SMD and NSS in deficit and nondeficit schizophrenia patients. According to these goals, we hypothesized that (1) the overall demographic and clinical characteristics of the deficit syndrome in drug-naive patients, parallels those reported in chronic and treated samples of schizophrenia patients and (2) patients with deficit schizophrenia present higher levels of primary neurological abnormalities than those with the nondeficit form of the disease.
Methods
Subjects and Design
The study population was drawn from 200 drug-naive psychotic patients who were consecutively admitted to the Psychiatry Section B of the Complejo Hospitalario de Navarra in Pamplona (Spain) between 1998 and 2006. The sample and assessment procedures used have been described in detail elsewhere.23 Briefly, the inclusion criteria were patients with a diagnosis of a nonaffective psychotic disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),24 no previous exposure to antipsychotic drugs as documented by the patient, close relatives, and medical records, and age between 15 and 65 years; exclusion criteria were lack of collaboration with the neurological examination, urgent need for starting antipsychotic treatment, a history of drug abuse confounding diagnosis, evidence of organic brain disorder, and meaningful somatic disease. This was a longitudinal and naturalistic study, in which patients were treated according to clinical choice after an initial trial with haloperidol, risperidone, or olanzapine. The study was conducted according to the declaration of Helsinki and approved by the local ethical committee. Patients or their legal representatives gave written informed consent to participate after complete description of the study that included the statement that the antipsychotic medication is going to be withheld a few hours until the psychopathological and neurological assessments are performed.
Patients were assessed for clinical symptoms, diagnosis, and neurological abnormalities at baseline and reassessed 1 year later for clinical symptoms, diagnosis, and neuromotor abnormalities. At baseline, clinical symptoms, including neurological signs, were all assessed immediately after admission, usually within a few hours after admission and before starting antipsychotic treatment. The 1-year assessment was particularly relevant because first-admission patients show some diagnostic instability, and deficit symptoms should ideally be rated on the basis of longitudinal and prospective assessments.
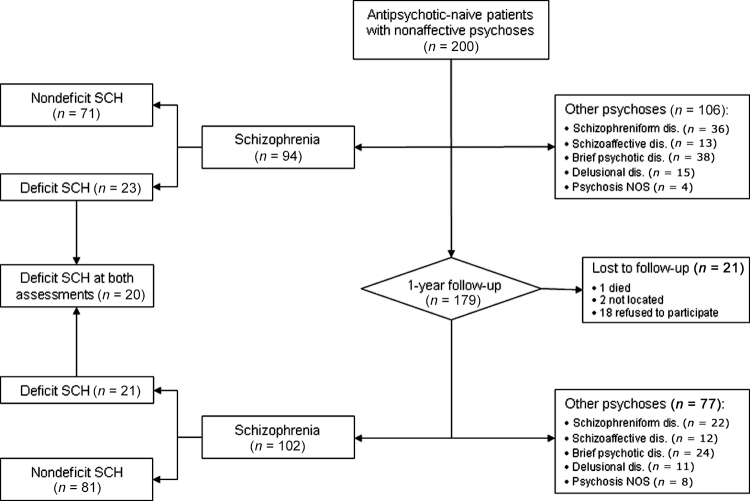
Figure 1 shows flow of patients through the study. Two hundred patients met inclusion and exclusion criteria and accepted to participate. Of the 179 patients that could be followed-up, 91 and 102 had a baseline and 1-year diagnosis of schizophrenia, respectively. One patient with a baseline schizophrenia diagnosis changed to a diagnosis of atypical psychosis and 12 patients with a baseline diagnosis of nonschizophrenic psychoses (4 with schizophreniform disorder, 3 with brief psychotic disorder, 5 with other psychotic disorders) changed their diagnosis to schizophrenia. Only those patients with a diagnosis of schizophrenia at 1-year follow-up were included in the study (n = 102), and only those patients meeting deficit syndrome criteria at both assessments were considered to have deficit syndrome (n = 20, 19.6%). Deficit and nondeficit groups were compared on the variables described below.
Fig. 1.
Flow of patients through the study.
Baseline Measures
Clinical Assessment.
Clinical assessments were performed on the basis of all available information procedure, which included al least two interviews with the subjects and information provided by close relatives and clinical records. The subjects were administered the Comprehensive Assessment of Symptoms and History (CASH) schedule,25 which served to assess demographic variables, diagnosis, and clinical symptoms. The CASH includes the Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS), whose subscale ratings were used to define the syndromes of reality distortion, disorganization, and negative.26
Socioeconomic status was assessed by means of the Hollingshead-Redlich Scale.27 The Premorbid Social Adjustment Scale28 was used to rate premorbid functioning for childhood (5–11 years) and adolescence (12–16 years). A change score was also computed, by subtracting the childhood score from the adolescence score, as a global measure of premorbid deterioration with age. Age at illness onset and duration of untreated illness (DUI) were assessed by means of the Symptom Onset in Schizophrenia Scale.29 We considered two DUI aspects: duration of untreated negative symptoms (DUNS) and duration of untreated psychosis (DUP). Because patients had not been previously exposed to antipsychotic medication, illness duration and DUI values are the same.
The deficit/nondeficit categorization was made by V.P. or M.J.C. on the basis of the Schedule for the Deficit Syndrome (SDS).30 The deficit syndrome is identified by the presence of two or more of six negative symptoms with at least moderate severity that have been determined to be primary and enduring. Interrater reliability for the different aspects of the deficit syndrome was assessed in a different sample and found to be good to excellent.31
Neurological Assessment.
SMD and NSS were rated on the basis of structured neurological examinations at the antipsychotic-naive status before starting antipsychotic treatment. The SMD examination was first completed by the senior researchers (V.P. or M.J.C.), and then NSS were blindly assessed by E.G.J. or M.S.C. We assessed four SMD domains: parkinsonism, dyskinesia, catatonia, and akathisia. Parkinsonism was rated according the Simpson-Angus Scale.32 The total score was used to determine severity, and a score >3 to determine the presence of parkinsonism. Dyskinetic movements were measured by the Abnormal Involuntary Movement Scale.33 We used the total score as a measure of severity, and the Schooler-Kane criteria34 to determine the presence of dyskinesia. Akathisia was rated with the Barnes Akathisia Rating Scale.35 The global rating was used to determine severity and a score ≥2 to determine the presence of akathisia. Catatonic symptoms were rated by means of the Modified Rogers Scale.36 The total score was used to determine severity, and the DSM-IV criteria to define the presence of catatonia. For specific analyses, three parkinsonian subdomains (hypokinesia, rigidity, and other signs) and three catatonia subdomains23 (negative, positive/hyperkinetic, and volitional) were considered.
NSS were assessed by means of the Neurological Evaluation Scale (NES),37 and four domains were considered: sensorial integration, motor coordination, motor sequencing, and release signs. We chose the later domain instead of the more general “other signs” NES subscale because of their increased reliability and homogeneity.20,38 For each NSS domain, patients were categorized on the basis of the median value into those with high vs low ratings. While all the 102 schizophrenia patients completed the SMD assessment, 19 patients had incomplete NES ratings. This was mainly because of patients’ uncooperativeness due to severe psychopathology and they were not included in specific analyses.
1-Year Follow-Up Assessment
Patients were reassessed 1 year after their first admission for clinical symptoms, diagnosis, deficit features, abnormal movement disorders, remission status, and functional outcome. Functional outcome was assessed by means of the Global Assessment of Functioning (GAF) Scale39 and criteria for symptomatic remission were adopted from Andreasen et al.40 on the basis of SAPS and SANS ratings. According to these criteria, for a patient to be considered symptomatically remitted, he or she must simultaneously score 2 (mild) or less over the last 6 months on all relevant items. We differentiated between overall symptomatic remission, which corresponds to the criteria defined above for positive and negative symptoms, and positive symptom remission. Deficit symptoms were rated over the previous 12 months (ie, in the period following the baseline assessment). Follow-up assessments were conducted by A.S.-T. or L.M.-I. who were blind to the baseline ratings and study hypotheses. Interrater reliability between baseline and 1-year raters was determined by 100% agreement on the diagnosis and 80% agreement for symptom presence.
Statistics
Diagnostic stability of the deficit syndrome was assessed using the prospective consistency (positive predictive value) that is the proportion of subjects in a category that maintain the same diagnosis at follow-up, and the retrospective consistency (sensitivity) that is the proportion of subjects in a category at follow-up who were in that same category at baseline. Stability of deficit symptoms was determined by means of the intraclass correlation coefficient (ICC). The factor structure of the six deficit symptoms was determined in deficit patients by means of principal component analysis (PCA), which was followed by promax rotation as independence of factors could not be assumed. Factor scores were obtained and summated scales were constructed on the basis of those items most loading on a given factor.
Categorical variables were compared using the chi-square or Fisher exact test statistics as appropriate. Because many dimensional variables were highly skewed, particularly those concerning deficit features, SMD and DUI ratings, together with unbalanced number of patients in the deficit/nondeficit categories, nonparametric tests were employed. The association between continuous variables and dichotomic variables was examined by means of Mann-Whitney U tests, and that for continuous variables was examined by means of Spearman rank-order correlation coefficients. Categorical measures of SMD and NSS were entered into a logistic regression model to identify domains of neurological abnormalities independently associated with the deficit/nondeficit categorization, reporting ORs and their 95% CIs.
In examining the associations between deficit features/diagnosis and neurological signs, we corrected for multiple testing using the family-wise Bonferroni method, by which domains of SMD and NSS, or their corresponding categorical and dimensional ratings, were considered as different family of hypotheses. All test were two-tailed, with statistical significance set at P < .05. Analyses were based on the Statistical Package for the Social Sciences, version 18.
Results
Stability of Deficit Features
Twenty-three patients met diagnostic criteria for the deficit syndrome at baseline and 21 at follow-up. Three patients with a baseline deficit syndrome did not longer meet the criteria at 1-year assessment, and one patient that did not meet the criteria at baseline did it at 1-year follow-up, which represents 83% agreement between initial and blind follow-up categorization of the deficit syndrome. The prospective and retrospective consistency of the deficit syndrome was 0.87 and 0.95, respectively. The ICC for deficit syndrome severity was 0.92, and the average ICC for individual deficit symptoms was 0.91, range between 0.89 (curving of interests) and 0.96 (diminished social drive).
Characteristics of Patients With and Without Deficit Schizophrenia
The two patient groups were not significantly different with respect to demographic variables and symptom severity, excepting negative symptoms at the two assessment points (table 1). Patients with the deficit form had poorer childhood and adolescence adjustment such as higher premorbid deterioration than nondeficit patients. DUNS and DUP were about three and two times lengthier, respectively, in deficit than in nondeficit patients (both, P < .001). At 1-year follow-up, deficit patients showed very poor functioning and no overall symptomatic remission; however, they showed a similar rate of positive symptom remission than nondeficit patients.
Table 1.
Demographic and Clinical Characteristics of 102 Schizophrenia Patients With and Without the Deficit Syndromea
| Deficit (n = 20) | Nondeficit (n = 82) | χ2 or W (df = 1) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Male gender, n (%) | 13 (65.0) | 56 (68.2) | 0.08 | .778 |
| Age | 31.7 (12.1) [15–61] | 28.4 (9.86) [15–65] | −0.82 | .409 |
| SES | 3.75 (0.91) [2–5] | 3.78 (1.01) [1–5] | −0.24 | .812 |
| Education (years) | 10.7 (3.09) [6–18] | 11.6 (4.0) [4–23] | −1.24 | .213 |
| Premorbid adjustment | ||||
| PSA, childhood total score | 17.3 (8.57) [5–29] | 11.5 (6.28) [5–29] | −2.64 | .008 |
| PSA, adolescence total score | 20.2 (9.02) [5–34] | 13.0 (6.72) [5–32] | −3.22 | .001 |
| PSA, change score | 2.85 (2.81) [0–15] | 1.50 (1.35) [−8–23] | −2.49 | .013 |
| Duration of untreated illness | ||||
| DUNS, years | 9.71 (8.49) [1–34] | 3.61 (5.79) [0–35] | −4.60 | <.001 |
| DUP, years | 6.29 (5.30) [0–18] | 3.10 (4.50) [0–24] | −4.72 | <.001 |
| Baseline psychopathologyb | ||||
| Reality-distortion symptoms | 3.70 (1.26) [1–5] | 3.91 (0.98) [0–5] | −0.03 | .975 |
| Disorganization symptoms | 2.25 (1.52) [0–5] | 1.67 (1.40) [0–5] | −1.72 | .085 |
| Negative symptoms | 3.60 (0.94) [3–5] | 1.80 (1.28) [0–4] | −6.04 | <.001 |
| Depressive symptoms | 1.15 (1.27) [0–4] | 0.82 (1.24) [0–4] | −1.68 | .092 |
| 1-year psychopathologyb | ||||
| Reality-distortion symptoms | 1.60 (1.14) [0–4] | 1.34 (1.16) [0–5] | −1.08 | .282 |
| Disorganization symptoms | 1.10 (1.29) [0–4] | 0.65 (0.92) [0–4] | −1.42 | .154 |
| Negative symptoms | 3.45 (0.83) [3–5] | 1.48 (1.10) [0–4] | −5.83 | <.001 |
| Depressive symptoms | 0.40 (0.94) [0–4] | 0.33 (0.61) [0–3] | −1.13 | .895 |
| 1-year pharmacological treatment | ||||
| Antipsychotic dose, CPZ equivalents | 305.2 (139.2) | 311.2 (134.8) [5–800] | −0.21 | .829 |
| Benzodiazepines/hypnotics, n (%) | 10 (50.0) | 44 (53.6) | 0.08 | .769 |
| Antidepressants, n (%) | 7 (35.0) | 19 (23.2) | 2.59 | .107 |
| Biperiden, n (%) | 5 (25.0) | 16 (19.5) | 0.30 | .586 |
| 1-year symptomatic remission | ||||
| Overall symptom remission, n (%) | 0 | 49 (59.8) | 23.0 | <.001 |
| Positive symptom remission, n (%) | 11 (55.0) | 58 (70.7) | 1.82 | .178 |
| 1-year functioning | ||||
| GAF Scale | 39.4 (16.7) [16–62] | 60.4 (18.9) [23–94] | −4.24 | <.001 |
Note: SES, socioeconomic status; PSA, premorbid social adjustment; DUNS, duration of untreated negative symptoms; DUP, duration of untreated psychosis; CPZ, chlorpromazine; GAF, Global Assessment of Functioning.
aUnless otherwise specified, values are mean (SD) [range].
bReality-distortion symptoms: mean rating of delusions and hallucinations; disorganization symptoms: mean rating of positive formal thought disorder, bizarre behavior, and inappropriate affect; negative symptoms: mean rating of affective flattening, alogia, and avolition; depressive symptoms: global severity rating of depression.
The distribution of the deficit/nondeficit categorization across classical schizophrenia subtypes varied significantly (χ2 = 7.83, df = 3, P = .049). Post hoc analyses showed that patients with deficit schizophrenia had a significantly higher proportion of catatonic (25% vs 8%, χ2 = 7.42, df = 1, P = .006) and disorganized schizophrenia (20% vs 11%, χ2 = 3.91, df = 1, P = .048) than those with the nondeficit form of the disease.
Factor Structure of Deficit Symptoms
PCA of baseline deficit symptoms resulted in two distinct and interpretable factors explaining 77% of the variance. Factor 1 (avolition) accounted for most of the variance explained (60%) and was made of curving of interests (0.89), diminished sense of purpose (0.94), and diminished social drive (0.93). The second factor (emotional expression) accounted for 17% of the variance and was made of restricted affect (0.93), diminished emotional range (0.97), and poverty of speech (0.88). The two factors were highly intercorrelated (r = .52). A similar factor structure was obtained using deficit symptom ratings at 1-year follow-up (data not shown).
Relationships Within and Between Neurological Domains
Overall, neurological ratings tended to be interrelated, both within and between neurological domains (supplementary table 1). Major exceptions were akathisia, which was unrelated to any other neurological rating, and sensorial integration, which was unrelated to SMD ratings and release signs. Summary ratings of SMD and NSS were significantly correlated (rs = .36, P < .001).
Relationships of Deficit Features With SMD and NSS
Overall, deficit features including individual symptoms, subscales, and global severity ratings were positively correlated with parkinsonism and catatonia ratings and with each NSS domain (table 2). Dyskinesia was only correlated with the SDS global severity rating, and akathisia was inversely correlated with diminished social drive and the avolition subscale.
Table 2.
Spearman Rank Correlations Coefficients of Baseline Deficit Features With Spontaneous Movement Disorders (SMD; n = 102) and Neurological Soft Signs (NSS; n = 83) Domains in Drug-Naive Schizophrenia Patients
| SMD | NSS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deficit Features | Mean (SD) | Parkinsonism | Dyskinesia | Catatonia | Akathisia | Sensorial Integration | Motor Coordination | Sequencing | Release Signs | ||
| Symptoms | |||||||||||
| Curbing of interest | 0.63 (0.99) | 0.25a,* | 0.06 | 0.17 | −0.19 | 0.30a,** | 0.19 | 0.33a,** | 0.24* | ||
| Diminished sense of purpose | 0.85 (1.17) | 0.29a,** | 0.14 | 0.31b,*** | −0.17 | 0.21 | 0.19 | 0.27* | 0.21 | ||
| Diminished social drive | 1.12 (1.31) | 0.21* | 0.07 | 0.22* | −0.20* | 0.14 | 0.18 | 0.23* | 0.23* | ||
| Restricted affect | 0.80 (1.07) | .25a,* | 0.15 | 0.40b,*** | −0.06 | 0.28a,** | 0.31a,** | 0.33a,** | 0.24* | ||
| Diminished emotional range | 0.72 (1.02) | 0.18 | 0.18 | 0.39b,*** | −0.04 | 0.30a,** | 0.34c,** | 0.37c,*** | 0.25* | ||
| Poverty of speech | 0.61 (0.91) | 0.24* | 0.18 | 0.23* | 0.07 | 0.08 | 0.30a,** | 0.26* | 0.26* | ||
| Factors | |||||||||||
| Avolition | 2.61 (3.25) | 0.25a,* | 0.11 | 0.26a,** | −0.22* | 0.16 | 0.18 | 0.27* | 0.25* | ||
| Emotional expression | 2.14 (2.82) | 0.23* | 0.15 | 0.36b,*** | −0.02 | 0.25* | 0.31a,** | 0.35c,*** | 0.25* | ||
| Global severity | |||||||||||
| Deficit symptoms total score | 4.75 (5.45) | 0.29a,** | 0.16 | 0.39b,*** | −0.16 | 0.28a,** | 0.31a,** | 0.39b,*** | 0.27* | ||
| Deficit syndrome global severity rating | 0.90 (1.16) | 0.35b,*** | 0.20* | 0.32b,*** | −0.13 | 0.23* | 0.41b,*** | 0.37c,*** | 0.32a,** | ||
a P < .05, b P < .001, c P < .01 (after Bonferroni correction).
*P < .05, **P < .01, ***P < .001.
Because associations between deficit features and some neurological domains such as parkinsonism and catatonia may be due to overlapping definitions or phenomenological resemblance, we explored more specifically this issue by examining such an associations at the subdomain level (supplementary table 2). Within parkinsonism, the subdomains of hypokinesia and rigidity were similarly related to deficit features. Within catatonia, the negative, positive/hyperkinetic, and volitional subdomains all were similarly related to deficit features, although the higher effect sizes were observed for the negative subdomain.
Prevalence and Severity of SMD and NSS in Deficit and Nondeficit Schizophrenia
Dimensional ratings of SMD, excepting akathisia, were significantly more severe in the deficit group than in the nondeficit group (table 3). Categorical ratings of parkinsonism, dyskinesia, and catatonia were more frequent in patients with deficit schizophrenia than in those with nondeficit schizophrenia. Thirteen patients with the deficit syndrome (65%) and 27 patients without the deficit syndrome (32.9%) had at least one spontaneous motor syndrome (χ2 = 6.94, df = 1, P = .008). All NSS domains were more severe and prevalent in patients with deficit schizophrenia than in those with nondeficit schizophrenia (table 4).
Table 3.
Dimensional and Categorical Ratings of Spontaneous Movement Disorders in 102 Drug-Naive Schizophrenia Patients With and Without the Deficit Syndrome
| Deficit (n = 20) | Nondeficit (n = 82) | |||
|---|---|---|---|---|
| Dimensional Ratings | Mean (SD) | Mean (SD) | Z | P |
| Parkinsonism | 4.40 (3.87) | 2.13 (2.93) | −3.11 | .002a |
| Dyskinesia | 3.55 (4.78) | 1.43 (2.92) | −2.63 | .007b |
| Catatonia | 6.55 (7.09) | 2.62 (4.15) | −2.92 | .004b |
| Akathisia | 0.10 (0.31) | 0.23 (0.57) | −0.83 | .408 |
| Categorical Ratings | n (%) | n (%) |

|
P |
| Parkinsonism | 9 (45.0) | 16 (19.5) | 5.64 | .039 |
| Dyskinesia | 6 (30.0) | 10 (12.2) | 3.85 | .080 |
| Catatonia | 5 (25.0) | 9 (11.0) | 2.67 | .142 |
| Akathisia | 0 | 4 (4.9) | 1.02 | .583 |
Note: Fisher exact test.
a P < .01, b P < .05 (after Bonferroni correction).
Table 4.
Dimensional and Categorical Ratings of Neurological Soft Signs in 83 Drug-Naive Schizophrenia Patients With and Without the Deficit Syndrome
| Deficit (n = 17) | Nondeficit (n = 66) | |||
|---|---|---|---|---|
| Dimensional Ratings | Mean (SD) | Mean (SD) | Z | P |
| Sensorial integration | 3.59 (1.87) | 2.44 (2.46) | −2.41 | .016 |
| Motor coordination | 2.53 (1.77) | 1.23 (1.68) | −3.03 | .002a |
| Motor sequencing | 6.76 (3.94) | 3.17 (3.26) | −3.20 | .001a |
| Release signs | 0.88 (1.11) | 0.34 (0.95) | −0.83 | .009b |
| Categorical Ratings | n (%) | n (%) |  |
P |
| Sensorial integration | 13 (76.5) | 29 (43.9) | 5.72 | .017 |
| Motor coordination | 14 (82.4) | 34 (51.5) | 5.27 | .022 |
| Motor sequencing | 15 (88.2) | 31 (47.0) | 9.32 | .002a |
| Release signs | 8 (47.1) | 9 (13.6) | 9.27 | .005b |
Note: Fisher exact test.
aP < .01, bP < .05 (after Bonferroni correction).
Given that deficit patients had higher levels of disorganization and depression than nondeficit patients with the differences not reaching statistical significance, and that these psychopathological domains may be secondary sources of deficit symptoms, we repeated the analyses excluding deficit patients with high levels (global score >3) of disorganization (n = 3) or depression (n = 2). The findings were very similar and there was no substantial change in the pattern or effect size of the associations between deficit symptoms/syndrome and neurological abnormalities.
Independent Associations of SMD and NSS Domains With Deficit Schizophrenia
Given that SMD and NSS tended to be interrelated both within and between domains, we examined independent associations of categorically defined SMD and NSS with deficit schizophrenia, first within each broad neurological domain and then considering all categories from the two broad neurological domains. This procedure was modeled by means of logistic regression analyses where the dependent variable was the deficit/nondeficit categorization and the independent variables the categorical ratings of SMD and/or NSS domains. Age and gender were used as covariates and entered at the first step of the regression model. Within SMD, the only category that was independently associated with the deficit syndrome was parkinsonism (OR = 3.74, 95% CI = 1.25–11.0, P = .018). Within NSS, the categories that were independently related to the deficit syndrome were motor sequencing (OR = 7.01, 95% CI = 1.42–34.6, P = .017) and release signs (OR = 4.34, 95% CI = 1.11–17.0, P = .017). When all SMD and NSS categories were entered in the regression model, the categories independently associated with the deficit syndrome remained the same: parkinsonism (OR = 7.41, 95% CI = 1.15–48.0, P = .036), motor sequencing (OR = 7.85, 95% CI = 1.50–41.2, P = .015), and release signs (OR = 5.05, 95% CI = 1.21–21.0, P = .026).
Relationships of Deficit Features With Abnormal Movement Disorders at 1-Year Follow-Up
The differential prevalence and severity of abnormal movement disorders in deficit and nondeficit schizophrenia (supplementary table 3), and the relationship of abnormal movement disorders with deficit features in the whole sample of schizophrenia patients (supplementary table 4) both followed a similar pattern of results than that observed at baseline, with minor variations in the effect size of the associations, the major difference being that abnormal movements tented to be more related to the emotional expression deficit subdomain.
Discussion
Deficit Syndrome Characterization
The pattern of demographic, premorbid, psychopathological, and outcome characteristics of our patients with deficit schizophrenia relative to those with the nondeficit subtype suggests that the deficit syndrome identified is highly consistent with the original deficit syndrome conceptualization.2,3,5 Furthermore, our study replicates the high temporal stability of the deficit syndrome,41 such as the 2-factor structure of deficit features comprising volitional and emotional expressivity dimensions.42,43 These findings confirm that the deficit/nondeficit categorization is replicable and reliable in first-admission, drug-naive patients and allows the identification of patients with consistent clinical characteristics across independent studies and differing samples. Moreover, the unique characteristics of our study sample—drug-naive patients—and design—two assessment points over 1-year follow-up—allowed us to eliminate the confounding factors of medication on the assessment of deficit and neurological symptoms, and the retrospective assessment bias of deficit symptoms.
Our data on the premorbid correlates of deficit features are consistent with a developmental model of the deficit syndrome and more specifically with Buchanan et al.’s hypothesis3 that deficit schizophrenia represents an early onset subgroup, in which poor premorbid functioning might represent the onset of deficit symptoms. Our study adds to previous studies of enduring negative44,45 and deficit symptoms46 that deterioration in functioning from childhood to adolescence is a core feature of the deficit syndrome, this suggesting that premorbid deterioration may represent either the onset of the schizophrenic illness or an ultra at-risk factor for the disorder.47 Deficit patients had a much lengthier DUNS and DUP than those with the nondeficit subtype. To the best of our knowledge, this finding has not been previously described for deficit schizophrenia and is consistent with evidence of a strong link between DUI and negative symptoms.48 Schizophrenia patients with the deficit syndrome appear to be at increased risk for protracted DUI, which clearly represents an unmet need for these patients and might be of relevance for the early detection and treatment of deficit patients. In fact, preliminary evidence indicates that early intervention programs may be effective for preventing deficit symptom escalation.49 We found that deficit patients do not show overall symptomatic remission and have a much worse functional outcome than nondeficit patients, which would be expected because of some redundancy among definitions of deficit syndrome, symptomatic remission, and functioning as assessed with the GAF. We found, however, that deficit patients have a similar rate of positive symptom remission than nondeficit patients, a finding consistent with the notion that psychotic and deficit symptoms represent different disease processes.50
Deficit Features and Primary Neurological Abnormalities
Levels of neurological abnormalities were in the mean range of those reported in previous studies of drug-naive schizophrenia patients.8,20 Ratings of SMD and NSS were substantially correlated, which supports their consideration as partially overlapping subdomains within a more broad neurological domain of schizophrenia.37 Overall, neurological ratings, excepting akathisia, tended to be consistently related to deficit schizophrenia and to deficit features in the whole population of schizophrenia patients. The association of deficit features with specific subdomains of catatonia (ie, volitional, positive/hyperkinetic) and parkinsonism (ie, rigidity), which do not bear phenomenological resemblance with deficit features, indicates that these associations are not merely due to phenomenological similarity among deficit and motor domains. Stronger associations were observed for dimensional SMD and NSS ratings than for categorical ones, which may be expected due to lower statistical power of the categorical approach. Categorical ratings of parkinsonism, motor sequencing, and release signs were all independently related to the deficit syndrome, and they were between five and seven times more likely to be present in deficit than in nondeficit patients.
Given the subdomain structure of deficit symptoms, a question arising is the relationship of these subdomains with primary neurological signs. Although no clear differential pattern emerged, the emotional expression domain showed more and stronger correlations with neurological signs than the avolition domain. Furthermore, sensorial integration and motor coordination were differentially related to the emotional expression domain, all of which would support the hypothesis of a somewhat differential neurocognitive profile for this domain.51 The lack of association between the avolition deficit domain and the volitional catatonia domain indicates that they are qualitatively distinct phenomena.
Interestingly, both SMD52,53 and NSS54,55 have been observed in children and adolescents at an elevated risk for schizophrenia. It has indeed been shown that there is a premorbid longitudinal progression of SMD that parallels that of negative symptoms in these subjects.53 Thus, it is rather likely that the neurological abnormalities observed in our first-admission sample already existed during the premorbid period. The association pattern of deficit psychopathology with premorbid negative symptoms, poor premorbid functioning, deterioration of premorbid adjustment, and neurological impairment, suggests that deficit symptoms join other markers of subtle but pervasive neurodevelopmental impairment that may share common neurobiological underpinnings.
Given that antipsychotic drugs may improve or unmask preexisting neurological abnormalities, such as cause “de novo” motor syndromes,56 the motor abnormalities assessed at follow-up are likely to be a complex mixture of primary and drug-induced features. This raises the interesting question of whether the deficit syndrome conveys an increased risk for developing drug-induced motor disorders. Our results are in line with those reported from other authors13 and all they would support this hypothesis.
The association between primary neurological abnormalities and deficit features supports an involvement of the basal ganglia in the pathophysiology of the deficit syndrome. Extrapyramidal symptoms are typical manifestations of basal ganglia pathology, where abnormal increment or reduction in the inhibitory output activity gives rise, respectively, to dyskinetic and parkinsonian movement disorders.57 Although less well studied, similar mechanisms have also been proposed for the negative and positive/hyperkinetic forms of catatonia.58,59 NSS are also indicative of basal ganglia dysfunction, because a reduction of grey matter volume in different basal ganglia structures has been reported in a number of studies.60–62 Furthermore, a recent study of functional and structural MRI in schizophrenia patients63 showed that those with high levels of negative symptoms had diminished striatal activity during a working memory task. Despite converging evidence of basal ganglia dysfunction in deficit schizophrenia, and given the marked influence of antipsychotic medication on basal ganglia structures,64 neuroimaging research on drug-naive patients with deficit schizophrenia is clearly warranted to confirm this hypothesis.
Limitations
Some limitations in this study should be noted. First, given that our study sample was made of inpatients, findings may not apply to the less severe patients not requiring hospitalization. In this regard, while it is possible that the more severe patients are also the more neurologically impaired, this does not necessarily influence the association between deficit symptoms and neurological impairment, because such an association has also been reported in subjects with schizotypal personality disorder.53 Second, SMD were not assessed blind to deficit features at baseline, which could bias results. This bias, however, may have been minimized by the highly structured instruments used to rate these symptoms and the longitudinal assessment of deficit features that was blindly performed in relation to baseline neurological ratings. Third, despite the multiple test performed, the possibility of some false positive findings is highly unlikely because most of the significant associations survived the Bonferroni correction, dimensional, and categorical analyses of neurological ratings tended to converge and we used the conservative nonparametric approach. Fourth, cognitive functioning was not examined, and this variable may represent a confounding factor mediating the relationship between deficit and neurological features. Fifth, factor analysis of SDS symptoms included a very low number of subjects; however, the factor structure obtained was the same than in previous studies,42,43 which reinforces the validity of our procedure. Finally, the relatively small sample size conveys a reduced power to detect statistically significant differences among groups. In fact, the effect size for the association between categories of SMD and the deficit syndrome may have been underestimated because of small sample size. Future studies of larger samples of drug-naive patients with deficit schizophrenia along with the use of the more sensitive instrumental measures of abnormal movements should help to clarify this issue.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
Ministerio de Educación y Ciencia (SAF2008-05674- C03-02); Departamento de Salud del Gobierno de Navarra (946-2005, 55-2007); Comissionat per a Universitats i Recerca del DIUE (2009SGR827).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge patients and their families for their participation in the study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Carpenter WT, Jr, Stephens JH. An attempted integration of information relevant to schizophrenic subtypes. Schizophr Bull. 1979; 5: 490–506 [DOI] [PubMed] [Google Scholar]
- 2. Carpenter WT., Jr Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988; 145: 578–583 [DOI] [PubMed] [Google Scholar]
- 3. Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT., Jr Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry. 1990; 147: 290–294 [DOI] [PubMed] [Google Scholar]
- 4. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994; 151: 351–356 [DOI] [PubMed] [Google Scholar]
- 5. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001; 58: 165–171 [DOI] [PubMed] [Google Scholar]
- 6. Galderisi S, Maj M, Mucci A, et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am J Psychiatry. 2002; 159: 983–990 [DOI] [PubMed] [Google Scholar]
- 7. Galderisi S, Maj M. Deficit schizophrenia: an overview of clinical, biological and treatment aspects. Eur Psychiatry. 2009; 24: 493–500 [DOI] [PubMed] [Google Scholar]
- 8. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009; 35: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen EY, Lam LC, Chen RY, Nguyen DG. Negative symptoms, neurological signs and neuropsychological impairments in 204 Hong Kong Chinese patients with schizophrenia. Br J Psychiatry. 1996; 168: 227–233 [DOI] [PubMed] [Google Scholar]
- 10. Sandyk R, Kay SR. The relationship of negative symptoms to parkinsonism. Int J Neurosci. 1990; 55: 1–59 [DOI] [PubMed] [Google Scholar]
- 11. Brown KW, White T. The association among negative symptoms, movement disorders, and frontal lobe psychological deficits in schizophrenic patients. Biol Psychiatry. 1991; 30: 1182–1190 [DOI] [PubMed] [Google Scholar]
- 12. McGlashan TH, Fenton WS. The positive-negative distinction in schizophrenia. Review of natural history validators. Arch Gen Psychiatry. 1992; 49: 63–72 [DOI] [PubMed] [Google Scholar]
- 13. Telfer S, Shivashankar S, McCreadie RG, Kirkpatrick B. Tardive dyskinesia and deficit schizophrenia. Acta Psychiatr Scand. 2011; 124: 357–362 [DOI] [PubMed] [Google Scholar]
- 14. Arango C, Kirkpatrick B, Buchanan RW. Neurological signs and the heterogeneity of schizophrenia. Am J Psychiatry. 2000; 157: 560–565 [DOI] [PubMed] [Google Scholar]
- 15. Tiryaki A, Yazici MK, Anil AE, Kabakçi E, Karaağaoğlu E, Göğüş A. Reexamination of the characteristics of the deficit schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2003; 253: 221–227 [DOI] [PubMed] [Google Scholar]
- 16. Schooler N. Deficit symptoms in schizophrenia: negative symptoms versus neuroleptic-induced negative deficits. Acta Psychiatr Scand. 1994; 380: 21–26 [DOI] [PubMed] [Google Scholar]
- 17. Ayd FJ. A survey of drug-induced extrapyramidal reactions. JAMA. 1961; 175: 1054–1060 [DOI] [PubMed] [Google Scholar]
- 18. Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry. 2002; 63:(suppl 4):12–19 [PubMed] [Google Scholar]
- 19. Gupta S, Andreasen NC, Arndt S, et al. Neurological soft signs in neuroleptic-naive and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry. 1995; 152: 191–196 [DOI] [PubMed] [Google Scholar]
- 20. Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry. 2002; 181:(suppl 43):50–57 [DOI] [PubMed] [Google Scholar]
- 21. Fenton WS, Wyatt RJ, McGlashan TH. Risk factors for spontaneous dyskinesia in schizophrenia. Arch Gen Psychiatry. 1994; 51: 643–650 [DOI] [PubMed] [Google Scholar]
- 22. Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011; 52: 139–145 [DOI] [PubMed] [Google Scholar]
- 23. Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010; 118: 168–175 [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). 4th ed Washington, DC:American Psychiatric Association; 1994; [Google Scholar]
- 25. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992; 49: 615–623 [DOI] [PubMed] [Google Scholar]
- 26. Peralta V, de Leon J, Cuesta MJ. Are there more than two syndromes in schizophrenia? A critique of the positive-negative dichotomy. Br J Psychiatry. 1992; 161: 335–343 [DOI] [PubMed] [Google Scholar]
- 27. Hollingshead AB, Redlich FC. Social Class and Mental Illness: A Community Study. New York, NY: John Wiley & Sons; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cannon M, Jones P, Gilvarry C, et al. Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am J Psychiatry. 1997; 154: 1544–1550 [DOI] [PubMed] [Google Scholar]
- 29. Perkins DO, Leserman J, Jarskog LF, et al. Characterizing and dating the onset of symptoms in psychotic illness: the Symptom Onset in Schizophrenia (SOS) inventory. Schizophr Res. 2000; 44: 1–10 [DOI] [PubMed] [Google Scholar]
- 30. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989; 30: 119–123 [DOI] [PubMed] [Google Scholar]
- 31. Peralta V, Cuesta MJ. The deficit syndrome of the psychotic illness. A clinical and nosological study. Eur Arch Psychiatry Clin Neurosci. 2004; 254: 165–171 [DOI] [PubMed] [Google Scholar]
- 32. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiat Scand. 1970; 212: 11–19 [DOI] [PubMed] [Google Scholar]
- 33. Guy WA. Abnormal Involuntary Movement Scale (AIMS). In: ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Department of Health, Education, and Welfare; 1976: 534–537 [Google Scholar]
- 34. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982; 39: 486–487 [DOI] [PubMed] [Google Scholar]
- 35. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989; 154: 672–676 [DOI] [PubMed] [Google Scholar]
- 36. Lund CE, Mortimer AM, Rogers D, et al. Motor, volitional and behavioural disorders in schizophrenia. 1: assessment using the Modified Rogers Scale. Br J Psychiatry. 1991; 158: 323–327 [DOI] [PubMed] [Google Scholar]
- 37. Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989; 27: 335–350 [DOI] [PubMed] [Google Scholar]
- 38. Peralta V, de Jalón EG, Campos MS, Basterra V, Sanchez-Torres A, Cuesta MJ. Risk factors, premorbid functioning and episode correlates of neurological soft signs in drug-naive patients with schizophrenia-spectrum disorders. Psychol Med. 2010; 22: 1–11 [DOI] [PubMed] [Google Scholar]
- 39. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976; 33: 766–771 [DOI] [PubMed] [Google Scholar]
- 40. Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Arch Gen Psychiatry. 2005; 162: 441–449 [DOI] [PubMed] [Google Scholar]
- 41. Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA. Stability of the diagnosis of deficit syndrome in schizophrenia. Am J Psychiatry. 1999; 156: 637–639 [DOI] [PubMed] [Google Scholar]
- 42. Kimhy D, Yale S, Goetz RR, McFarr LM, Malaspina D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr Bull. 2006; 32: 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakaya M, Ohmori K. A two-factor structure for the schedule for the deficit syndrome in schizophrenia. Psychiatry Res. 2008; 158: 256–259 [DOI] [PubMed] [Google Scholar]
- 44. Kelley ME, Gilbertson M, Mouton A, van Kammen DP. Deterioration in premorbid functioning in schizophrenia: a developmental model of negative symptoms in drug-free patients. Am J Psychiatry. 1992; 149: 1543–1548 [DOI] [PubMed] [Google Scholar]
- 45. Malla AK, Norman RM, Takhar J, et al. Can patients at risk for persistent negative symptoms be identified during their first episode of psychosis? J Nerv Ment Dis. 2004; 192: 455–463 [DOI] [PubMed] [Google Scholar]
- 46. Strauss GP, Allen DN, Miski P, Buchanan RW, Kirkpatrick B, Carpenter WT., Jr Differential patterns of premorbid social and academic deterioration in deficit and nondeficit schizophrenia. Schizophr Res. 2012; 135: 134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004; 67: 131–142 [DOI] [PubMed] [Google Scholar]
- 48. Perkins DO, Gu H, Lieberman JA. Relationship between duration of untreated illness and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005; 162: 1785–1804 [DOI] [PubMed] [Google Scholar]
- 49. Melle I, Larsen TK, Haahr U., et al. Prevention of negative symptom psychopathologies in first-episode schizophrenia. Arch Gen Psychiatry. 2008; 65: 634–640 [DOI] [PubMed] [Google Scholar]
- 50. Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998; 155: 751–760 [DOI] [PubMed] [Google Scholar]
- 51. Kirkpatrick B, Fisher B. Subdomains within negative symptoms of schizophrenia: commentary. Schizophr Bull. 2006; 32: 2006–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994; 20: 441–451 [DOI] [PubMed] [Google Scholar]
- 53. Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008; 65: 165–171 [DOI] [PubMed] [Google Scholar]
- 54. Obiols JE, Serrano F, Caparrós B, Subirá S, Barrantes N. Neurological soft signs in adolescents with poor performance on the continous performance test: markers of liability for schizophrenia spectrum disorders? Psychiatry Res. 1999; 86: 217–228 [DOI] [PubMed] [Google Scholar]
- 55. Prasad KM, Sanders R, Sweeney J, et al. Neurological abnormalities among offspring of persons with schizophrenia: relation to premorbid psychopathology. Schizophr Res. 2009; 108: 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peralta V, Cuesta MJ. The effect of antipsychotic medication on neuromotor abnormalities in neuroleptic-naive nonaffective psychotic patients: a naturalistic study with haloperidol, risperidone, or olanzapine. Prim Care Companion J Clin Psychiatry. 2010; 12:. pii: PCC.09m00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Arbizu J, Giménez-Amaya JM. The basal ganglia and disorders of movement. News Physiol Sci. 2002; 17: 51–55 [DOI] [PubMed] [Google Scholar]
- 58. Caroff SN, Mann SC, Francis A, Friccione GL. Catatonia: From Psychopathology to Neurobiology. Washington, DC: American Psychiatric Publishing Inc,; 2004. [Google Scholar]
- 59. Scheuerecker J, Ufer S, Käpernick M, et al. Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res. 2009; 43: 607–614 [DOI] [PubMed] [Google Scholar]
- 60. Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004; 127: 143–153 [DOI] [PubMed] [Google Scholar]
- 61. Thomann PA, Wüstenberg T, Santos VD, Bachmann S, Essig M, Schröder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med. 2009; 39: 371–379 [DOI] [PubMed] [Google Scholar]
- 62. Janssen J, Diaz-Caneja A, Reig S, et al. Brain morphology and neurological soft signs in adolescents with first-episode psychosis. Br J Psychiatry. 2009; 195: 227–233 [DOI] [PubMed] [Google Scholar]
- 63. Ehrlich S, Yendiki A, Greve DN, et al. Striatal function in relation to negative symptoms in schizophrenia. Psychol Med. 2012; 42: 267–282 [DOI] [PubMed] [Google Scholar]
- 64. Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009; 39: 1763–1777 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.