Abstract
A comprehensive understanding of the phenomenology of auditory hallucinations (AHs) is essential for developing accurate models of their causes. Yet, only 1 detailed study of the phenomenology of AHs with a sample size of N ≥ 100 has been published. The potential for overreliance on these findings, coupled with a lack of phenomenological research into many aspects of AHs relevant to contemporary neurocognitive models and the proposed (but largely untested) existence of AH subtypes, necessitates further research in this area. We undertook the most comprehensive phenomenological study of AHs to date in a psychiatric population (N = 199; 81% people diagnosed with schizophrenia), using a structured interview schedule. Previous phenomenological findings were only partially replicated. New findings included that 39% of participants reported that their voices seemed in some way to be replays of memories of previous conversations they had experienced; 45% reported that the general theme or content of what the voices said was always the same; and 55% said new voices had the same content/theme as previous voices. Cluster analysis, by variable, suggested the existence of 4 AH subtypes. We propose that there are likely to be different neurocognitive processes underpinning these experiences, necessitating revised AH models.
Key words: auditory verbal hallucinations, memory, schizophrenia
Introduction
The causes of hearing a voice with a compelling sense of reality in the absence of an appropriate external stimulus, formally termed an auditory verbal hallucination (AVH), remain poorly understood. Such experiences often cause significant distress, and when social/occupational impairment ensues, they will most commonly result in a diagnosis of schizophrenia.1 Because AVHs often persist when antipsychotic medication is administered,2 there is a need to better understand their causal mechanisms and to translate this knowledge into improved interventions.3
Insufficient knowledge of the phenomenology of AVHs forms one barrier to understanding and modeling the experience.1,4 Although AVH phenomenology was richly descriptively explored by Esquirol, Bleuler, and Kraepelin,1 it was not until Nayani and David’s5 seminal study that systematic, quantitative data on the phenomenology of AVHs (and nonverbal auditory hallucinations [NVAHs]), from a large sample of people with schizophrenia-spectrum diagnoses (N = 100), became available. Since then, studies have employed smaller samples6 and/or focused on specific questions such as comparing the phenomenology of AVHs to thoughts.7,8 Despite the improved understanding of AVH phenomenology this has resulted in,9 there are a number of reasons to revisit this topic.
First, many aspects of AVH phenomenology have not been inquired about, forming a barrier to the development and evaluation of contemporary models. For example, models that argue AVHs result from “the failure to inhibit memories of prior events”10 (p. 132) or claim parallels with the repetitive thoughts found in obsessive compulsive disorder (OCD)11,12 cannot be evaluated phenomenologically due to an absence of large-scale studies on these aspects of AVH phenomenology. We hence first aimed to document such phenomenological properties of AVHs, using the Mental Health Research Institute Unusual Perceptions Schedule (MUPS).13
Second, although Nayani and David’s study5 has been influential in the development of models of AVHs, no other large-scale study has yet attempted replication. Despite this, many researchers base theories/models on these findings. For example, Badcock14 has offered an innovative neural-based explanation for Nayani and David’s finding that AVHs are typically experienced as a male’s voice, yet this finding of male voice-predominance remains to be replicated. Therefore, the second aim of our study was to examine pattern of similarities and differences to the findings of Nayani and David with respect to aspects of AVH phenomenology assessed by both their phenomenological survey and the MUPS.
Third, the heterogeneity of AVH phenomenology5,9 has led many authors to propose that distinct AVH subtypes may exist, with each having distinct underlying neurocognitive mechanisms (with some shared components) and potentially requiring different clinical interventions.1,4,15–17 Although case studies have presented preliminary evidence for the existence of AVH subtypes,12,15 only 1 study has statistically investigated their existence. In this study, Stephane and colleagues16 collected data on 21 aspects of AVH phenomenology in a sample of 30 people diagnosed with schizophrenia, clustered these variables using cluster analysis, and identified 2 AVH subtypes. The first was characterized by having repetitive content, low linguistic complexity (hearing single words), an outer space location, and clear acoustics; being accompanied by other hallucinations; and being attributed to the self. The second had systematized (ie, nonrepetitive) content, had high and intermediate linguistic complexity (hearing sentences and conversations), had an inner space location, involved multiple voices, which were episodic (ie, were not constant), spontaneous (ie, did not have clear triggers), and attributed to another.
However, this study had limitations. First, there was an extremely low ratio of 1.4 participants to each variable entered into the cluster analysis. While there are no formal rules as to what this ratio should be, a general rule of thumb would be at least 10:1, possibly greater when using binary data including items with low endorsement rates, hence raising questions over the reliability of these findings. Second, it did not use a formal mathematical technique to inform how many clusters should be extracted. Third, it did not include NVAHs, which commonly co-occur with AVHs.5 Finally, their selection of phenomenological variables was not able to be theoretically driven by the literature on AVH subtypes because most of it was published subsequent to their study.
The third aim of our study was therefore to replicate Stephane and colleagues’ approach to testing for the existence of AVH subtypes, with an improved design involving examining AH subtypes generally (ie, including AVHs and NVAHs), selecting phenomenological variables on the basis of relevant theory, clustering using a participant: variable ratio high enough to likely guarantee meaningful results, and using a formal mathematical technique to inform the decision as to the number of clusters to be extracted.
Method
The basis for this study was a data set, facets of which have already been reported on by Copolov and colleagues.18,19 All analyses performed here have not previously been reported.
Participants
Participants were 199 individuals (134 male), with a mean age of 32.75 years (SD = 10.67, range 15–63) with psychiatric diagnoses and experience of AHs. Only 3 reported solely NVAHs. The majority of participants were recruited from a psychiatric hospital (126), a clubhouse for the mentally ill (17), and private psychiatrists (15). Diagnoses were made using the Structured Clinical Interview for DSM-III-R.20 Diagnoses related to the time of the MUPS interview, but AHs reported could be from the past. Although the mean age of onset of AHs was 23.2 years (9.5 years prior to interview), the MUPS interview typically focused on AHs experienced during the most recent illness episode, with interviewers focusing only on AHs that the participant could remember details of. Schizophrenia was the most common principal psychiatric diagnosis (80.9%), with others being affective psychosis (13.6%), other nonorganic psychoses (3%), and borderline personality disorder (2.5%). For the 191 subjects for whom information was available, 91.6% were prescribed antipsychotic medication, the mean daily dose of antipsychotic medication of which, in chlorpromazine equivalents, was 474mg (SD = 287, range 25–1500). Most (84.9%) were born in an English-speaking country.
Procedure
The MUPS,13 a semistructured interview that investigates many aspects of AHs, was administered to participants by professionals trained in its use. Its 365 questions are organized into 7 main sections: physical characteristics, personal characteristics, relationship and emotive aspects, form and content, cognitive processes, personal perceptions, and psychosocial issues. Response formats (fixed or free) vary according to the question being asked. The reliability and acceptability of the MUPS have been reported previously.13
Cluster Analysis
Methodology.
Following Stephane and colleagues,16 we explored the internal structure of AH phenomenology using hierarchical cluster analysis (clustering by variable). Analyses were performed using ClustanGraphics.21 We employed Ward’s method, argued to be one of the best methods for cluster structure recovery.22 Because the data were binary, we used Jaccard’s coefficient as the similarity measure, previously been shown to work well with Ward’s method in recovering cluster structure in binary data.23 The number of clusters to be extracted was to be informed by both the dendogram and the objective criterion offered by ClustanGraphics (Mojena’s upper tail rule).24
Variables.
In a necessary compromise between reliability (ie, not having too great a participant:variable ratio) and meaningfulness of analyses (ie, having enough variables to yield informative clusters), we determined to enter 13 variables into the analysis, resulting in a participant:variable ratio of 15:1. These variables were selected based on both the established phenomenologies of AHs,1,5 existing theorizing in the area of AH subtypes12,16,17 and clinical experience, but were limited to the aspects of AH phenomenology assessed by the MUPS.13
First, we included measures of the commonest forms of AVHs, namely commands and running commentaries (items #1 and #2 in table 1).5 Second, following Stephane and colleagues,12,16 we included a measure of the repetitiveness of AVHs (item #3). Third, following Badcock and colleagues’10 research showing that AVHs may be memories of prior events, we included 2 items on the extent to which AVHs were identified as either identical (item #4) or similar to memories (item #5). Fourth, given that AHs have been proposed to result from spontaneous activity in the superior temporal gyrus25 (STG), we also included facets of AH phenomenology found in Penfield and Perot’s study26 of direct stimulation of the STG, including NVAHs (eg, music, bangs; item #6) and AVHs, which did not make sense (ie, were like gibberish; item #7). We also included the form of address the voice took, ie, first/second/third person (items #8–10), or voices not addressing the person (item #11). We further included a measure of whether participant felt AVHs were actually their own thoughts (item #12), based on work suggesting that AVHs exist on a continuum with thoughts.8 Finally, following Hoffman and Hampson’s proposal27 that AH subtypes can be distinguished on the basis of whether they are constant or intermittent, we included a measure of how constant AHs were (item #13). These items were operationalized using MUPS items as described in table 1, and we used binary coding, following Stephane and colleagues’ approach.16
Table 1.
Operationalization of Variables Entered Into Hierarchical Cluster Analysis
| Item | Variable | Operationalization | % scored “1” |
|---|---|---|---|
| 1 | Command hallucinations | Responses “often” or “sometimes” to MUPS item 25a, “Did the voices ever tell you what to do?” scored 1 | 67 |
| 2 | Running commentary | Responses “often” or “sometimes” to MUPS item 21a, “Did the voice(s) ever take the form of a running commentary?” scored 1 | 55 |
| 3 | Repetitive themes and content | MUPS item 24b, “How repetitive were the voices with regard to the general theme or content of what the voices said?”, is scored on a 5-point Likert scale ranging from “Never the same voice” to “Extremely repetitive/ always the same voice.” Participants who rated four or five on this scale were scored 1 | 72 |
| 4 | Identical to memories | Participants who endorsed MUPS item 18c indicating that they believed “the voices seemed in some way to be ‘replays’ of memories of previous conversations you’ve had? Or have overheard?” and on MUPS item 18d indicated that these “replays” were identical to the original conversations were scored 1 | 12 |
| 5 | Similar to memories | Participants who endorsed MUPS item 18c indicating that they believed “the voices seemed in some way to be ‘replays’ of memories of previous conversations you’ve had? Or have overheard?” and on MUPS item 18d indicated that these “replays” were similar to the original conversations were scored 1 | 31 |
| 6 | Nonverbal auditory hallucinations | The response “yes” to MUPS item 26, “During your last illness episode did you ever hear sounds that you suspect others didn’t hear or report hearing, either together with the voices or at another time?” scored 1 | 32 |
| 7 | Voices which don’t make sense | “No” to MUPS item 13c, “Did the words spoken by the voice(s) make sense [ie, not gibberish]?” was scored 1 | 21 |
| 8 | First person | Positive response to MUPS item 16a-i was scored 1 | 28 |
| 9 | Second person | Positive response to MUPS item 16a-ii/-iii was scored 1 | 80 |
| 10 | Third person | Positive response to MUPS item 16a-iv was scored 1 | 65 |
| 11 | Voice does not address patient | Positive response to MUPS item 16a-v was scored 1 | 28 |
| 12 | Could be one’s own voice/thoughts | A “yes” response to MUPS item 23c, “Is it possible that it was actually your own voice and thoughts you heard?” was scored 1 | 34 |
| 13 | Constantly occurring | Participants who endorsed “constantly with you” to MUPS item 4b “During this last episode with the voices/sounds, how frequently were the voices/sounds with you?” were scored 1 | 48 |
Note: All responses not scored 1 were scored 0. MUPS, Mental Health Research Institute Unusual Perceptions Schedule.
Results
AH Phenomenology
Duration, Frequency, and Location.
Excluding the 28% of participants who reported an uncountable number of voices, a mean of 4.3 voices were heard (SD = 4.9, range 1–40). The number of voices heard during the last episode was constant for 48% of participants, but varied for 52%. AH location was approximately equally likely to be internal, external, or both during the latest episode although voices were most likely to be internal when they were first heard (table 2). Externally located voices were most commonly heard in both ears (75%). The most common duration of voices/sounds was hours (59%), with fewer participants reporting voice/sound durations of minutes (31%) or seconds (12%). The duration of these voices/sounds varied during the day for 59% of participants. Of the 58 participants who recalled the very first AH they had experienced, 48% had first heard a voice and 33% first heard a Nonverbal AH. The remainder either first heard both together (8%) or were not sure (11%).
Table 2.
Basic Properties of Auditory Verbal Hallucinations
| Property (MUPS question #, n) | Response option | % endorsed |
|---|---|---|
| Number of voices heard (Q9a, 196) | 1 | 18 |
| 2 | 12 | |
| 3 | 12 | |
| 4 | 11 | |
| 5+ | 19 | |
| Uncountable | 28 | |
| Frequency of heard voices/sounds (Q4b, 199) | Constantly with you | 48 |
| Often (11–20 times/week) | 26 | |
| Occasionally (once a day) | 16 | |
| Rarely (0–5 times/week) | 10 | |
| Voices had a pattern/or a rhythm (Q7a, 196) | Yes | 47 |
| No | 45 | |
| Location voice first heard in (Q8, 120) | Inside head (internal) | 47 |
| Outside head (external) | 38 | |
| Both | 15 | |
| Location of voice during most recent episode (Q8, 197) | Inside head (internal) | 34 |
| Outside head (external) | 28 | |
| Both | 38 | |
| Specific location of voices/sounds of those with only internal AHs (Q8a, 57) | Inside all over | 46 |
| Inside middle | 23 | |
| Specific location of voices/sounds of those with only external AHs (Q8b, 58) | Outside all around | 38 |
| Outside left | 29 | |
| Outside right | 22 | |
| Average loudness of predominant voice/sound (Q11a, 199) | Whisper or soft | 31 |
| Normal | 35 | |
| Loud | 25 | |
| Yelling/screaming | 4 | |
| Tone of predominant voice (Q12A, 197) | Angry | 52 |
| Authoritative | 58 | |
| Malicious/nasty | 52 | |
| Bossy | 45 | |
| Loving | 40 | |
| Gentle | 37 | |
| Kind | 39 | |
| Friendly | 40 | |
| Tone changed over time? (Q12B, 193) | Yes | 31 |
| No | 69 | |
| Did the words spoken by the voice(s) make sense (ie, not gibberish)? (Q13c, 195) | Yes | 77 |
| No | 21 | |
| Clarity of voice/sounds (Q13a, 199) | Very sharp/unusually clear | 24 |
| Clear | 46 | |
| Varies | 23 |
Note: Where total does not equal 100% this is due to “unsure” or “other” responses.
Loudness, Tone, Clarity, and Reality.
While voices most commonly spoke at a normal conversational volume, this could vary with many participants having soft/whispering or loud voices (table 2). For participants who heard more than 1 voice, 52% said that their different voices spoke at the same volume/loudness. Around 50% of participants had voices with a predominantly negative tone, eg, angry, nasty, and bossy and around 40% had positive voices, eg, loving, kind, and gentle (table 2). Voices typically made sense, ie, weren’t gibberish (table 2). The voices/sounds were typically clear or very clear (table 2), with their reality rated as “very real” by 85% of participants, with 11% saying they were “somewhat real” or “dream-like.”
Forms of Address.
The most common forms of address were the first and third person, with form of address being highly stable over time (table 3). Forty percent of participants reported that voices talked about themselves in relation to them (eg, “We are normal, you are mad”). The majority of participants either often (35%) or sometimes (32%) had heard voices that told them what to do (ie, command hallucinations), with only 25% never experiencing this. Of the participants who had experienced voices giving them commands, 76% said they were able to resist them. While most participants (53%) never heard voices just talking among themselves without referring to them, 17% experienced this often. The majority of participants (75%) who heard voices conversing also heard voices that directly addressed them. For those who heard more than 1 voice, each voice would typically address them separately (table 3), and although 53% never had their voices all speak at the same time (like a chorus), 25% sometimes had this experience, and 15% had it often.
Table 3.
Forms of Address, Identity, and Relationship With Auditory Verbal Hallucinations
| Property (MUPS question #, n) | Response option | % endorsed |
|---|---|---|
| In what “person” did the predominant voice(s) usually speak to you? (Q16a, 193) | First person (“I am bad”) | 28 |
| Second person (“You are bad”) | 73 | |
| Implied first person (“Time for you to sit”) | 36 | |
| Third person (“Steve is bad”) | 65 | |
| Not addressed (“The grass is green”) | 28 | |
| Form of address changed over time (Q16b, 187) | Yes | 15 |
| No | 85 | |
| Hear two (or more) voices commenting to each other (or one another) about you? (Q19a, 166) | Never | 33 |
| Rarely | 8 | |
| Sometimes | 29 | |
| Often | 30 | |
| Did the different voices address you separately? (Q20a, 161) | Never | 11 |
| Rarely | 4 | |
| Sometimes | 26 | |
| Often | 59 | |
| Did the voice(s) ever take the form of a running commentary (commenting on your behavior or thoughts)? (Q21a, 196) | Never | 38 |
| Rarely | 7 | |
| Sometimes | 25 | |
| Often | 30 | |
| Gender of the voice(s) (Q15, 183) | Male | 25 |
| Female | 5 | |
| Both | 63 | |
| Unsure | 7 | |
| Are the voices anonymous, ie, unknown to the person? (Q18a, 197) | Yes | 31 |
| No | 37 | |
| Mix of known & unknown | 32 | |
| Voices like people who’ve spoken to you in the past? (Q18b, 188) | Yes | 70 |
| No | 30 | |
| If the voice is unknown to you do you picture the appearance of this unknown person behind the voice? (Q30a, 150) | Never | 52 |
| Rarely | 4 | |
| Sometimes | 22 | |
| Often | 22 | |
| Do any of your voices have accents? (Q34a, 194) | Never | 74 |
| Rarely | 6 | |
| Sometimes | 11 | |
| Often | 9 | |
| Relationship between you and the voice(s)? (Q31b, 195) | Yes | 64 |
| No | 28 | |
| Has the relationship changed over time? (Q31c, 154) | Yes | 40 |
| No | 55 |
Note: Where total does not equal 100%, this is due to “unsure” or “other” responses.
Identity and Relationship.
It was most common for participants to hear both male and female voices (table 3), however a related-samples McNemar test indicated that significantly more participants heard male voices than heard female voices, P < .001. Voices were almost equally likely to be those of people known to the person, unknown, or a mix of these (table 3). Of the sample, 71% only heard adult’s voices, 2% only children’s voices, and 26% a mix of both. The majority of participants said their voices were similar to people who had spoken to them in the past, but the voices rarely had accents (table 3). The majority of participants (62%) said that the same voices returned, but 38% said that there were different voices from time to time. If the voices stopped, 39% of participants said they would miss them, and of these, 47% said they would miss them a lot or often.
Content.
While around 60% of participants endorsed each negative adjective relating to their voice content, around 40% endorsed each positive adjective (table 4). The content of voices did not change over time in the majority of participants (table 4). While the majority acknowledged it was possible that voices reflected thoughts they may have had, the majority were clear that the voices were not actually their own voice/thoughts (table 4). The majority of participants thought that the voice was linked to an influential figure in their life (table 4).
Table 4.
Content of Auditory Verbal Hallucinations
| Property (MUPS question #, n) | Response option | % endorsing |
|---|---|---|
| Best description of content of the voices you hear (Q22, 196) | Persecutory | 54 |
| Abusive/insulting | 61 | |
| Obscene | 41 | |
| Derogatory | 67 | |
| Accusatory | 49 | |
| Threatening | 63 | |
| Critical | 65 | |
| Helpful | 45 | |
| Guiding | 49 | |
| Affirming | 37 | |
| Inspiring | 40 | |
| Changeable | 44 | |
| Has the content changed over time? (Q22B, 193) | Yes | 27 |
| No | 73 | |
| Different voices say different or similar sorts of things? (Q20c, 157) | Different sorts of things | 54 |
| Same sorts of things | 46 | |
| Voices reflect thoughts you may have had? (Q23a, 197) | Yes | 50 |
| Unsure/maybe | 17 | |
| No | 33 | |
| Is it possible that it was actually your own voice and thoughts you heard? (Q23c, 196) | Yes | 34 |
| Unsure/maybe | 15 | |
| No | 51 | |
| Is it possible that the idea behind the message/content of the voices is linked or connected to someone who is or was influential in your life? (Q23b, 196) | Yes | 56 |
| Unsure/maybe | 11 | |
| No | 33 | |
| When you hear voices, is the mood of the voices like your mood at the time? (Q37a, 195) | Yes | 40 |
| No | 55 | |
| If yes, how often is the mood of the voices like your mood at the time? (Q37a, 79) | Rarely | 7 |
| Sometimes | 23 | |
| A lot or often | 71 |
Note: Where total does not equal 100%, this is due to “unsure” or “other” responses.
Memory and Repetitiveness.
A large proportion of participants believed that their voices were in some way replays of their previous experiences (table 5). Yet, the majority of those who believed this said that they were similar but not identical to these earlier conversations. In terms of the repetitiveness of voices, participants typically heard the same voice speaking on the same theme (table 5).
Table 5.
Memory and Repetitive Nature of Auditory Verbal Hallucinations
| Property (MUPS question #, n) | Response option | % endorsing |
|---|---|---|
| Memory | ||
| Did you believe the voices seemed in some way to be “replays” of memories of previous conversations you’ve had? Or have overheard? (Q18c, 196) | Yes | 39 |
| No | 54 | |
| Unsure | 7 | |
| If yes | ||
| How were these “memory replays” related to these previous conversations? (Q18d, 79) | Identical | 23 |
| Similar | 71 | |
| A mix of both | 6 | |
| To what extent do the “memory replays” make up the content of the voices? (Q18e, 83) | Not at all | 7 |
| Slightly | 33 | |
| Moderately | 46 | |
| Completely | 14 | |
| Repetitiveness | ||
| How repetitive were the voices with regard to | ||
| which voice it was? (Q24a, 196) | Never the same | 3 |
| Rarely the same | 8 | |
| Sometimes the same | 27 | |
| Mostly the same | 33 | |
| Always the same | 29 | |
| The general theme or content of what the voices said (Q24b, 195) | Never the same | 3 |
| Rarely the same | 7 | |
| Sometimes the same | 18 | |
| Mostly the same | 27 | |
| Always the same | 45 | |
| The general theme of the predominant voice (Q24c, 154) | Never the same | 3 |
| Rarely the same | 7 | |
| Sometimes the same | 11 | |
| Mostly the same | 24 | |
| Always the same | 55 | |
| If different voices present, do the “new” voices carry the same content/message as the “old” voices? (Q36b, 71) | Yes | 55 |
| No | 45 | |
| Did the voice(s) use the same words/phrases repeatedly (as if “stuck”)? (Q21b, 194) | Never | 44 |
| Rarely | 7 | |
| Sometimes | 22 | |
| Often | 27 | |
Nonverbal Auditory Hallucinations.
Thirty-two percent of participants reported Nonverbal AHs (table 1). Of these, 46% reported music, 43% ringing, 29% animal sounds, 27% clicks, 24% humming, 10% water, and 56% other nonverbal sounds. We found 20% reported their Nonverbal AHs occurred at a different time to voices, 19% at precisely the same time as voices, and 61% during the same episode in a general way. Fifty-three percent said their Nonverbal AHs worried them less than their voices, 18% said they were equally worrying, and 25% said they were more worrying.
Cluster Analysis
Clustering by Variable.
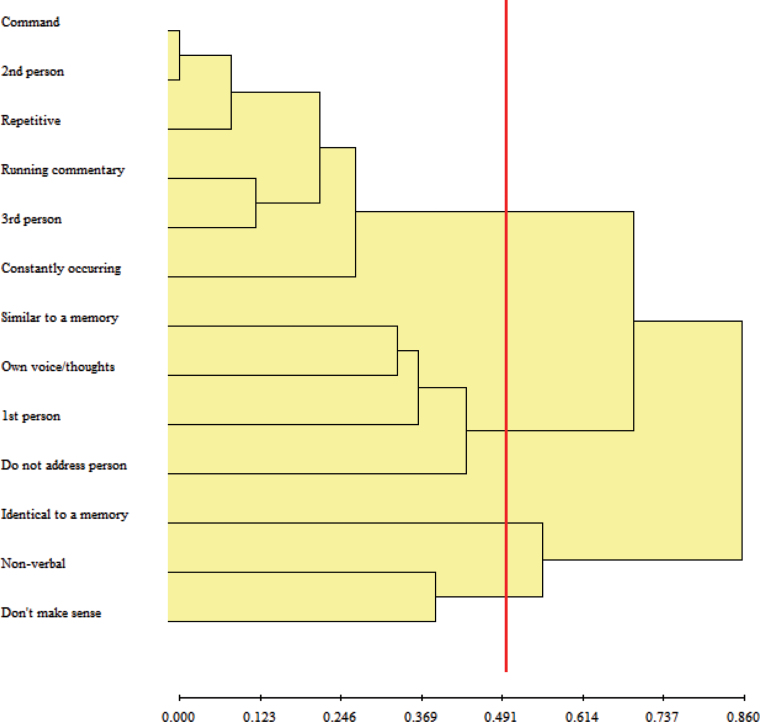
The proportion of participants endorsing the variables entered into the cluster analysis are shown in table 1. Missing data comprised 2.2% of the total data points. Both Mojena’s upper tail, t(11) = 2.67, P < .05, and inspection of the dendogram (figure 1) suggested the existence of 4 clusters. The first cluster (“Constant Commanding and Commenting AVHs”) consisted of the properties of repetitiveness, commands, first or third-person voices, and running commentaries, which were constantly with the person. The second cluster (“Own Thought AVHs”) consisted of the properties of not addressing the person, being first-person voices, being similar to a memory, and possibly being one’s own voice/thoughts. The third cluster (“Nonverbal AHs”) comprised the properties of not making sense or being Nonverbal AHs. The final cluster (“Replay AVHs”) consisted of the property of AVHs being identical to a memory of heard speech. The same 4-cluster structure was found when the analyses were repeated, including only people with diagnosis of schizophrenia (n = 161), t(11) = 3.00, P < .05.
Fig. 1.
Dendogram of cluster analysis.
Vertical line indicates level at which clustering was stopped, as per Mojena’s Upper Tail rule, resulting in 4 clusters.
For indicative purposes, participants were assigned positive status on an AH cluster if they endorsed ≥50% of the items constituting that cluster. By this criterion, Constant Commanding and Commenting AVHs were near universal being reported by 86% of participants, whereas Replay AVHs were relatively uncommon (12%). Own Thought AVHs were endorsed by 36%, and 42% endorsed Nonverbal AHs. While 5% of participants fell into none of these clusters, 36% fell into only 1 (with 92% of these being Constant Commanding and Commenting AVHs). Most participants were positive for multiple clusters: 38% for 2, 19% for 3, and 2% for all 4.
Discussion
This study performed the most comprehensive phenomenological analysis to date of AHs in people with predominantly schizophrenia-spectrum diagnoses. We quantified aspects of AH phenomenology not previously enquired about on a large scale, documented patterns of similarity and difference in AH phenomenology in our sample compared with Nayani and David’s5 findings, and found evidence for the existence of AH subtypes. We turn now to a consideration of the implications of these findings.
Phenomenological Findings and AH Subtypes
Our cluster analysis first identified a cluster that we termed “Constant Commanding and Commenting AVHs.” This included variables that were inherently likely to cluster together (ie, running commentaries and third-person voices; commands and second-person voices). Within this, the property of repetitiveness clustered with second-person commands. The existence of such an AVH supported Stephane and colleagues case study of such a subtype.12 Our descriptive statistics gave a finer grained analysis of the nature of AVH repetitiveness than has been previously reported. The majority of participants heard voices whose theme or content was mostly or always the same, and almost half said that their voices would sometimes/often use the same words/phrases repeatedly, as if “stuck.” Most heard the same voice, most or all of the time, saying the same things. New voices often continued the theme of the old voices. AVHs with this highly repetitive form and content offer some support for arguments that they may share common cognitive process with OCD.10
In addition to this cluster, representing the prototypical AVH associated with schizophrenia, we also found evidence for 3 less prominent AH subtypes. The first was a cluster made up of a single property, namely that of being identical to a memory, which we termed a “Replay AVH.” This was not tested for or examined in Stephane and colleagues’ original cluster analytic study.16 Our descriptive statistics showed that 12% of participants heard some voices that they identified as being identical to memories. It is possible that a subset of people diagnosed with schizophrenia have reexperiencing symptoms that are more commonly associated with posttraumatic stress disorder, potentially grounded in situationally accessible memories28 resulting from trauma.
Whereas only 12% of participants reported AVHs as like identical replays from memory, 31% reported AVHs as similar to memories. Given that memory tends to be “gist,” reconstructing the past rather being verbatim recall,29 this finding is to be expected. The property of being similar to a memory fell into a cluster we termed “Own Thought AVHs,” which included voice properties of not addressing the person, being spoken in the first person, and possibly being one’s own voice/thoughts. Such AVHs appear to share some commonalities with both inner speech and memory, suggesting the need for AVH models to attend more to how inner speech and memory process are related.1 Overall, the subjective experience of AVHs being identical/similar to memories is consistent with memory-based cognitive models of AVHs,10 as well as neuroimaging findings showing involvement of hippocampal/parahippocampal regions immediately prior to30 and during31 AVHs.
We also found one further AH subtype, which we termed “Nonverbal AHs.” This comprised the properties of being words that did not make sense, or being Nonverbal AHs. It seems plausible that the mechanism underpinning such AHs is spontaneous activity of the superior temporal gyrus25 and is distinct from the intra-/interhemispheric fronto-temporal connectivity model of AVHs,32,33 perhaps more suited to explaining Constant Commanding and Commenting AVHs.
Relation to Nayani and David’s Findings
In terms of commonalities between our findings and those of Nayani and David,5 we also found that participants were more likely to hear a male voice than a female voice; that the majority of participants experienced command AVHs, had voices which were constantly with them; that around 60% endorsed each negative adjective relating to their voice’s content; and that around 40% endorsed each positive adjective. In terms of differences, whereas Nayani and David found a mean of 3.2 voices per participant, we found a mean of 4.3 voices. Because our figure of 4.3 voices was likely to be an underestimate (being calculated after excluding the 28% of participants who said their number of voices was uncountable), this suggests Nayani and David’s figure may be an underestimate. Second, we found a lower percentage of voices being heard external to the head (28% vs Nayani and David’s 49%) and more being heard both internally and externally (38% vs Nayani and David’s 12%). Third, while Nayani and David reported a trend for AVHs becoming more likely to be internally located over time, in our sample, the most significant shift over time was from voices moving from first being heard either internally or externally to later being heard both internally and externally. Finally, whereas Nayani and David found 73% of voices usually spoke in normal conversational volume, we found that only 35% reported voices speaking at a normal volume, with similar numbers of participants saying that their voices were either louder (29%) or softer (31%) than normal conversational volume.
Other Novel Phenomenological Findings
While there is not space to discuss all the new AH data presented here, we note a number of further novel findings. Although the majority of participants indicated that their voices lasted for hours at a time, 59% indicated that the duration of their voices varied throughout the day. This interday variability remains to be clearly accounted for.34 We also reported data on the under-researched area of change in AVHs over time.17,35 Around 70% of participants reported the tone and content of their voices had not changed over time. Although an even higher percentage (85%) reported their voices’ form of address had not changed over time, relationships with voices were more changeable, highlighting the need for formal longitudinal research into how and why such relationships may change.
Theoretical Implications
The range of AH subtypes identified by this study suggest that existing neurocognitive models of AH may need amendment to allow that the output of different (although at some level, related) cognitive systems involved in the production of inner experience may result in the output of phenomenologically distinct AHs. The spectrum of our inner experiences, whether these be “classical” inner speech processes (associated with behavior control and evaluation, and fitting with Constant Commanding and Commenting AVHs), more general verbal mentation (fitting with Own Thought AVHs), verbal memory processes (fitting with Replay AVHs), or the output of processes creating nonverbal representations (fitting with Nonverbal AHs), all appear to have AH equivalents. In this sense, existing box-and-arrow neurocognitive models of AHs that converge on a single AH output box36 may need to be amended to show how inputs from distinct, different cognitive systems may lead to outputs that are phenomenologically discernible as distinct AH subtypes. Across patient groups, the preferential involvement of a particular cognitive system may account for transdiagnostic differences in AH phenomenology.9 Yet the fact that most participants endorsed multiple AH subtypes suggests there are extensive shared and related mechanisms between AH subtypes in schizophrenia. More generally, as Waters and colleagues36 suggest that deficits in intentional inhibition may play a role in allowing the output of such systems to enter awareness, models of AHs are hence likely to benefit from a greater consideration of exactly how this range of internally generated information (ie, such as that produced by the default network) comes to enter consciousness and the barriers that act as inhibitory. The integration of existing neurocognitive models with leading contemporary models of consciousness, such as Baars’ Global Workspace Theory,37 may hence be fruitful.
As our cluster analysis suggests but does not confirm the existence of AH subtypes, useful next steps might include examining if the neural correlates of these subtypes differ (using fMRI symptom capture studies), and whether these AH subtypes emerge from qualitative analyses of AH phenomenology.
Therapeutic Implications
The existence of AH subtypes suggests that therapeutic interventions may benefit from some shaping according to the subtype that dominates the clinical presentation. For example, the high prevalence of repetitive AVHs suggests that formal trials of antiobsessional medication may be worthwhile12 and that cognitive behavioral techniques (CBT) employed with OCD patients may also be beneficial.38 People presenting prominent AVHs that they identify as being identical to a memory may be helped by techniques more traditionally associated with posttraumatic stress disorder, such as trauma-based CBT or eye movement desensitization and reprocessing. However, such a tailored strategy is likely to be problematic practically due to the high number of participants with multiple AH subtypes. Treating AH subtypes using individually tailored approaches may often not be clinically feasible.
Our finding that the majority of participants (64%) had some relationship with their voices, and almost half had seen a change in this relationship, suggests that forms of psychotherapy that attempt to change the relationship between the person and their voices, 38 or aid disengagement from unhelpful patterns of responding to voices39 may be beneficial for many (but perhaps not all) voice hearers. It was also notable that half of the participants believed that their voices may have reflected thoughts they themselves had, and over half believed that the message/content of the voice was linked to someone who is (or was) influential in their lives. This suggests that examining links between the identity and content of the voice, and the life of the voice hearer may be informative.39
Limitations
First, because the majority of people in this study had diagnoses of schizophrenia, it is unclear the extent to which our finding have transdiagnostic validity.9 Second, because our study was limited to only entering specific variables into the cluster analysis that were assessed by the MUPS,13 further research is needed using a tailored phenomenological interview to thoroughly assess all AH subtypes proposed by contemporary research1 (eg, with questions addressing properties that characterize hypervigilance AHs, such as locus of attention and level of environmental noise during AH)15. Third, because we converted some continuous variables into binary variables, this will have caused some loss in power. However, given the large sample size, this effect is likely to be negligible. Fourth, our study was also open to reporting biases, as some participants reported about AH experiences that were no longer current. Finally, our theory-driven approach to variable selection for our cluster analysis may have driven the results toward existing models of AHs. Use of other subsets of variables might well produce other or additional clusterings.
Conclusion
This article has reported new data on AH phenomenology and evidence for AH subtypes, which contemporary AH models need to account for. However, the co-occurrence of multiple AH subtypes in many participants makes it less clear how we may tailor specific interventions to AH subtypes in practice.
Funding
This study was made possible by a grant from the National Health and Medical Research Council (923301), and a Macquarie University Research Fellowship awarded to Dr S.M.-J.
Acknowledgments
We thank the participants, the NHMRC for funding, Dorothy Carter and Rosemary Thomas who were also involved in conducting the interviews, and Summer Schrader for bringing Hoffman and Hampson’s paper27 to our attention. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. McCarthy-Jones S. Hearing Voices: The Histories, Meanings and Phenomenologies of Auditory Verbal Hallucinations. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 2. Shergill SS, Murray RM, McGuire PK. Auditory hallucinations: a review of psychological treatments. Schizophr Res. 1998; 32: 137–150 [DOI] [PubMed] [Google Scholar]
- 3. McCarthy-Jones S. Taking back the brain: could neurofeedback training be effective for relieving distressing auditory verbal hallucinations in patients with schizophrenia? Schizophr Bull. 2012; 38: 678–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laroi F. The phenomenological diversity of hallucinations: some theorertical and clinical implications. Psychologia Belgica. 2006; 46: 163–183 [Google Scholar]
- 5. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996; 26: 177–189 [DOI] [PubMed] [Google Scholar]
- 6. Leudar I, Thomas P, McNally D, Glinski A. What voices can do with words: pragmatics of verbal hallucinations. Psychol Med. 1997; 27: 885–898 [DOI] [PubMed] [Google Scholar]
- 7. Hoffman RE, Varanko M, Gilmore J, Mishara AL. Experiential features used by patients with schizophrenia to differentiate ‘voices’ from ordinary verbal thought. Psychol Med. 2008; 38: 1167–1176 [DOI] [PubMed] [Google Scholar]
- 8. Moritz S, Larøi F. Differences and similarities in the sensory and cognitive signatures of voice-hearing, intrusions and thoughts. Schizophr Res. 2008; 102: 96–107 [DOI] [PubMed] [Google Scholar]
- 9. Larøi FSI, Blom JD, Fernyhough C, et al. The characteristic features of auditory verbal hallucinations in clinical and nonclinical groups: state-of-the-art overview and future directions. Schizophr Bull. 2012; 38: 724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badcock JC, Waters FA, Maybery MT, Michie PT. Auditory hallucinations: failure to inhibit irrelevant memories. Cogn Neuropsychiatry. 2005; 10: 125–136 [DOI] [PubMed] [Google Scholar]
- 11. Badcock JC, Waters FA, Maybery M. On keeping (intrusive) thoughts to one’s self: testing a cognitive model of auditory hallucinations. Cogn Neuropsychiatry. 2007; 12: 78–89 [DOI] [PubMed] [Google Scholar]
- 12. Stephane M, Polis I, Barton SN. A subtype of auditory verbal hallucinations responds to fluvoxamine. J Neuropsychiatry Clin Neurosci. 2001; 13: 425–427 [DOI] [PubMed] [Google Scholar]
- 13. Carter DM, Mackinnon A, Howard S, Zeegers T, Copolov DL. The development and reliability of the Mental Health Research Institute Unusual Perceptions Schedule (MUPS): an instrument to record auditory hallucinatory experience. Schizophr Res. 1995; 16: 157–165 [DOI] [PubMed] [Google Scholar]
- 14. Badcock JC. The cognitive neuropsychology of auditory hallucinations: a parallel auditory pathways framework. Schizophr Bull. 2010; 36: 576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodgson G, Gordon S. Avoiding false negatives: are some auditory hallucinations an evolved design flaw? Behav Cogn Psychother. 2009; 37: 325–334 [DOI] [PubMed] [Google Scholar]
- 16. Stephane M, Thuras P, Nasrallah H, Georgopoulos AP. The internal structure of the phenomenology of auditory verbal hallucinations. Schizophr Res. 2003; 61: 185–193 [DOI] [PubMed] [Google Scholar]
- 17. Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull. 2010; 36: 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Copolov D, Trauer T, Mackinnon A. On the non-significance of internal versus external auditory hallucinations. Schizophr Res. 2004; 69: 1–6 [DOI] [PubMed] [Google Scholar]
- 19. Copolov DL, Mackinnon A, Trauer T. Correlates of the affective impact of auditory hallucinations in psychotic disorders. Schizophr Bull. 2004; 30: 163–171 [DOI] [PubMed] [Google Scholar]
- 20. Spitzer RL, Williams J, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R. Washington, DC: American Psychaitric Press; 1990. [Google Scholar]
- 21. Wishart D. ClustanGraphics. [computer program]. Version. Edinburgh, UK: Clustan Limited; 2006. [Google Scholar]
- 22. Milligan GW. A review of Monte Carlo tests of cluster analysis. Multivariate Behavioural Research. 1981; 16: 379–407 [DOI] [PubMed] [Google Scholar]
- 23. Finch H. Comparison of distance measures in cluster analysis with dichotomous data. Journal of Data Science. 2005; 3: 85–100 [Google Scholar]
- 24. Mojena R. Hierarchical grouping methods and stopping rules: an evaluation. Computer Journal. 1977; 20: 359–363 [Google Scholar]
- 25. Nazimek JM, Hunter MD, Woodruff PW. Auditory hallucinations: expectation-perception model. Med Hypotheses. 2012; 78: 802–810 [DOI] [PubMed] [Google Scholar]
- 26. Penfield W, Perot P. The brain’s record of auditory and visual experience. A final summary and discussion. Brain. 1963; 86: 595–696 [DOI] [PubMed] [Google Scholar]
- 27. Hoffman RE, Hampson M. Functional connectivity studies of patients with auditory verbal hallucinations. Front Hum Neurosci. 2012; 6:6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brewin CR, Holmes EA. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev. 2003; 23: 339–376 [DOI] [PubMed] [Google Scholar]
- 29. Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond, B, Biol Sci. 2007; 362: 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diederen KMJ, Neggers SFW, Daalman K, et al. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry. 2010; 167: 427–435 [DOI] [PubMed] [Google Scholar]
- 31. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011; 168: 73–81 [DOI] [PubMed] [Google Scholar]
- 32. Mulert C, Kirsch V, Whitford TJ, et al. Hearing voices: a role of interhemispheric auditory connectivity? World J Biol Psychiatry. 2012; 13: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whitford TJ, Mathalon DH, Shenton ME, et al. Electro physiological and diffusion tensor imaging evidence of delayed corollary discharges in patients with schizophrenia. Psychol Med. 2011; 41: 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varese F, Udachina A, Myin-Germeys I, Oorschot M, Bentall RP. The relationship between dissociation and auditory verbal hallucinations in the flow of daily life of patients with psychosis. Psychosis. 2011; 3: 14–28 [Google Scholar]
- 35. Milligan D, McCarthy-Jones S, Winthrop A, Dudley R. Time changes everything? A qualitative investigation of the experience of auditory verbal hallucinations over time. Psychosis. 2012. 10.1080/17522439.2012.667438. [Google Scholar]
- 36. Waters F, Allen P, Aleman A, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012. 38;683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baars BJ. A Cognitive Theory of Consciousness. Cambridge, MA: Cambridge University Press; 1988. [Google Scholar]
- 38. Whittal ML, McLean PD. CBT for OCD: the rationale, protocol, and challenges. Cogn Behav Prac. 1999; 6: 383–396 [Google Scholar]
- 39. Longden E, Corstens D, Escher S, Romme M. Voice hearing in a biographical context: a model for formulating the relationship between voices and life history. Psychosis. 2012; 4: 224–234 [Google Scholar]