Abstract
Background: An inverse relationship between risk of schizophrenia and premorbid IQ is a robust empirical finding. Cognitive impairment may be a core feature of schizophrenia in addition to the clinical symptoms that have historically defined the disorder. Aims: To evaluate whether risk of schizophrenia increases linearly or nonlinearly with the lowering of premorbid IQ after adjustment for a range of confounding factors. Methods: IQ data from the 1958 National Child Development Study, a prospective national birth cohort (n = 17 419), were linked with psychiatric admissions in England and Wales over a 20-year period. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnoses were derived from case notes. Results: A clear nonlinear inverse relationship between general intelligence at ages 7 and 11 and risk of adult psychosis was found even after adjustment for potential social, behavioral, or demographic confounding factors. No such relationship was found for affective disorders. Conclusions: The nonlinear relationship suggests an excess risk of schizophrenia in children with premorbid IQ in the learning disabilities range. Previous reports of a linear relationship are likely to be a result of less sensitive statistical methods for detecting nonlinearity.
Key words: risk of schizophrenia, premorbid IQ, birth cohort, epidemiology
Introduction
Firm evidence for some impairment in cognitive functioning in children and adolescents who later develop schizophrenia has accumulated over the past three decades.1–3 According to a recent meta-analysis,4 premorbid adolescents who later develop schizophrenia score on average 5.3 IQ points lower than matched healthy individuals. Other studies have reported markedly elevated rates of intellectual disability among those with a diagnosis of schizophrenia5 (ie, dual diagnosis). An association between low premorbid IQ and the risk for developing a psychiatric disorder is not confined to schizophrenia,6 but it is significantly less marked in nonpsychotic, primarily affective disorders.2,7,8
Cognitive impairments are found in all forms of psychosis and extend to all domains of cognition, suggesting a general impairment such as that measured by general intelligence.9,10 There does not appear to be any qualitative difference in type of impairment across the different forms of psychosis, but in schizophrenia it is significantly more severe.10 Accumulating evidence suggests that cognitive impairment is a dimension of psychosis, separate from clinical symptoms. It remains stable, varying little with symptom change during recovery from schizophrenia. It also influences the transition from attenuated symptoms to the clinical form of psychosis,9,11 and has been nominated as a distinct target for developing new therapeutics for schizophrenia.12 Recent works using causal pathway models have shown that cognitive ability influences real-world functioning in psychosis via a direct or primary route as well as indirectly by enhancing various competency skills.13
Understanding the causal path between premorbid cognitive function and the development of schizophrenia is less well developed but of great theoretical importance. In the past decade, a number of cohort studies have reported evidence for an increase in the risk of schizophrenia with decreasing premorbid IQ scores (for a review, see14). Most studies concluded that the relationship was linear in nature extending across the whole range of IQ scores.8,15–19 One explanation for this linear relationship is the cognitive reserve hypothesis,20 in which either IQ influences the development of symptoms per se or the degree of personal control over emerging psychotic symptoms. However, two recent studies reported evidence for a significant nonlinear relationship suggesting an elevated risk for schizophrenia only for the lower range of IQ with no apparent benefit of a reduced risk for higher levels in IQ.21,22 Although a recent meta-analysis23 of cohort studies concluded in favor of a linear dose-response relationship between IQ and risk, it also reported marked heterogeneity between the studies in respect of a nonlinear risk function. Most, but not all, of the studies considered were based on conscript, as opposed to birth cohorts in which there is a risk of excluding participants with marked learning or physical disabilities because they will not be conscripted24 and their IQ is typically assessed in late adolescence.
We investigated in this article the predictive relationship between general intelligence in childhood and the risk of a later diagnosis of schizophrenia or nonpsychotic affective disorder using data from the National Child Development Study (NCDS), a large-scale population-based birth cohort25 with IQ measured in childhood at ages 7 and 11. Our major aim was to carefully investigate this risk relationship for any evidence of nonlinearity, which would suggest a differential impact of intelligence on the underlying psychopathological processes. We first explored how the risk of psychosis or affective disorders was distributed across the whole range of IQ and used nonparametric regression to reveal the shape of the underlying risk function. We then employed logistic regression to test for nonlinearity of the risk function and finally compared the risk of schizophrenia or affective disorder between IQ groups after adjustment for social, behavioral, or demographic confounding factors.
Methods
Study Population and Participants
Participants are members of the NCDS, an ongoing longitudinal birth cohort study following up children from the British Perinatal Mortality Survey, which included about 98% (N = 17 419) of all registered births in England, Scotland, and Wales during March 3–9, 1958.25 Participants’ physical, educational, and social development, as well as their health, was assessed at eight time points, the most recent being in 2004–2005. In this study, we utilize data from the first two sweeps, when participants were 7 (N = 15 439) and 11 years old (N = 15 145).
Obstetric Complications, Socioeconomic Status, Family Characteristics, and Behavior Difficulties
Data were obtained at ages 7 and 11 using questionnaires completed by parents, school doctors, or teachers. A weighted index of obstetric complications26 was derived from the British Perinatal Mortality Survey. Multiple births were recorded separately. The socioeconomic status of the family was derived from the occupation of the father or male head according to the General Registrar’s Office Classification of Occupations (1960). Parents’ educational level was recorded as the age at which they left full-time education. An index of family distress was calculated as a sumscore (24 items) involving specific family difficulties (eg, marriage and housing problems, mental illness, alcoholism, unemployment). The parent-child relationship was coded in terms of three groups, children living with both biological parents, with one biological parent only (mostly the mother), or with neither biological parent. Children’s behavior problems at ages 7 and 11 were measured by a total score of the Bristol Social Adjustment Guide, a standardized psychometric test of social maladjustment derived from 150 descriptions of behavior in school, and completed by the teacher.27
Assessments of Cognitive Performance
Cognitive Tests.
At age 7, the Southgate Reading Test,28 a 30-item test of word recognition, as well as the 10-item Problem Arithmetic Test29 were administered by teachers in class. At age 11, a 35-item reading comprehension test and a 40-item arithmetic test were administered by the class teacher of the child, both specifically constructed for use in the NCDS.
Teacher Assessment of Children’s Academic and Creative Abilities.
School attendance at age 7 was rated by the class teacher as a percentage of total days attended. Teachers also rated children’s academic abilities at both ages 7 and 11 on a 5-point scale in relation to reading, number working, oral expression, awareness of the world, and creativity. These ratings were substantially intercorrelated, and a principal component analysis (PCA) revealed only one general factor at both ages explaining 62% and 70% of the total variance, respectively, enabling data reduction into a highly reliable single total score (Cronbach’s alpha = .90 at both ages).
Overall Cognitive Performance at Ages 7 and 11.
The cognitive test scores for reading and arithmetic (see above) were substantially correlated among themselves and also with the teachers’ total score of children’s academic and creative abilities (minimum r = .53). As has been done before,30 a PCA of these three scales was performed to obtain component scores as an overall measurement for cognitive performance. A strong general factor emerged at ages 7 (73% of total variance) and 11 (84% of total variance), with all component loadings ≥0.80 suggesting high convergent validity toward general intelligence. Component scores were transformed to a scale with a mean of 100 (SD = 15) in the total sample. The reliabilities of the two component scales were high (age 7: Cronbach’s alpha = .82; age 11: Cronbach’s alpha = .90). The component scores at age 11 were highly correlated (r = .84) with a test of general intelligence—the General Ability Test31 administered in this sweep only, implicating very good concurrent validity.
Psychiatric Status After Age 16.
After obtaining relevant approval from regional ethics committees, a systematic search of computerized records held by Regional Health Authorities in England, Wales, and Scotland took place to identify individuals, with dates of birth indicating they were potential cohort members, who had been admitted to psychiatric hospitals between 1974–1994 when cohort members were between ages 16 and 36.32 Although such a data linkage approach can miss out less severe cases of mental disorder, which might be treated in primary care or not seek help at all, it is unlikely to miss cases of schizophrenia, as only a small proportion of such patients did not have contact with hospitals in this period before the rise of Early Intervention in Psychosis and other community teams in the UK.33 Casenote diagnoses were made independently by two psychiatrists using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, followed by a consensus exercise with a third psychiatrist to derive a single agreed diagnosis when there was disagreement. For the current study, we selected only those who met DSM-IV criteria for schizophrenia or affective disorder (with or without psychotic features).
Statistical Analyses
Having identified the NCDS cases that met DSM-IV criteria for a psychiatric disorder, we first generated bivariate scatterplots to explore the conditional distribution of risk for schizophrenia and affective disorders depending on general intelligence scores at ages 7 and 11. For these scatterplot analyses, the individual IQ scores (minimum 53, maximum 139 at age 7) were recoded into discrete units equivalent to five IQ points, and the risk of schizophrenia or affective disorders was then calculated for each unit (ie, cases as nominator, remainder of the cohort as denominator). Nonparametric regression technique (ie,. LOWESS smoothing algorithm)34 was employed to reveal the best fitting underlying bivariate relationship, which was decided on the basis of its smoothness, the bandwidth parameter and the AIC (Akaike’s Information Criterion) as an index for accomplishment.35
Logistic regression analyses were then performed to test for nonlinearity of the predicted risk function (on a logit scale) for a diagnosis of schizophrenia or affective disorder depending on IQ at ages 7 or 11. In the first step of these analyses, IQ was entered as a continuous linear predictor; in the second step, a Box-Tidwell transformed predictor was added to the model to test for unspecified nonlinearity,36 and we then examined the fit of a quadratic polynomial logistic regression model. Thereafter, we used logistic regression to obtained risk estimates for 4 SD IQ groups represented as dummy coded predictors (<−2 SD, <−1 SD, ±1 SD, +1 SD) and tested those for differences in their ORs. Demographic and other potential confounders that had a significant association (P < .01) with schizophrenia or affective disorders on their own were used to adjust the ORs for the IQ groups.
Results
Our national screening of case notes delivered 65 cases of DSM-IV schizophrenia. Some of these were misclassified by date of birth or they were immigrants not included in the NCDS. Thus, 54 were identified as NCDS cases and 49 with data for the study (see table 1). The best estimate of national cumulative incidence from the England and Wales population by age 36, given our sampling procedure, should be based on 65 cases in the nominator and hence an estimate of 65/15 439 = 0.0042 (According to Pogue-Guile,37 only 71% of the lifetime risk has accumulated by age 36.), which extrapolates to an estimated lifetime morbid risk of 6 per 1000. This compares favorably to the median population risk estimate of 7.2 per 1000.38
Table 1.
Risk Ratio Estimates (OR) for Schizophrenia and Affective Disorders Depending on IQ at Ages 7 and 11
| IQ Groups (Group Mean) | N | Schizophrenia | Affective Disorders | ||||
|---|---|---|---|---|---|---|---|
| Risk (Number of Cases) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Risk (Number of Cases) | Crude OR (95% CI) | Adjusted ORb (95% CI) | ||
| Age 7 years | 15 061 | 49 | N = 14 167 | N = 14 167 | |||
| <−2 SD<70 (63.5) | 509 | 0.0157 (8) | 8.40 (3.66–19.28) | 7.89 (3.00–20.72) | 0.0079 (4) | 1.93 (0.69–5.40) | 1.23 (0.36–4.26) |
| <−1 SD70−84 (78.2) | 1969 | 0.0081 (16) | 4.31 (2.21–8.39) | 2.72 (1.18–6.25) | 0.0066 (13) | 1.62 (0.86–3.02) | 1.12 (0.54–2.34) |
| <±1 SD85–115 (101.0) | 10 017 | 0.0019 (19) | 1.00 | 1.00 | 0.0041 (41) | 1.00 | 1.00 |
| >+1 SD>115 (119.9) | 2566 | 0.0023 (6) | 1.23 (0.49–3.09) | 1.18 (0.45–3.10) | 0.0016 (4) | 0.38 (0.14–1.06) | 0.34 (0.10–1.09) |
| Age 11 years | 14 1246 | 40 | N = 13 880 | N = 13 880 | |||
| <−2 SD<70 (65.8) | 206 | 0.0194 (4) | 10.69 (3.57–32.05) | 9.01 (2.75–29.50) | 0.0097 (2) | 2.43 (0.58–10.13) | 1.64 (0.38–7.14) |
| <−1 SD70−84 (79.1) | 2121 | 0.0056 (12) | 3.07 (1.47–6.44) | 2.51 (1.10–5.75) | 0.0061 (13) | 1.53 (0.81–2.882) | 1.16 (0.59–2.28) |
| <±1 SD85–115 (99.1) | 9193 | 0.0018 (17) | 1.00 | 1.00 | 0.0040 (37) | 1.00 | 1.00 |
| >+1 SD>115 (121.7) | 2726 | 0.0025 (7) | 1.39 (0.58–3.35) | 1.27 (0.49–3.29) | 0.0022 (6) | 0.55 (0.23–1.30) | 0.69 (0.29–1.66) |
Note: BSAG, Bristol Social Adjustment Guide.
aConfounders: father’s years in education; multiple birth; BSAG total score at age 7; relation with natural parents at age 7.
bConfounders: multiple birth; BSAG total score at age 7; relation with natural parents at age 7.
cConfounders: father’s years in education; BSAG total score at age 11; relation with natural parents at age 11.
dConfounders: multiple birth; BSAG total score at age 11; relation with natural parents at age 11.
In addition, we identified 65 cases with a DSM-IV diagnosis of any affective disorder with/without psychotic symptoms, 22 with stress disorder, 7 with anxiety disorder, and 51 received any other DSM-IV diagnosis. The attrition rates from 7 to 11 years were 22% (12 participants) in the schizophrenia group, 9% (6 participants) in the affective disorder group, and 8% (1388 participants) in the remainder of the cohort. Attrition was significantly greater for the schizophrenia group (χ2(2 df) = 13.2, exact-P = .003). IQ scores for each group together with demographic and potential confounder variable information are presented in table 2 and significant group differences are indicated.
Table 2.
Descriptive Statistics for IQ Performance, Sociodemographic Status, Birth Condition, and Family Risk Factors
| Controls | Schizophrenia | Affective Disorders | ||||
|---|---|---|---|---|---|---|
| N | Mean (SD), % (n) | N | Mean (SD), % (n) | N | Mean (SD), % (n) | |
| Child variables | ||||||
| Cognition age 7 | 14 950 | 100.1 (15.0) | 49 | 89.1 (19.6)* | 62 | 95.9 (14.9)* |
| Cognition age 11 | 14 148 | 100.0 (15.0) | 40 | 92.1 (19.1)* | 58 | 94.9 (13.7)* |
| Gender (females) | 16 432 | 48.4% (7959) | 54 | 55.6% (30) | 65 | 60% (39) |
| School attendance age 7 | ||||||
| Below 71% attendance | 14 716 | 3.2% (477) | 49 | 6.0% (3) | 61 | 8.1% (5) |
| 71%–90% attendance | 14 716 | 30.6% (4503) | 49 | 34.6% (17) | 61 | 31.2% (19) |
| >90% attendance | 14 716 | 66.2% (9736) | 49 | 59.2% (29) | 61 | 60.7% (37) |
| BSAG age 7 | 14 821 | 8.8 (8.9) | 49 | 14.9 (11.0)** | 62 | 11.5 (9.7)*** |
| BSAG age 11 | 14 063 | 8.5 (8.10) | 40 | 14.2 (13.1)** | 57 | 12.5 (12.0)** |
| Social and economic status | ||||||
| SES (I) professional | 15 871 | 5.2% (825) | 44 | 6.8% (3) | 62 | 3.2% (2) |
| SES (II) managerial | 15 871 | 14.5% (2297) | 44 | 13.6% (6) | 62 | 22.6% (14) |
| SES (III) skilled | 15 871 | 53.1% (8425) | 44 | 54.6% (24) | 62 | 41.9% (26) |
| SES (IV) semiskilled | 15 871 | 17.4% (2761) | 44 | 11.4% (5) | 62 | 17.7% (11) |
| SES (V) unskilled | 15 871 | 6.5% (1031) | 44 | 2.3% (1) | 62 | 6.5% (4) |
| Number of male head of family | 15 871 | 3.4% (532) | 44 | 11.4% (5) | 62 | 8.1% (5) |
| Father left FT education at age 16 or younger | 10 711 | 88.3% (9458) | 22 | 63.6% (14)** | 41 | 85.4% (35) |
| Mother left FT education at age 16 or younger | 11 016 | 89.9% (9902) | 24 | 83.3% (20) | 46 | 91.3% (42) |
| Birth variables | ||||||
| OCs | 16 431 | 0.4 (0.6) | 54 | 0.5 (0.7) | 65 | 0.5 (0.8) |
| Multiple births | 16 432 | 2.2% (366) | 54 | 7.4% (4)* | 65 | 6.2% (4)*** |
| Family variables | ||||||
| Family adversity | 14 278 | 0.6 (1.4) | 44 | 1.4 (2.5)** | 58 | 0.8 (1.5) |
| Children living with both parents | 14 562 | 92.3% (13 441) | 44 | 77.3% (34)** | 58 | 81.0% (47)* |
| Children living with one parent | 14 562 | 5.5% (804) | 44 | 11.4% (5) | 58 | 12.1% (7) |
| Children living without parents | 14 562 | 2.2% (317) | 44 | 6.9% (4) | 58 | 6.9% (4) |
Note: Abbreviation is explained in the first footnote to table 1. SES, socioeconomic status; FT, full-time education; OCs, obstetric complications.
Significance of the comparison with controls: *P < .01, **P < .001, ***P < .05.
The average IQ of children later diagnosed with either schizophrenia or with affective disorder was significantly lower than the remainder of the cohort at age 7 (see table 2). In the case of the schizophrenia group, these mean differences amounted to medium effect sizes at ages 7 and 11 (d = 0.64; 95% CI: 0.34−0.91 and d = 0.46; 95% CI: 0.15−0.77, respectively). For affective disorder, modest effect sizes are noted at ages 7 and 11 (d = 0.27; 95% CI: 0.03−0.52 and d = 0.36; 95% CI: 0.10−0.62, respectively).
Exploring the Risk Functions for Schizophrenia and Affective Disorders Depending on IQ Scores at Ages 7 and 11
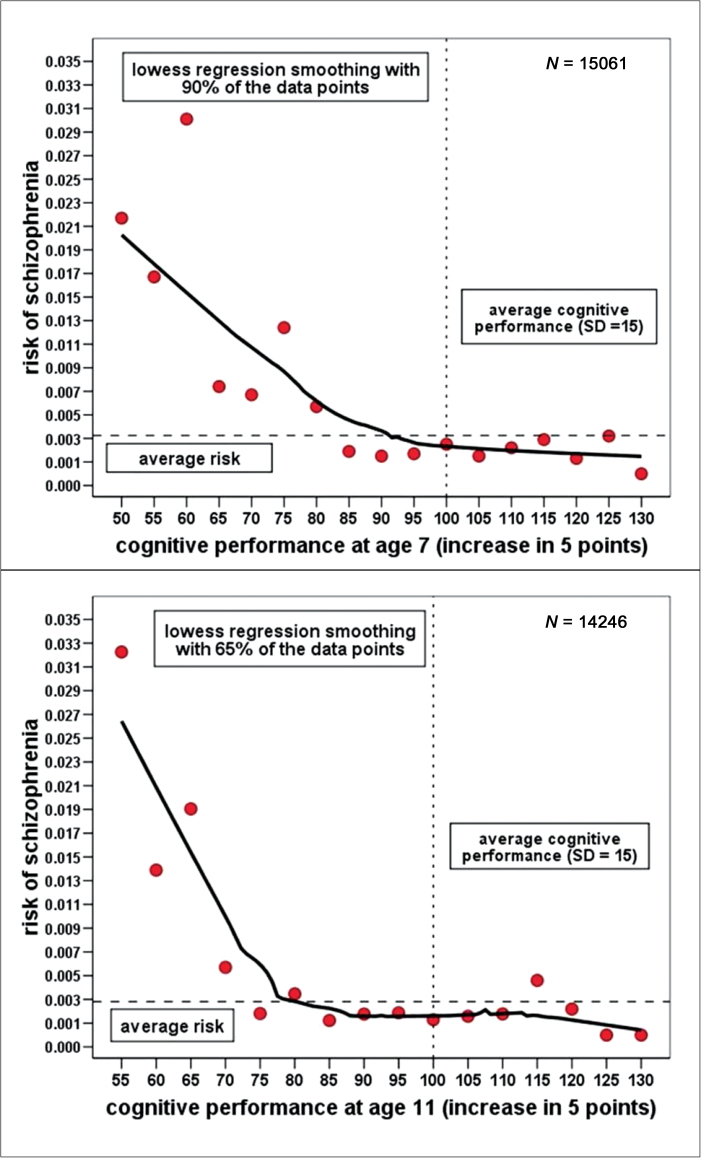
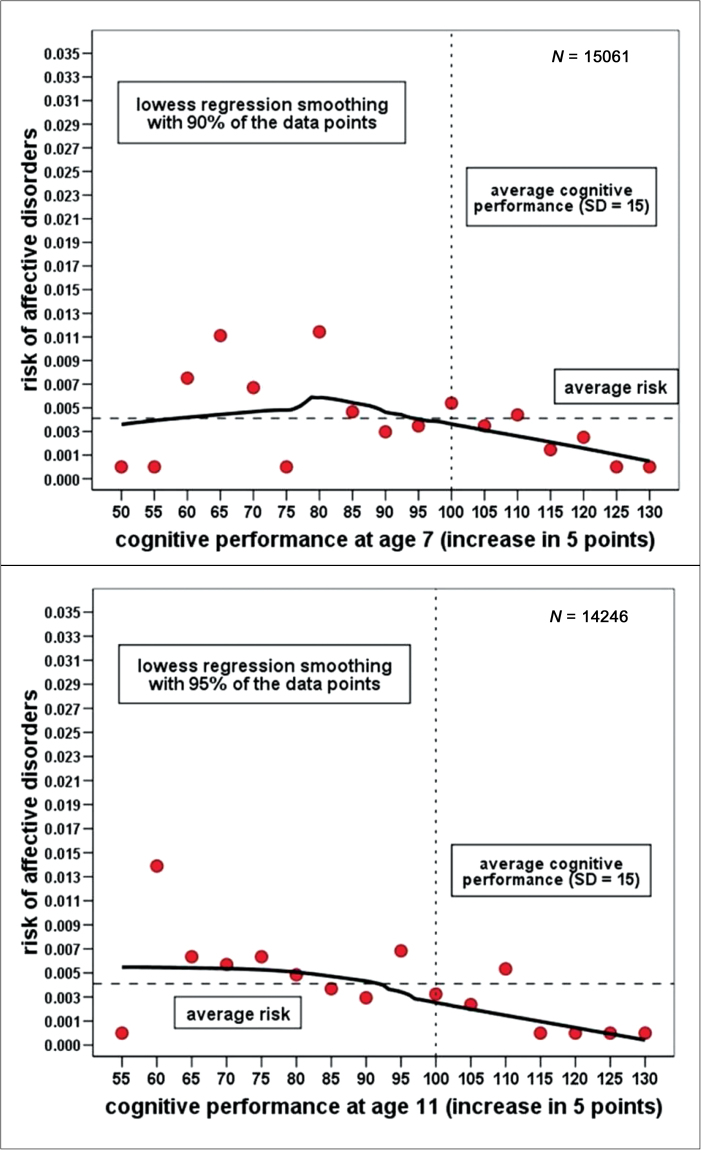
Figure 1 displays the results of smoothing scatterplots for the risk (ie, the conditional probability) of schizophrenia depending on IQ (grouped into units of five points) at ages 7 and 11. Both scatterplots revealed similar nonlinear relationships resembling an exponential decline curve suggesting an elevated risk only at the lower end of the IQ range. At ages 7 and 11, the highest risk of schizophrenia was found in the group of children scoring 2 SD (<70) below the mean (100 points). From this peak, the risk function is falling with increasing IQ up to around 85 points at age 7 and 75 points at age 11, from where on it is levelling off slightly below the average risk. In stark contrast, the smoothing scatterplots for affective disorder at ages 7 and 11 (see figure 2) did not reveal any systematic relation between IQ and the probability of a diagnosis; both risk functions appear almost flat across the whole IQ range.
Fig. 1.
Smoothing scatterplots of the risk of schizophrenia depending on IQ at ages 7 and 11 (LOWESS smoothing).
Fig. 2.
Smoothing scatterplot of the risk of affective disorders depending on IQ at ages 7 and 11 (LOWESS smoothing).
Nonlinearity in the relationship between cognitive performance and the logits for schizophrenia was confirmed by a logistic regression model at age 7 (LRT (Likelihood Ratio Test), χ2(2 df) = 27.7, P < .001, Nagelkerke-R2 = .043) and age 11 (LRT, χ2(2 df) = 21.6, P < .001, Nagelkerke-R2 = .040) that included both the individual IQ scores as a linear predictor (P < .05) and a Box-Tidwell transformed predictor (P < .05). Subsequent analysis of the shape of this relationship using a second-order polynomial logistic regression model revealed that it followed very closely a quadratic curve with both the linear and quadratic predictor making significant (P < .05) contributions to the fit of the model. The same analysis was repeated for affective disorders, but no statistical evidence for any nonlinearity was found; only a very weak negative linear relationship emerged between IQ scores and the risk of affective disorders.
Risk Estimates for IQ Groups
In addition to modeling, the risk function for schizophrenia and affective disorders using IQ as a continuous predictor, we also conducted logistic regression analyses using IQ as a categorical predictor (ie, 4 SD groups) and the results are presented in table 1. Highly significant differences in the ORs for schizophrenia between the 4 SD groups were revealed at the age of 7 (LRT, χ2(3 df) = 15.9, P = .001) and similarly at the age of 11 (LRT, χ2(3 df) = 11.4, P = .01), even when adjusted for several confounders. At age 7, a trend in the ORs emerged such that a significantly higher risk for schizophrenia was found for children with IQ scores lower than 1 SD in comparison with the normal range of IQ (adjusted OR: 2.72; 95% CI: 1.18–6.25) and that risk increased further to almost 8-fold for the group of children below 2 SD from the average IQ range (adjusted OR: 7.89; 95% CI: 3.00–20.72). A similar trend was found at age 11; children with IQ 1 SD lower than the average had a modestly higher risk for schizophrenia (adjusted OR: 2.51; 95% CI: 1.10–5.25), but for the group of children performing 2 SD lower than the normal range, the increase in risk was 9-fold (adjusted OR: 9.01; 95% CI: 2.75–29.50). For affective disorders, no significant differences in the adjusted ORs were found between the four groups at either age (P-values for the LRT ≥ .10).
Discussion
We found robust evidence for a nonlinear risk relationship between IQ in childhood and the risk of schizophrenia replicated at two time points in childhood (ages 7 and 11). We have reported an exponential decline curve leveling off as it approached average IQ; children with IQ scores < 2 SD (ie, IQ < 70) were eight times (at 7 years) and nine times (at 11 years) more likely to develop schizophrenia in comparison with children with average IQ, whereas no evidence was found for a significant risk reduction for children with above average intelligence. By contrast, risk of affective disorders was unrelated to IQ in childhood.
Several birth and conscript cohort studies have investigated the relationship between premorbid IQ and the risk of schizophrenia. Among the conscript cohorts, most of these studies concluded that the relationship was linear,6,8,16–19,39 although both Gunnell et al.21 and Urfer-Parnas et al.22 did report a supralinear trend, and others have commented on an apparent nonlinear trend, which did not reach conventional criteria for statistical significance.8,16,39 A meta-analysis of five of these cohort studies23 confirmed this picture of an overall linear relationship, but also found significant heterogeneity across studies as some had reported evidence for a nonlinear trend.
It is important to elucidate the exact shape of the risk function between schizophrenia and premorbid IQ given its clinical implications. The nonlinear relationship indicates that only the learning disabled (IQ < 70) are at greater risk due to their low premorbid level of IQ, and their risk is many times greater than those with average or above average IQ. A linear relationship, however, would suggest a dose-response relationship across the whole range of IQ scores.
The current lack of clarity about the risk function is likely to be due to a considerable variation in the selected statistical methods for detecting or testing a nonlinear trend in risk estimates, and partly down to differences in study design. Only a minority of studies reported results for IQ as a continuous predictor,17,22,39 whereas most studies used grouped IQ data based on 4–6 SD groups8,16,18,19 to compare adjusted risk parameters and some reported in addition the results for a linear trend test, but very few studies actually tested for nonlinearity (notable exceptions).8,16,22 Furthermore, a linear trend in logistic regression corresponds to a nonlinear trend of ORs or predicted probabilities.36,40 Another problem could be insufficient statistical power to detect nonlinearity due to the small number of cases with schizophrenia in the lower and higher SD groups.
Although all studies that we reviewed provided clear evidence for a significant increase in risk when IQ fell below −1 SD, few studies8,19,39 reported significant risk reduction for higher levels (+1 SD) of premorbid IQ relative to normal IQ. Without providing evidence for both a significant increase as well as decrease in the risk depending on low or high IQ, the suggestion of a linear relationship in the risk of schizophrenia across the whole range of IQ is, as yet, empirically not justified, and so the current emphasis in the literature on a “linear relationship” between IQ and risk of schizophrenia is in need of further clarification.
Differences between the studies in design, in particular birth vs conscript cohorts (for a critical summary, see McCabe)14 and the method and age of cognitive assessment will also have led to variation in the risk estimates as well as their trend patterns. Furthermore, in contrast to birth cohorts, conscript cohorts exclude a small number of males on the grounds of mental/physical handicap, early onset of psychosis or other psychological dysfunction,8,24,39 and hence will underrepresent the learning disability subpopulation (ie, IQ ≤ 70) which, however, is of particular relevance to the current research question.
Risk Mechanism
A number of explanations have been suggested as to how normal or high IQ can protect against schizophrenia including better coping with stressful life events41 and cognitive reserve.20 Explanations focusing more on low IQ as a risk factor for schizophrenia note that it may be a proxy measure of impaired neurodevelopment or abnormal neuroanatomy.41
Edelman and Gally42 hypothesized that degeneracy (ie, the ability of different structural elements or processes to perform the same function or yield the same output) was a fundamental aspect for the evolution of biological and functional complexity, especially in the nervous system because degeneracy would enable more effective computations in perception and action. Hence, development of a high or low level of degeneracy is linked to development of high or low intelligence, respectively. We conjecture that children with average, or above average, intelligence develop sufficient levels of degeneracy in all or most circuits in the brain, not only those serving the computations required in intelligence testing. Hence, these children are somewhat buffered against the development of psychosis because abnormal neurodevelopment of one or some systems is compensated for by the normal development in other systems that serve similar cognitive, social, or emotional functions. However, children with low IQ develop inadequate degeneracy and hence have little or no buffering capacity putting them at greater risk from etiological factors that cause or contribute to psychosis. This formulation would predict a nonlinear relationship between premorbid cognitive ability and risk of schizophrenia due to the compensation inherent in degenerate systems. It does not contradict the cognitive reserve hypothesis of Barnett et al.20 but is a variant of it.
Strength and Limitations
Using a prospective birth cohort we applied sensitive exploratory data analysis well suited for uncovering unusual nonlinear relationships as well as established statistical methods. Overall, the effect size we found for cognitive impairment in childhood of later schizophrenia patients is in line with the one reported recently by Woodberry et al.4 The advantage of a prospective birth cohort study is that it covers the whole natural range of IQ scores stretching well into the area of learning disability, whereas in conscript cohorts the exclusion due to learning/ physical disabilities or gross psychological dysfunction will introduce some selection bias. On the other hand, birth cohorts have considerably smaller numbers of cases of psychiatric disorders compared with those in conscript cohorts, which impacts on the accuracy of risk estimates.
A further limitation of our research was the use of hospital admission registry to define cases. While this is unlikely to bias the rates for schizophrenia,41 it is likely to result in more severe cases of affective disorder being included in this study because inpatient care for nonpsychotic disordered patients is less common in the UK.8 Therefore, cases of affective disorders may represent a particularly disturbed group of patients and those with poor coping skills or lack of social support. Finally, the nonlinear risk function revealed in this cohort study is representative only for age of onset up to 36 years, hence the late onset cases might somewhat alter the shape of the risk function. In conclusion, according to our results there is compelling evidence for a nonlinear risk relationship between childhood IQ and later schizophrenia, but no such relationship with affective disorders.
Funding
Stuart Leask was funded by the Medical Research Council, UK.
Acknowledgments
Dr Mike Blows who provided independent DSM-IV diagnoses for each case and Prof. Tim J. Crow on guiding the diagnostic consensus process where there was disagreement between Mike Blows and S.L. The authors are also grateful to (1) NHS Regional office database managers for their contribution to case ascertainment, (2) the Centre for Longitudinal Studies, Institute of Education for the use of the NCDS data, and (3) the UK Data Archive and Economic and Social Data Service for making them available. However, they bear no responsibility for the analysis or interpretation of these data. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull. 1984; 10: 430–459 [DOI] [PubMed] [Google Scholar]
- 2. Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002; 59: 449–456 [DOI] [PubMed] [Google Scholar]
- 3. Sørensen HJ, Mortensen EL, Schiffman J, Ekstrøm M, Denenney D, Mednick SA. Premorbid IQ and adult schizophrenia spectrum disorder: verbal performance subtests. Psychiatry Res. 2010; 178: 23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008; 165: 579–587 [DOI] [PubMed] [Google Scholar]
- 5. Morgan VA, Leonard H, Bourke J, Jablensky A. Intellectual disability co-occurring with schizophrenia and other psychiatric illness: population-based study. Br J Psychiatry. 2008; 193: 364–372 [DOI] [PubMed] [Google Scholar]
- 6. Gale CR, Batty GD, Tynelius P, Deary IJ, Rasmussen F. Intelligence in early adulthood and subsequent hospitalization for mental disorders. Epidemiology. 2010; 21: 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker E, Kestler L, Bollini A, Hochman KM. Schizophrenia: etiology and course. Annu Rev Psychol. 2004; 55: 401–430 [DOI] [PubMed] [Google Scholar]
- 8. Zammit S, Allebeck P, David AS, et al. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 2004; 61: 354–360 [DOI] [PubMed] [Google Scholar]
- 9. Keefe RS. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry. 2008; 7: 22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bora E, Yücel M, Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: implications for DSM-V criteria and beyond. Schizophr Bull. 2010; 36: 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carpenter WT. Conceptualizing schizophrenia through attenuated symptoms in the population. Am J Psychiatry. 2010; 167: 9 [DOI] [PubMed] [Google Scholar]
- 12. Hyman SE, Fenton WS. What are the right targets for psychopharmacology? Science. 2003; 299: 350–351 [DOI] [PubMed] [Google Scholar]
- 13. Bowie CR, Depp C, McGrath JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010; 167: 1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maccabe JH. Population-based cohort studies on premorbid cognitive function in schizophrenia. Epidemiol Rev. 2008; 30: 77–83 [DOI] [PubMed] [Google Scholar]
- 15. Batty GD, Mortensen EL, Osler M. Childhood IQ in relation to later psychiatric disorder: evidence from a Danish birth cohort study. Br J Psychiatry. 2005; 187: 180–181 [DOI] [PubMed] [Google Scholar]
- 16. David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997; 27: 1311–1323 [DOI] [PubMed] [Google Scholar]
- 17. Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999; 156: 1328–1335 [DOI] [PubMed] [Google Scholar]
- 18. Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994; 344: 1398–1402 [DOI] [PubMed] [Google Scholar]
- 19. Tiihonen J, Haukka J, Henriksson M, et al. Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. Am J Psychiatry. 2005; 162: 1904–1910 [DOI] [PubMed] [Google Scholar]
- 20. Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006; 36: 1053–1064 [DOI] [PubMed] [Google Scholar]
- 21. Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia. Cohort study. Br J Psychiatry. 2002; 181: 298–305 [DOI] [PubMed] [Google Scholar]
- 22. Urfer-Parnas A, Lykke Mortensen E, Saebye D, Parnas J. Pre-morbid IQ in mental disorders: a Danish draft-board study of 7486 psychiatric patients. Psychol Med. 2010; 40: 547–556 [DOI] [PubMed] [Google Scholar]
- 23. Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 2011;132:220–227; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nilsson PM, Nyberg P, Ostergren PO. Increased susceptibility to stress at a psychological assessment of stress tolerance is associated with impaired fetal growth. Int J Epidemiol. 2001; 30: 75–80 [DOI] [PubMed] [Google Scholar]
- 25. Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol. 2006; 35: 34–41 [DOI] [PubMed] [Google Scholar]
- 26. Done DJ, Johnstone EC, Frith CD, Golding J, Shepherd PM, Crow TJ. Complications of pregnancy and delivery in relation to psychosis in adult life: data from the British perinatal mortality survey sample. BMJ. 1991; 302: 1576–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Done DJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ. 1994; 309: 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Southgate V. Manual of the Southgate Group Reading Tests. London: University of London Press; 1962; [Google Scholar]
- 29. Pringle MK, Butler N, Davie R. 11000 Seven Year Olds. London: Longman; 1966; [Google Scholar]
- 30. Jones P. Risk factors for schizophrenia in childhood and youth.. In: Haeffner H, ed. Risk and Protective Factors in Schizophrenia. Darmstadt, Germany: Steinkopf; 2002; 140–162 [Google Scholar]
- 31. Douglas JWB. The Home and the School. London, England: MacGibbon & Kee; 1964; [Google Scholar]
- 32. Leask SJ. On the Presentation and Relevance of Laterality: A Study of Psychosis. Nottingham, UK: Medicine, University of Nottingham; 2005; [Google Scholar]
- 33. Eaton WW. Epidemiology of schizophrenia. Epidemiol Rev. 1985; 7: 105–126 [DOI] [PubMed] [Google Scholar]
- 34. Cleveland WS. LOWESS: a program for smoothing scatterplots by robust locally weighted regression. Am Stat. 1981; 35: 54 [Google Scholar]
- 35. Fox J. Nonparametric Simple Regression. Smoothing Scatterplots. New York, NY: Sage; 2000; [Google Scholar]
- 36. Hosmer DW, Lemeshow S. Applied Logistic Regression 2nd ed New York, NY: John Wiley; 2000; [Google Scholar]
- 37. Pogue-Guile MF. Developmental aspects of schizophrenia.. In: Keshavan MS, Murray RM, eds. Neurodevelopment and Adult Psychopathology Cambridge, England: Cambridge University Press; 1997; 137–155 [Google Scholar]
- 38. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;;2: 413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reichenberg A, Weiser M, Caspi A, et al. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol. 2006; 28: 193–207 [DOI] [PubMed] [Google Scholar]
- 40. Greenland S. Introduction to regression models.. In: Rothmann KJ, Greenland S, eds. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven Publishers; 1998; 359–399 [Google Scholar]
- 41. Koenen KC, Moffitt TE, Roberts AL, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009; 166: 50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci U S A. 2001; 98: 13763–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]