Abstract
Background:
Previous research has provided compelling support for olfactory dysfunction in schizophrenia patients, their first-degree relatives, and youth at-risk for psychosis. A previous meta-analysis revealed large effect sizes across olfactory tasks but was limited to 2 olfactory tasks and did not examine moderator variables. Thus, the current meta-analysis was undertaken to incorporate additional studies, risk cohorts, olfactory test domains, and moderator variable analyses.
Method:
A meta-analysis was conducted on 67 publications examining olfactory function in schizophrenia patients and 15 publications examining olfactory functioning in youth at-risk for psychosis, first-degree relatives of schizophrenia patients, and individuals with schizotypy.
Results:
Results revealed medium-to-large olfactory deficits in schizophrenia patients though significant heterogeneity was evident. Several variables moderated overall study effects. At-risk youths similarly demonstrated medium-to-large effect sizes, whereas first-degree relatives and individuals with schizotypy showed small effects.
Conclusions:
Findings suggest robust olfactory deficits in schizophrenia and at-risk youths. In schizophrenia, several variables had significant impact on these deficits and warrant consideration in prospective studies. Our findings also indicate that olfactory measures may be a useful marker of schizophrenia risk status.
Key words: schizophrenia prodrome, smell, olfaction, schizotypy, psychosis
Introduction
Previous research has provided compelling support for the presence of olfactory dysfunction in patients with schizophrenia.1 Thus far, impairments across a wide variety of olfactory tasks have been well-documented, including reduced odor identification,2,3 odor detection threshold sensitivity,4 odor discrimination,4 odor memory,5 and odor hedonic judgments.6–8 Importantly, the nature of this impairment extends beyond the behavioral level as structural and physiological abnormalities in the underlying neurocircuitry of the olfactory system have been documented, ranging from reduced volume in the olfactory bulbs,9 posterior nasal cavity, and olfactory eloquent brain regions10,11 to abnormal olfactory event-related potentials and electro-olfactograms.12,13
Olfactory deficits have also been documented in ultra high-risk cohorts, nonpsychotic first-degree relatives of schizophrenia patients, and in individuals scoring high on psychometrically defined measures of schizotypal personality features.14,15 While studies examining olfactory dysfunction in high-risk adolescents are still in their infancy, the few existing articles do suggest that olfactory dysfunction may be a marker of schizophrenia risk status. For example, Brewer and colleagues16 found that those high-risk subjects who subsequently developed schizophrenia showed a greater disturbance in olfactory performance compared with those who progressed to another psychotic diagnosis. Similarly, Woodbury et al15 found that high-risk youths were significantly impaired on a brief measure of odor identification. Notably, the magnitude of olfactory impairment was much larger than deficits in other neuropsychological test measures and was greater for those high-risk subjects who subsequently became psychotic compared with those who did not. A study from our laboratory14 included tests of odor discrimination and odor identification and found that clinical risk subjects showed robust impairments across both tasks. Collectively, these findings suggest that olfactory impairments are a sensitive vulnerability marker of risk for psychosis that may have predictive utility in determining those individuals who develop schizophrenia.
The first published meta-analysis1 on olfactory dysfunction in schizophrenia was conducted in order to examine group differences in olfactory performance across various types of olfactory performance measures. Our prior results indicated comparable deficits for different psychophysical measures examined including odor detection threshold, discrimination, identification, and memory. In addition, olfactory impairment in schizophrenia was largely independent of sex, medication status, and smoking history. While the results of the previous meta-analysis addressed several unanswered questions concerning olfactory performance in schizophrenia, several limitations were also apparent. First, only 25 studies were available for analysis, with an overrepresentation of odor identification studies. In addition, the effects of several moderator variables could not be reliably assessed given the lack of presented data and appropriate meta-analytic tools available at the time of publication.
Since the publication of the first meta-analysis, olfactory research in schizophrenia has grown exponentially, with over 100 studies published since that time. This exponential growth has coincided with a greater refinement in reporting medication and smoking variables. For example, the calculation of pack years and pack days as measures of smoking dosages have now been reported across several studies. In addition, new meta-analytic techniques have emerged that enable the examination of moderator variables such as duration of illness, age of illness onset, and antipsychotic medication type. The growing body of olfactory studies in first-degree family members, youths at-risk for psychosis, and individuals with psychometrically defined schizotypy has also allowed for the analysis of study effect sizes in these subgroups. Finally, research on olfactory hedonic judgments has also gained interest in schizophrenia, with studies examining qualitative ratings of odor characteristics.
A recently conducted meta-analysis examined olfactory performance in schizophrenia patients and in groups deemed by the authors to be “at risk” for schizophrenia.17 However, their analysis was limited only to studies of odor identification and odor detection threshold and did not include a comprehensive analysis of moderator variables, such as sex, smoking, medication use, and illness characteristics. Thus, the current meta-analysis was undertaken to provide a comprehensive examination of olfactory psychophysical performance in the schizophrenia spectrum, and given the increase in the number of studies available and emergence of new research questions, apply more sophisticated ways to analyze moderator variables. Several moderator variables were examined including task type, task presentation (birhinal vs unirhinal), smoking, sex, medication, schizophrenia symptomatology, and other illness characteristics in an effort to understand how these variables influence impairment in olfactory performance. We also compared olfactory study effect sizes in individuals at clinical risk for psychosis, first-degree family members of schizophrenia patients, and individuals with schizotypy given that an ideal marker of vulnerability for schizophrenia should reliably distinguish between these groups.
Materials and Methods
Literature Search and Study Selection for Schizophrenia Studies
A computerized literature search using combinations of the keywords “olfaction” or “smell” and “schizophrenia” was conducted on PubMed and Embase databases. The search parameters were limited to English-language publications with human participants published through February 2012. In addition, manual searches were performed by examining article references elicited through the databases. The search yielded 113 publications that were later reviewed for inclusion by 3 authors (DMM, VK, and PJM). The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) standard18 was followed in the extraction of relevant studies and data. Inclusion criteria were as follows: (1) a focus on formal tests of olfactory function in patient groups with schizophrenia or schizophrenia-plus (cohort included patients with schizophrenia, schizophreniform, or schizoaffective [excluding bipolar type] disorders), (2) a healthy comparison group, and (3) statistical information that allowed for effect size calculation and use of meta-analytic procedures. Based on the aforementioned criteria, 39 publications were excluded for (1) presenting no data (eg, review article) or data that were incomplete/unrelated (n = 20), (2) lacking a control group (n = 11), and (3) lacking a schizophrenia patient group (eg, schizotypy; n = 8). In addition, 4 publications were excluded for having mixed patient populations: schizoaffective-bipolar type,19 affective psychosis,20 and psychosis not otherwise specified.21,22
A large number of publications included either more than 1 relevant measure of olfactory function (eg, both odor identification and discrimination assessed) or included unirhinal testing, in which data for each nostril were obtained and analyzed separately. Therefore, 161 studies (out of 70 publications) were found to be appropriate for meta-analytic review. Relevant data, including statistical values on olfactory tasks and information about demographics, smoking, medication status, task type, and other moderator variables of interest were extracted by 3 individuals (VK, DMM, and ZDL) and cross-checked and reviewed by another author (PJM).
Methodological Variables
Consistent with our 1999 analysis, we examined odor identification, odor discrimination, odor detection threshold, and odor memory. In addition, we included an analysis of odor hedonics tasks, in which individuals are asked to make pleasantness ratings of the odors presented using Likert scales.
Moderator Variables
In the current meta-analysis, a detailed coding system was employed in order to examine the potential effects of psychophysical task type and stimuli presentation methods, clinical characteristics, medication status, and general demographics. The following psychophysical olfactory task and stimuli presentation method moderator variables were coded: (1) psychophysical task type (ie, odor identification, detection threshold sensitivity, discrimination, memory, and hedonics), (2) stimuli presentation method (ie, birhinal and unirhinal assessment), and (3) lateralized testing results (ie, left vs right nostril performance in unirhinal studies).
Within the patient population, the following clinical characteristics were coded: (1) diagnosis of the patient sample (ie, schizophrenia sample or schizophrenia-plus sample), (2) hospitalization status (ie, inpatient, outpatient, or mixed status), (3) mean age of onset, (4) mean duration of illness, and (5) positive and negative symptom rating total score, assessed from the Scales for the Assessment of Positive (SAPS)23 and Negative Symptoms (SANS)24 or the Positive and Negative Symptom Scale25 scores (PANSS-Pos and PANSS-Neg). In order to assess the effects of medication on olfactory function, medication information was coded in 3 ways: (1) antipsychotic medication status (ie, medicated on antipsychotic medications and unmedicated: not currently on an antipsychotic medication), (2) medication regimen (ie, all subjects on typical and all subjects on atypical antipsychotics), and (3) mean dosage defined in chlorpromazine equivalents.
Demographic information was assessed separately within the patient and control group as follows: (1) sex (% male), (2) mean age, (3) mean years of education, and (4) handedness (% right handed). Smoking status, a variable of substantial interest in olfactory studies, was categorized in 2 ways. First, the percent of patient smokers and percent of control smokers were entered. Second, mean pack years, calculated by multiplying the daily pack percentage by the number of smoking years, were coded when provided.
Statistical Analysis
All analyses were conducted using Comprehensive Meta-Analysis, Version 2.026. In order to standardize group differences on measures of olfactory performance, an effect size (Cohen’s d) was calculated by calculating the mean difference in scores for studies reporting contrasts of schizophrenia patients and healthy comparison participants and dividing this value by the pooled SD. Studies were weighted according to their inverse variance estimates in order to control for study differences in sample size when mean effect sizes were computed. Based on Cohen’s criteria,27 effect sizes are categorized as small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Confidence intervals (CI) and z-transformations of the effect size were used to determine whether mean effect sizes were statistically significant.
In order to assess homogeneity across studies for each olfactory domain, the Cochran Q-statistic was utilized.28 The significance level of the mean effect sizes was computed using fixed effects linear models except when the Q-statistic revealed significant within-group heterogeneity, in which case a random effects model was used. To determine the effect of publication bias, tests were conducted graphically using a funnel plot and mathematically using an adjusted rank correlation test, according to the methods delineated by Begg and Mazumdar29 and Eggers et al.30
In domains with significant heterogeneity, possible effect size moderators were examined based on the Q-statistic. Analysis of olfactory performance differences was performed on all eligible studies. Further subgroup analysis was conducted to compare studies grouped by task design and those reporting on relevant demographic and clinical characteristics.
Literature Search and Study Selection for Youths At-Risk for Psychosis, First-Degree Family Members, and Schizotypy
Search criteria for identification of (1) at-risk youths, (2) first-degree family members, and (3) individuals with psychometrically defined schizotypy included the following search terms: “olfaction,” “smell,” “olfactory,” “familial schizophrenia,” “schizophrenia prodrome,” “first-degree family member,” “schizotypy,” “schizotypal,” and “ultrahigh risk.” The search parameters were limited to English-language publications. The search yielded 37 publications that were later reviewed for inclusion by 2 authors (VK and PJM). A manual search of references in articles was performed, which elicited 5 additional publications; 1 unpublished data set was also included.31 Once again, the MOOSE standard18 was followed in the extraction of relevant studies and data. Publications presenting no data or data that were incomplete/unrelated were excluded (n = 26). Additionally, 1 publication was excluded for including second-degree family members in their family cohort,21 and another for including a family member cohort of a patient group with diagnoses other than schizophrenia.19 This left 14 publications and 1 unpublished article from which 37 individual effects were extracted.
Results
Overall Meta-Analysis Results in Schizophrenia
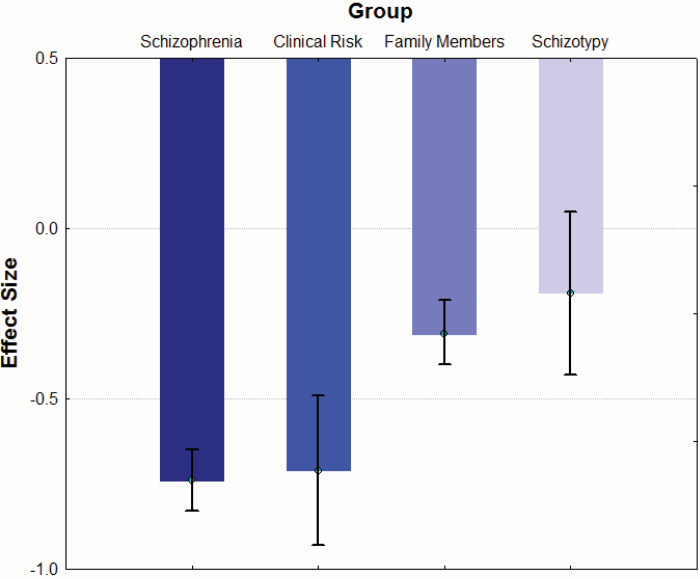
Across all studies with a schizophrenia or schizophrenia-plus cohort, the overall effect size for olfactory processing tasks (k = 161; 4 491 schizophrenia patients and 4 408 controls) was −0.74 [95% CI = −0.83 < δ < −0.65], which is moderate to large in strength (see figure 1). An analysis of homogeneity including all studies revealed significant variance among study effect sizes that would support examining the effects of moderator variables (QB[160] = 630.0, P < .001). In order to understand the variability among effect sizes, we examined psychometric, clinical, and demographic variables that might explain this heterogeneity.
Fig. 1.
Differences in study effect sizes across schizophrenia and related subgroups.
Mean (± CI) study effect sizes for schizophrenia (k = 161 studies), clinical risk (k = 6 studies), first-degree family members (k = 37 studies), and individuals with psychometrically defined schizotypy (k = 8 studies) across task type.
Publication Bias
Publication bias was assessed using the Begg and Mazumdar29 rank correlation and Egger et al30 tests. Results showed a significant rank correlation test (P < .001, one tailed) and Egger test (P < .001, one tailed), indicating evidence of possible publication bias. In order to assess the potential effect of such bias on study results, an Orwin’s fail-safe N was calculated, indicating that 7 103 studies reporting a zero effect would be needed to reduce the observed effect to 0.20. The large number of “null” studies needed to eliminate the observed effects suggest that any possible publication bias had a minimal to nonexistent influence on the current results.
Moderator Analysis
Psychophysical Methods.
Olfactory Task Type
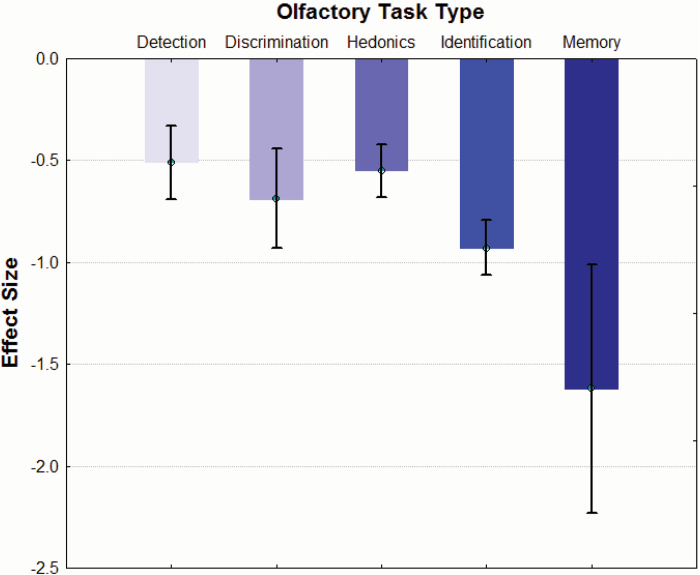
Included studies employed a variety of tasks to assess olfactory impairment. Task types included (1) odor detection threshold sensitivity (k = 40 studies, d = −0.51, 95% CI = −0.69 < δ < −0.33), (2) odor discrimination (k = 13 studies, d = −0.69, 95% CI = −0.93 < δ < −0.44), (3) odor hedonics (k = 30 studies, d = −0.55, 95% CI = −0.68 < δ < −0.42), (4) odor identification (k = 76 studies, d = −0.93, 95% CI = −1.06 < δ < −0.79), and (5) odor memory (k = 2 studies, d = −1.62, 95% CI = −2.23 < δ < −1.01; figure 2). Homogeneity analysis revealed a significant influence of task type on effect size (QB[4] = 29.10, P < .001). Post hoc testing revealed that odor memory tests yielded significantly larger effect sizes than measures of odor identification (QB[1] = 4.79, P = .03), odor detection threshold sensitivity (QB[1] = 11.77, P = .001), odor hedonics (QB[1] = 11.44, P = .001), and odor discrimination (QB[1] = 7.83, P = .005).
Fig. 2.
Study effect sizes across olfactory task types in schizophrenia patients.
Mean (± CI) study effect sizes for tasks of odor identification (k = 76 studies), odor discrimination (k = 13 studies), odor memory (k = 2 studies), odor detection (k = 40 studies), and odor hedonics (k = 30 studies).
With the exception of odor memory as noted above, odor identification measures showed significantly greater impairment than odor detection threshold sensitivity (QB[1] = 12.93, P < .001) and odor hedonics (QB[1] = 15.67, P < .001). Differences between odor identification and odor discrimination tasks were not statistically significant (QB[1] = 2.86, P = .10).
Odor hedonics was not observed to be significantly different from odor detection or discrimination (all P’s >.33). Similarly, odor discrimination tasks did not show greater impairment than odor detection tasks (QB[1] = 1.28, P = .26). Notably, results revealed that odor hedonic studies employing pleasant odors (k = 12 studies, d = −0.78, 95% CI = −0.97 < δ < −0.58) were associated with significantly larger effect sizes than those employing unpleasant odors (k = 11 studies, d = −0.33, 95% CI = −0.50 < δ < −0.16; QB[1] = 11.21, P = .001).
Presentation Type
The impact of method on odorant presentation was analyzed by comparing studies that presented odors to subjects birhinally (both nostrils at the same time; k = 90 studies, d = −0.86, 95% CI = −0.97 < δ < −0.75) to studies employing a unirhinal presentation method (each nostril separately; k = 71 studies, d = −0.59, 95% CI = −0.73 < δ < −0.45). Results showed that studies presenting odors birhinally were associated with significantly larger effect sizes than those presented unirhinally (QB[1] = 8.98, P = .003).
Left/Right Nostril
In those studies that presented odors unirhinally, left nostril impairments (k = 32 studies, d = −0.61, 95% CI = −0.83 < δ < −0.39) and right nostril deficits (k = 33 studies, d = −0.57, 95% CI = −0.78 < δ < −0.36) were not significantly different (QB[1] = 0.06, P = .81).
Clinical Characteristics.
Diagnosis
Studies varied in their diagnosis as either schizophrenia-only (k = 139 studies, d = −0.75, 95% CI = −0.85 < δ < −0.65) or schizophrenia-plus (k = 22 studies, d = −0.65, 95% CI = −0.87 < δ < −0.43) groups that included patients with schizophrenia in addition to schizophreniform and/or schizoaffective disorder. Contrasts illustrated no difference between the studies with schizophrenia-plus or schizophrenia-only populations (QB[1] = 0.69, P = .41).
Inpatient/Outpatient Status
Hospitalization status was also examined as several studies recruited inpatients only (k = 32 studies, d = −0.66, 95% CI = −0.78 < δ < −0.53), outpatients only (k = 54 studies, d = −0.71, 95% CI = −0.83 < δ < −0.58), or a mixture of both inpatients and outpatients (k = 35, d = −0.48, 95% CI = −0.63 < δ < −0.33). Analysis of effect sizes between these subgroups revealed differences at only a trend-level (QB[2] = 5.68, P = .06).
Age of Onset/Duration of Illness
Age of illness onset and illness duration were examined to determine whether these variables influenced the magnitude of the overall effect size. Age of onset (k = 70 studies) was shown to not significantly influence effect sizes (Z = −0.94, P = .35). Conversely, duration of illness (k = 64 studies) significantly moderated overall effect sizes (Z = −4.98, P < .001), with a longer duration of illness being associated with a greater magnitude of olfactory impairment on formal testing.
Clinical Characteristics/Symptoms
Studies included various indicators of the severity of the illness such as positive and negative symptoms scores from the SANS (k = 36 studies), SAPS (k = 36 studies), PANSS-Neg (k = 37), and PANSS-Pos (k = 37). SAPS scores were significant (Z = 2.19, P = 0.03), indicating that a higher level of positive symptoms coincided with a smaller study effect size. In contrast, findings for the PANSS-Pos showed the opposite effects, such that a higher degree of positive symptoms was associated with a larger study effect size (Z = −2.58, P < .01). Analysis revealed that study effect size did not vary significantly by negative symptomatology on the PANSS-Neg or SANS (all P’s >.18).
Antipsychotic Medications. Medication Status
In order to examine the moderating effect of antipsychotic medication status on olfactory performance, studies were classified according to their reported medication use at the time of the study. Groups included medicated (k = 59 studies, d = −0.87, 95% CI = −1.09 < δ < −0.66) and unmedicated (k = 15 studies, d = −0.87, 95% CI = −1.08 < δ < −0.65) patients. No significant differences in effect size were observed between these 2 medication status groups (QB[1] = 0.003, P = .96).
Chlorpromazine Equivalents
In those studies that reported chlorpromazine equivalents (k = 44 studies), analysis showed no significant influence on study effect size (Z = −1.29, P = .20).
Medication Type
A more finely grained analysis of medication effects was performed by further delineating medicated patients according to antipsychotic medication type. The 2 groups included all patients on atypical (k = 9 studies, d = −0.61, 95% CI = −0.92 < δ < −0.29), and all patients on typical (k = 12 studies, d = −1.60, 95% CI = −2.32 < δ < −0.88) antipsychotics. Effect sizes for these 2 groups were found to be heterogeneous (QB[1] = 6.13, P = .013). Post hoc contrasts revealed that studies examining patients on a regimen of typical antipsychotics displayed significantly more olfactory impairment than studies with patients on only atypical antipsychotic regimens (QB[1] = 6.13, P = .01)
Demographic Characteristics.
Several demographic variables theorized to influence olfactory performance were analyzed as well.
Sex
In order to account for any effect of sex composition on observed effect size, the percentage of male patients (k = 151 studies) and healthy male participants (k = 151) were analyzed. Both variables proved to be significant (Z = 2.64, P < .01 and Z = 2.37, P = .02, respectively), indicating that a higher percentage of males in a given study coincided with a smaller study effect size.
Age
Increased age in patients (k = 154 studies) was observed to be associated with greater olfactory deficit (Z = −2.87, P < .01), while no such relationship was seen in healthy subjects (k = 157 studies; Z = −1.20, P = .23).
Education
The average years of education for patients (k = 94 studies) and healthy comparison subjects (k = 93 studies) were also examined. While patient education did not reveal a significant influence on effect size (Z = −1.04, P = .30), a significant relationship was seen between higher education levels and better olfactory performance in healthy subjects (Z = 2.11, P = .03).
Handedness
The influence of hand dominance on study effect size was also evaluated. Neither healthy subjects (k = 23 studies) or patients (k = 23 studies) showed a relationship between handedness and olfactory performance (all P’s >.08).
Smoking History
In order to assess for the possible influence of smoking, the percentage of patient smokers (k = 107 studies) and healthy subject smokers (k = 108 studies) were analyzed. Patient smokers did not have a significant impact on study effect size (Z = −0.17, P = .86). Similarly, percent of healthy subject smokers did not significantly moderate study effect size (Z = 0.27, P = .79).
Patients and controls were then analyzed according to the number of pack years. In controls, smoking burden as measured by pack years did not appear to significantly moderate effect size (k = 49 studies; Z = 0.79, P = .43). However, patient pack-year history (k = 47 studies) was statistically significant (Z = 2.22, P = .03), indicating that higher levels of smoking in patients were associated with smaller deficits in olfactory performance.
Overall Meta-Analysis Results in At-Risk Youths, First-Degree Family Members, and Schizotypy
Across studies of at-risk youths, schizotypy and first-degree family members of schizophrenia patients, the overall effect size for olfactory processing tasks (k = 37; 875 subjects and 1190 controls) was −0.33 [95% CI = −0.42 < δ < −0.23], which is a small-to-medium strength effect (see figure 1). An analysis of homogeneity revealed significant variance among study effect sizes that would support examining the effects of moderator variables (QB[36] = 59.66, P < .01). Clinical risk youths (k = 6 studies, d = −0.71, 95% CI = −0.93 < δ < −0.49), first-degree family members (k = 23 studies, d = −0.25, 95% CI = −0.36 < δ < −0.13), and individuals with schizotypy (k = 8 studies, d = −0.19, 95% CI = −0.43 < δ < 0.05) were subsequently compared. Olfactory impairment was significantly greater in the at-risk cohort compared with that in either first-degree family members (QB[1] = 13.64, P < .001) or schizotypy groups (QB[1] = 9.92, P < .01). The latter 2 cohorts were not significantly different from each other (QB[1] = 0.16, P = .69).
Publication Bias
There was no evidence of any possible publication bias as indicated by nonsignificant Begg and Mazumdar rank correlation (P = .17) and Egger et al (P = .33) tests. Regardless, calculation of a fail-safe N revealed that a total of 393 null studies would be required to reduce the observed effect to 0.20. The latter analyses indicate that the observed effects are accurate representation of the extant literature on olfactory function in these populations.
Discussion
Consistent with our prior quantitative review,1 robust olfactory deficits were seen across diverse olfactory tasks, suggesting a medium-to-large effect size for these deficits in schizophrenia. The current results could not be accounted for by any potential publication bias given the large volume of null studies needed to reduce the magnitude to a small effect. Looking at the construct of olfaction broadly, this meta-analysis supports the notion that dysfunction in the central and peripheral olfactory system in schizophrenia is ubiquitous. We did, however, observe that the magnitude of the effect size varied significantly by olfactory task type. Notably, tests of odor memory and identification continue to reveal the largest and most robust effect sizes relative to tasks of odor detection threshold, odor hedonics, and odor discrimination. The primary explanation for these findings could be that tasks assessing odor identification, odor memory, and odor discrimination require increased integration and recruitment of associated language function, working memory, and short-term memory functions. Thus, the greater deficit in odor memory may reflect this task’s reliance on primary olfactory cortex and temporal brain regions, which are both closely associated with the pathophysiology of the disorder. In addition, odor identification and odor memory deficits can result from dysfunction at any level of peripheral or higher order processing (eg, retrieving a semantic label or remembering an odor that was presented previously). Furthermore, the additional language and memory processing involved in odor identification and memory tasks are processes that are also reported as deficient in schizophrenia19, 32 and may contribute to the larger effect sizes seen for odor identification and odor memory tasks. These hypotheses, however, must be tempered by the fact that different olfactory measures appear to have different reliabilities, with odor identification tasks showing better reliabilities relative to other olfactory measures.33 As such, additional research is needed to examine how these factors, including the psychometric properties, task difficulty, and nonolfactory components of olfactory tasks, influence the study effect sizes.
Several moderator variables appeared to have a significant positive or negative impact on olfactory deficits. We found that birhinal presentation of odors produced larger study effects than unirhinal presentation. Prior work has indicated that birhinal processing requires additional interaction of both hemispheres,34 while unirhinal processing (ie, testing nostrils in isolation) may be more difficult.35 Indeed, a study by Bromley and Doty36 reported that birhinal testing of odor memory is easier than unirhinal testing in healthy individuals. Abnormalities of interhemispheric transfer of information are seen in schizophrenia for visual information,37 tactile information,38 and auditory information (dichotic listening),39,40 so it may be that the transfer of olfactory information between the cerebral hemispheres in patients is also disrupted and more prominent when olfactory stimuli are simultaneously processed by both nostrils. The differences in study effects produced by birhinal and unirhinal presentation method suggest that additional research on the differences in unirhinal and birhinal processing of odors in schizophrenia are warranted.
With respect to clinical features of the illness, our results indicate that longer duration of illness in the schizophrenia samples was associated with larger study effect sizes. Studies reporting an association between olfactory abilities and illness duration have been well-documented,3,41,42 and seem to support a slow degradation of the olfactory system over the course of illness. Negative symptoms did not moderate study effect sizes, which was unexpected given the widely reported relationship between increased negative symptoms and greater olfactory impairment.42–44 One reason for this lack of an effect may be the relatively low number of studies reporting negative symptom scores. In addition, moderator analysis was collapsed across all olfactory task types. Thus, it is possible that any potential domain-specific relationships were confounded or minimized (eg, the bulk of studies reporting a relationship with negative symptoms have examined odor identification). In contrast, the positive symptoms of psychosis (eg, hallucinations, delusions, etc.) were found to significantly moderate effect size, with higher levels of these symptoms being associated with reduced levels of olfactory deficit. A previous study from our group42 examining the clinical, neuropsychological, and structural brain correlates of olfactory functioning found that increased positive symptoms were related to better odor detection threshold sensitivity. This finding was hypothesized to reflect an increased vigilance to external and internal stimuli often seen in patients with more predominant Schneiderian symptomatology. It would be helpful in future studies to examine the subscales that make up these composite symptom measures in order to better understand the items that drive or mitigate any potential relationships with olfaction.
Consistent with our prior meta-analysis in 1999, we found that overall medication status (ie, medicated and unmedicated) was not a significant moderator of study effect size. Similarly, no significant moderation of effect size was seen in those studies that reported chlorpromazine equivalents. In this study, however, we sought to further explore and refine the medication moderator variable by logging the general antipsychotic class used in a given study. This approach revealed that studies in which patients were on a regimen of typical or first-generation antipsychotics showed significantly greater olfactory deficits than those in which atypical or second-generation antipsychotics were prescribed. There is evidence from pharmacologic,45 genetic,46 and animal studies47 that suggest that performance on odor processing tasks may be modulated, in part, by dopamine-related neural mechanisms. While the precise mechanisms underlying this differential medication response are unclear, it does appear that there is specific dopaminergic modulation of primary olfactory afferents. Further work detailing the acute effects of antipsychotic medication on tests of odor identification, detection threshold sensitivity, memory, and hedonics in schizophrenia is therefore warranted.
Smoking has been an important variable of consideration in olfactory studies given the reported adverse influence of cigarette use on olfactory abilities.48 Consistent with the findings of our prior meta-analysis, this study found no effect of smoking on study effect size as assessed by percentage of patient and control smokers in a given sample. This variable, however, does not account for the intensity and frequency of subjects’ smoking. Our group, as well as others,1,49 have advocated for the use of “pack years” where smoking burden is quantified by both duration and intensity of smoking. Using this approach, we found that, in patients, increased burden of smoking was associated with significantly decreased study effect size. This relationship was not seen in healthy subjects. While this finding may seem counterintuitive, it is notable that most studies of olfaction in schizophrenia have treated smoking as a nuisance variable—to be controlled for—as opposed to being a primary variable of interest. Notably, a recent study by McLean and colleagues reported an enhancing effect of smoking on olfactory ability in individuals with psychosis.20 These authors speculated that the central nervous system effects of nicotine might have a “normalizing” effect on olfactory function, which perhaps outweigh the adverse locally mediated aspects of smoking. Regarding neuropsychological functions in schizophrenia, there has been considerable interest in the potentially remediating effects of nicotine on cognition.50–52 The current data suggest that studying smoking burden as a variable of interest in olfactory studies may help elucidate some of the pharmacologic underpinnings or mitigating factors of this deficit in schizophrenia.
While the literature examining olfactory dysfunction in individuals at risk for schizophrenia is still in its infancy, several studies have noted olfactory impairment in at-risk youths.14,15,16 However, a recently conducted meta-analysis of olfactory performance across 16 studies of “schizophrenia risk status” concluded that odor identification deficits were not useful as a schizophrenia vulnerability marker.18 Notably, the 16 studies analyzed by Cohen et al18 included studies examining olfactory performance in otherwise-healthy individuals with psychometrically defined schizotypal personality features and in nonpsychotic first-degree biological relatives of schizophrenia patients and only 2 studies of youths with prodromal symptomatology. Consistent with their findings, we found significant heterogeneity in study effect sizes across the 3 groups, with the clinical risk cohort showing a level of olfactory impairment that was comparable with that observed in patients and significantly greater than that seen in first-degree relatives and schizotypal individuals. These findings suggest that olfactory measures may have utility in distinguishing clinical risk youths from symptom-free family members and individuals with psychometrically defined schizotypy. Indeed, a recent meta-analysis of baseline and longitudinal follow-up neurocognitive data in at-risk youths found that olfactory scores showed the largest effect sizes in those individuals who subsequently converted to psychosis.53 Based on these limited though robust findings, we believe that olfactory dysfunction may be a useful marker of schizophrenia risk status.54
Consistent with the notion that olfactory measures may be important in those people at risk for the development of schizophrenia, a recent epidemiologic study by Nguyen-Khoa and colleagues55 examined 2 large medical insurance databases in the United States detailing the rates of claims for disturbances of the sense of smell and their association with various diseases and medications. The authors found that schizophrenia and psychosis were strongly associated with olfactory dysfunction in both database cohorts (OR = 6.2; 95% CI = 2.0–19.7 and OR = 5.9; 95% CI = 1.5–22.6). These data converge in suggesting that people presenting to their physician with complaints of smell dysfunction have roughly 6 times the risk of developing schizophrenia than people who do not have any chemosensory complaints. As it is well known that most patients with olfactory dysfunction are not aware of their smell loss, the actual impact of this factor in the latter study is likely an underestimate of risk.
Overall, the results of the current meta-analysis confirm the presence of olfactory deficits across the largest number of studies to date. Contrary to the results of our prior meta-analytic review, we found that several moderators influenced the magnitude of olfactory deficits observed across studies, including (1) olfactory task type, (2) method of task presentation (birhinal vs unirhinal), (3) antipsychotic medication regimen type, (4) age of onset, (5) duration of illness, (6) education, (7) sex, and (8) patient smoking. In light of the latter findings, there are a number of specific factors that appear to warrant attention in future studies of olfactory dysfunction in schizophrenia. First, while we added tests of odor hedonics to the current meta-analysis, there are other olfactory domains that are still relatively unexplored (eg, odor intensity, memory, and recognition threshold). These measures may hold promise in further delineating the scope and magnitude of the olfactory dysfunction seen in patients. Second, examination of smoking burden in pack years and focusing on this as a predictor variable as opposed to a nuisance variable may help to explore any differential impact on olfactory function in patients and healthy controls. Third, while the studies in the literature have focused on overall positive and negative symptom scores, further delineation of this effect by examining the specific items or subscales that comprise these global scores seems prudent. In addition, examination of other clinical measures of affective functioning such as anhedonia or empathy would also appear to hold promise.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
National Institutes of Health (MH63381 to P.J.M., MH59852 to B.I.T.); Independent Investigator Award from the Brain & Behavior Research Foundation on Schizophrenia and Depression to P.J.M.
Supplementary Material
Acknowledgments
We wish to thank Zach D. Liapis for his assistance with data extraction and the Hofmann Trust for their support of this research through the BBRF. Conflict of interest: B.I.T. and R.E.G. report investigator-initiated research support from AstraZeneca Pharmaceuticals and Pfizer Inc.
References
- 1. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340 [DOI] [PubMed] [Google Scholar]
- 2. Brewer WJ, Pantelis C, Anderson V, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115 [DOI] [PubMed] [Google Scholar]
- 3. Moberg PJ, Doty RL, Turetsky BI, et al. Olfactory identification deficits in schizophrenia: correlation with duration of illness. Am J Psychiatry. 1997; 154: 1016–1018 [DOI] [PubMed] [Google Scholar]
- 4. Rupp CI, Fleischhacker WW, Kemmler G, et al. Various bilateral olfactory deficits in male patients with schizophrenia. Schizophr Bull. 2005;31:155–165 [DOI] [PubMed] [Google Scholar]
- 5. Wu J, Buchsbaum MS, Moy K, et al. Olfactory memory in unmedicated schizophrenics. Schizophr Res. 1993; 9: 41–47 [DOI] [PubMed] [Google Scholar]
- 6. Doop ML, Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophr Res. 2006;81:317–319 [DOI] [PubMed] [Google Scholar]
- 7. Moberg PJ, Arnold SE, Doty RL, et al. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003; 160: 1784–1789 [DOI] [PubMed] [Google Scholar]
- 8. Plailly J, d’Amato T, Saoud M, Royet JP. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage. 2006; 29: 302–313 [DOI] [PubMed] [Google Scholar]
- 9. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830 [DOI] [PubMed] [Google Scholar]
- 10. Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003; 60: 1193–1200 [DOI] [PubMed] [Google Scholar]
- 11. Rupp CI, Fleischhacker WW, Kemmler G, et al. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005; 74: 149–161 [DOI] [PubMed] [Google Scholar]
- 12. Turetsky BI, Hahn CG, Arnold SE, Moberg PJ. Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology. 2009; 34: 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003; 53: 403–411 [DOI] [PubMed] [Google Scholar]
- 14. Kamath V, Turetsky BI, Calkins ME, et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at risk for psychosis. World J Biol Psychiatry. 2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res. 2010;123:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brewer WJ, Wood SJ, McGorry PD, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003; 160: 1790–1794 [DOI] [PubMed] [Google Scholar]
- 17. Cohen AS, Brown LA, Auster TL. Olfaction, “olfiction,” and the schizophrenia-spectrum: an updated meta-analysis on identification and acuity. Schizophr Res. 2012; 135: 152–157 [DOI] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 19. Compton MT, McKenzie Mack L, Esterberg ML, et al. Associations between olfactory identification and verbal memory in patients with schizophrenia, first-degree relatives, and non-psychiatric controls. Schizophr Res. 2006; 86: 154–166 [DOI] [PubMed] [Google Scholar]
- 20. McLean D, Féron F, Mackay-Sim A, et al. Paradoxical association between smoking and olfactory identification in psychosis versus controls. Aust N Z J Psychiatry. 2004; 38: 81–83 [DOI] [PubMed] [Google Scholar]
- 21. Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158:1286–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Good KP, Leslie RA, McGlone J, Milliken HI, Kopala LC. Sex differences in olfactory function in young patients with psychotic disorders. Schizophr Res. 2007; 97: 97–102 [DOI] [PubMed] [Google Scholar]
- 23. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 24. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261–276 [DOI] [PubMed] [Google Scholar]
- 26. Comprehensive Meta-Analysis. [computer program]. Version. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 27. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 28. Hedges L, Olkin I. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985. [Google Scholar]
- 29. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50: 1088–1101 [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamath V, Turetsky BI, Calkins ME, et al. The influence of valence and intensity on odor identification ability in schizophrenia patients, their nonpsychotic first-degree relatives, and youths at-risk for psychosis. Unpublished manuscript
- 32. Good KP, Martzke JS, Milliken HI, Honer WG, Kopala LC. Unirhinal olfactory identification deficits in young male patients with schizophrenia and related disorders: association with impaired memory function. Schizophr Res. 2002; 56: 211–223 [DOI] [PubMed] [Google Scholar]
- 33. Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995; 20: 645–656 [DOI] [PubMed] [Google Scholar]
- 34. Cain WS. Bilateral interaction in olfaction. Nature. 1977; 268: 50–52 [DOI] [PubMed] [Google Scholar]
- 35. Doty RL, Bromley SM, Moberg PJ, Hummel T. Laterality in human nasal chemoreception. In: Christman S, ed. Cerebral Asymmetries in Sensory and Perceptual Processing. Amsterdam: North Holland Publishing; 1991; 497–542 [Google Scholar]
- 36. Bromley SM, Doty RL. Odor recognition memory is better under bilateral than unilateral test conditions. Cortex. 1995; 31: 25–40 [DOI] [PubMed] [Google Scholar]
- 37. David AS. Tachistoscopic tests of colour naming and matching in schizophrenia: evidence for posterior callosum dysfunction? Psychol Med. 1987;17:621–630 [DOI] [PubMed] [Google Scholar]
- 38. Carr SA. Interhemispheric transfer of stereognostic information in chronic schizophrenics. Br J Psychiatry. 1980;136:53–58 [DOI] [PubMed] [Google Scholar]
- 39. Green P, Kotenko V. Superior speech comprehension in schizophrenics under monaural versus binaural listening conditions. J Abnorm Psychol. 1980;89:399–408 [DOI] [PubMed] [Google Scholar]
- 40. Hallett S, Green P. Possible defects of interhemispheric integration in children of schizophrenics. J Nerv Ment Dis. 1983;171:421–425 [DOI] [PubMed] [Google Scholar]
- 41. Ugur T, Weisbrod M, Franzek E, Pfüller U, Sauer H. Olfactory impairment in monozygotic twins discordant for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005; 255: 94–98 [DOI] [PubMed] [Google Scholar]
- 42. Moberg PJ, Arnold SE, Doty RL, et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006; 28: 1444–1461 [DOI] [PubMed] [Google Scholar]
- 43. Brewer WJ, Pantelis C, Anderson V, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001; 158: 107–115 [DOI] [PubMed] [Google Scholar]
- 44. Malaspina D, Coleman E, Goetz RR, et al. Odor identification, eye tracking and deficit syndrome schizophrenia. Biol Psychiatry. 2002; 51: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sirota P, Davidson B, Mosheva T, Benhatov R, Zohar J, Gross-Isseroff R. Increased olfactory sensitivity in first episode psychosis and the effect of neuroleptic treatment on olfactory sensitivity in schizophrenia. Psychiatry Res. 1999;86:143–153 [DOI] [PubMed] [Google Scholar]
- 46. Kamath V, Moberg PJ, Gur RE, Doty RL, Turetsky BI. Effects of the val(158)met catechol-O-methyltransferase gene polymorphism on olfactory processing in schizophrenia. Behav Neurosci. 2012; 126: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doty RL, Risser JM. Influence of the D-2 dopamine receptor agonist quinpirole on the odor detection performance of rats before and after spiperone administration. Psychopharmacology (Berl). 1989; 98: 310–315 [DOI] [PubMed] [Google Scholar]
- 48. Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990; 263: 1233–1236 [PubMed] [Google Scholar]
- 49. Martzke JS, Kopala LC, Good KP. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry. 1997; 42: 721–732 [DOI] [PubMed] [Google Scholar]
- 50. Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858 [DOI] [PubMed] [Google Scholar]
- 51. Smith RC, Warner-Cohen J, Matute M, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006; 31: 637–643 [DOI] [PubMed] [Google Scholar]
- 52. Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002; 27: 479–497 [DOI] [PubMed] [Google Scholar]
- 53. Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415 [DOI] [PubMed] [Google Scholar]
- 54. Turetsky BI, Kamath V, Calkins ME, et al. Olfaction and schizophrenia clinical risk status: just the facts. Schizophr Res. 2012;139:260–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nguyen-Khoa BA, Goehring EL, Jr, Vendiola RM, Pezzullo JC, Jones JK. Epidemiologic study of smell disturbance in 2 medical insurance claims populations. Arch Otolaryngol Head Neck Surg. 2007; 133: 748–757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.