Abstract
Background: Schizophrenia and bipolar disorder share aspects of phenomenology and neurobiology and thus may represent a continuum of disease. Few studies have compared connectivity across the brain in these disorders or investigated their functional correlates. Methods: We used resting-state functional magnetic resonance imaging to evaluate global and regional connectivity in 32 healthy controls, 19 patients with bipolar disorder, and 18 schizophrenia patients. Patients also received comprehensive neuropsychological and clinical assessments. We computed correlation matrices among 266 regions of interest within the brain, with the primary dependent measure being overall global connectivity strength of each region with every other region. Results: Patients with schizophrenia had significantly lower global connectivity compared with healthy controls, whereas patients with bipolar disorder had global connectivity intermediate to and significantly different from those of patients with schizophrenia and healthy controls. Post hoc analyses revealed that compared with healthy controls, both patient groups had significantly lower connectivity in the paracingulate gyrus and right thalamus. Patients with schizophrenia also had significantly lower connectivity in the temporal occipital fusiform cortex, left caudate nucleus, and left thalamus compared with healthy controls. There were no significant differences among the patient groups in any of these regions. Lower global connectivity among all patients was associated with worse neuropsychological and clinical functioning, but these effects were not specific to any patient group. Conclusions: These findings are consistent with the hypothesis that schizophrenia and bipolar disorder may represent a continuum of global disconnectivity in the brain but that regional functional specificity may not be evident.
Key words: resting-state fMRI, schizophrenia, bipolar disorder, connectivity
Introduction
Since the time of Kraeplin,1 a dichotomy between schizophrenia (SCZ) and bipolar disorder (BD) has been emphasized, which continues today in the nosology of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).2 There is increasing evidence, however, that these disorders share similar epidemiologic features such as incidence3 and genetic risk.4 Moreover, several risk genes may be common to both disorders, including Disrupted in Schizophrenia 1,5,6 neuregulin 1,7,8 catechol-o-methyl transferase,9 and the G72/G30 loci.10 Recent genome-wide association studies also report overlap in the genes implicated in both disorders.11,12 Similarly, several studies reported family-based evidence in genetic overlap of these disorders.4
In vivo magnetic resonance imaging (MRI) studies have provided information regarding the potential overlap and the distinct brain regions that contribute to the neurobiology of these disorders. For instance, there is some evidence that gray matter structural alterations within the thalamus13 and medial prefrontal cortex14 may be common to both disorders. Other data, however, suggest that frontotemporal cortical abnormalities may be unique to SCZ,15 whereas BD may be characterized by disturbances in regions responsible for emotional processing, including the orbital–frontal16 cortex. Along these lines, a meta-analysis of volumetric brain-imaging studies17 reported that compared with patients with SCZ, patients with BD had larger amygdala volumes, thus supporting the hypothesis that the neurobiology of BD may be characterized by abnormalities in regions critical to emotional processing.
Functional MRI (fMRI) has provided additional insights into the overlapping and distinct patterns of brain connectivity that contribute to these disorders. In a recent review, Whalley and colleagues18 reported that among 21 studies, there was evidence for overactivation in the medial temporal lobe in patients with BD compared with patients with SCZ on emotion or memory tasks. Using functional network analysis to assess differential connectivity in resting-state networks in patients with SCZ and those with psychotic BD and healthy subjects, Meda et al19 found unique patterns of connectivity specific to SCZ (meso/paralimbic to sensory–motor) and BD (meso/paralimbic to frontotemporal/paralimbic). Chai et al20 reported that resting-state activities between the medial prefrontal cortex and insula and between the medial prefrontal cortex and ventrolateral prefrontal cortex were positively correlated in BD, whereas patients with SCZ did not demonstrate any resting-state associations between these regions.
Functional connectivity measures are being used in creasingly to distinguish patients with psychiatric disorders by focusing on deficits in global and local connectivity.21 This approach has been utilized most often in studies of SCZ.22–26 For instance, van den Heuvel et al27 reported evidence that patients with SCZ demonstrate less well-integrated global connectivity such that the frontal hubs play a less central role. Similarly, Cole et al28 reported that global disconnectivity may contribute to prefrontal cortical abnormalities in patients with SCZ. Moreover, Venkataraman et al29 reported that less parietotemporal connectivity correlated with positive symptoms in SCZ, whereas greater connectivity in the frontoparietal circuit was correlated with worse negative symptoms, suggesting that different brain circuits may mediate aspects of phenomenology. In contrast, functional connectivity studies in BD have not emphasized the investigation of global connectivity patterns.
We examined patterns of connectivity using resting-state fMRI across the entire brain in 266 predefined regions of interest (ROIs), encompassing cortical and subcortical regions, to investigate similarities and differences in global and regional connectivities in patients with SCZ and BD and in healthy controls (HCs). We also examined the functional correlates of these connectivity patterns. We hypothesized that patients with SCZ would be characterized by the greatest pattern of global disconnectivity across the entire brain and that patients with BD would demonstrate a pattern of disconnectivity intermediate to that in HCs and patients with SCZ.
Methods
Subjects
The study included 19 patients with BD, 18 SCZ patients, and 32 HCs (see table 1). All subjects provided written informed consent and the protocol was approved by the Institutional Review Board of the North Shore–Long Island Jewish Health System. Patients and HCs were assessed using the Structured Clinical Interview for DSM-IV2 Axis I Disorders (SCID). Patient diagnoses were determined through consensus among 3 senior clinicians and confidence in the accuracy of the agreed-upon diagnosis was assigned on a scale of 1–4 (4 denotes highest confidence). Patients with diagnoses assigned a high confidence (3 or 4) were included in the current study. In addition, HCs had no history of an Axis I disorder, as determined from the nonpatient edition SCID-NP. Most BD patients were of the psychotic subtype (16 of 19 had a history of psychosis). The average disease duration was 20.80±9.67 years in SCZ and 14.76±9.43 years in BD (in BD, disease duration information was available for only 9 patients). Subjects were also evaluated with the Brief Psychiatric Rating Scale (BPRS) and the Hamilton Rating Scale for Depression (HRSD; 17-item scale). In addition, BD patients were assessed using the Clinician-Administered Rating Scale for Mania (CARS-M). Average symptom scores for the SCZ and BD groups for these scales and the medication information are provided in table 1. None of the HCs was receiving psychotropic medication. All patients were administered the MATRICS Consensus Cognitive Battery (MCCB).30,31 Further details regarding this battery are provided in the supplementary data.
Table 1.
Sample Characteristics and Psychotropic Medications at the Time of Scan
| N (M/F) | Age ± SD (Age of Onset ± SD) | BPRS-18 | HRSD-17 | CARS-M | Antipsychotics (Chlorpromazine Equivalent) | GABA Agonists | Benztropine | Mood Stabilizers | Lithium |
|---|---|---|---|---|---|---|---|---|---|
| HC: 32 (20/12) | 43.6±8.2 | — | — | — | — | — | — | — | — |
| BD: 19 (11/8) | 40.6±13.3 (24.1±9.4) | 25.7±5.0 | 5.6±4.4 | 5.25±6.50 | 11/19 (228±275mg/d) | 5/19 | 1/19 | 4/19 | 4/19 |
| SCZ: 18 (13/5) | 45.0±10.9 (25.3±6.1) | 31.3±5.4 | 7.9±4.4 | — | 17/18 (664±535mg/d) | 3/18 | 7/18 | 5/18 | 0/18 |
Note: BPRS, Brief Psychiatric Rating Scale; HRSD, the Hamilton Rating Scale for Depression (17-item scale); CARS-M, Clinician-Administered Rating Scale for Mania; GABA, gamma amino butyric acid; HC, healthy control; BD, bipolar disorder patients; SCZ, schizophrenia patients. Antipsychotics included haloperidol, fluphenazine, asenapine, risperidone, quetiapine, olanzapine, ziprasidone, aripiprazole, clozapine. GABA agonists included clonazepam, alprazolam, lorazepam, zolpidem. Mood stabilizers included valproate, carbamazepine, lamotrigine, topiramate.
Resting-State fMRI Image Acquisition
MRI examinations were conducted on a 3T scanner (GE Signa HDx). For image registration, we acquired anatomical scans in the coronal plane using an inversion-recovery-prepared 3-dimensional (3D) fast spoiled gradient (IR-FSPGR) sequence (repetition time [TR] = 7.5ms, echo time [TE] = 3ms, time to inversion [TI] = 650 ms, matrix = 256 × 256, field of view [FOV] = 240mm), producing 216 contiguous images (slice thickness = 1 mm) through the whole brain. We also acquired resting-state functional scans comprising a total of 150 echo-planar imaging (EPI) volumes with the following parameters: TR = 2000ms, TE = 30ms, matrix = 64 × 64, FOV = 240mm, slice thickness = 3mm, 40 continuous axial oblique slices (1 voxel = 3.75 × 3.75 × 3.00 mm). During these image acquisitions, the subjects were asked to close their eyes and instructed “not to think of anything in particular.”
Image Analysis and Preprocessing
We used FSL (http://www.fmrib.ox.ac.uk)/AFNI(http://afni.nimh.nih.gov/afni)-based script libraries from the 1000 Functional Connectomes Project (http://www.nitrc.org/projects/fcon_1000)32 for preprocessing and a laboratory-developed script in the R statistical language for additional analysis. Resting-state scans were preprocessed using the scripts from the 1000 Functional Connectomes Project (“fcon scripts”). Standard preprocessing included removal of the first 4 “dummy” scans, motion correction, and spatial smoothing (6-mm full width at half-maximum Gaussian kernel). This was followed by standard registration and normalization to MNI152 space, with the resulting transformation then applied to each individual’s functional data set (12 parameter affine transformation). The resulting time series were then filtered using high- and low-pass filters (cutoff frequencies were 0.05 and 0.1 Hz, respectively). Each individual’s 4D time series data were regressed on 8 predictors: white matter (WM), cerebrospinal fluid, and 6 motion parameters. We did not regress out the global signal because it would have shifted the correlation distribution to the middle and interfered with the connectivity strength calculation described below. Given the recent concerns raised by Power et al33 regarding the effects of small motion on functional connectivity measures, we conducted careful motion analysis of our scans (supplementary data and supplementary figures 1–4).
Regional Connectivity Strength
We computed regional mean time series in the resting-state fMRI data by using a set of predefined regions.34 In their functional network study, Power et al demonstrated that these regions (n = 264, diameter: 10mm) are not only functionally relevant but can eliminate artificial short-distance correlations. In addition to these regions, we also added caudate nucleus ROIs bilaterally. After obtaining 266 time series per subject, we decomposed these signals with maximal overlap discrete wavelet transform35 similar to the method of Lynall et al.26 We used the Daubechies wavelet transform filter of length 4 and used the 0.060- to 0.125-Hz scale wavelet coefficients for further analysis (due to the preprocessing cutoff frequency at 0.1 Hz, in our case, this covers the range of 0.06–0.1 Hz). We used the R wavelet package to implement these calculations.36 We then estimated the correlation of these wavelet-transformed signals (wavelet coefficients) between each possible pair of regions. For each region i, we then averaged the correlation coefficients that the i region had with all the other j regions (ie, we averaged each row of the correlation matrix), and according to its usual name in the connectivity literature, we named this metric “connectivity strength” (CS). Indeed, this reflects how strongly one region is connected to other regions and what is often referred to as ROI-based connectivity strength.
We also used complementary voxel-wise analyses to compare these results with the ROI-based approach. Using this approach, we computed correlations and connectivity strength in all 181144 voxels restricted to a gray matter mask (FSL MNI atlas: MNI-maxprob-thr25-2mm.nii.gz) in contrast to computing and averaging correlations in only 266 regions. We thus obtained a complete map of connectivity strength in each individual.37 Further description of the ROI-based analyses and voxel-based connectivity strength/correlation profiles is provided in the supplementary data.
Statistical Analysis
All ROI analyses were conducted using SAS (version 9.1) and R (version 2.15.1) programs. We used repeated-measures analysis of variance (ANOVA) with a general linear model (ie, PROC GLM procedure with the REPEATED statement). The between-subjects factor was group (patients with SCZ, patients with BD, and HCs). The within-subjects factor was region. Post hoc tests were conducted using the SLICE option within the LSMEANS (least-squares means) statement in SAS. Raw P values obtained from the LSMEANS output were adjusted for multiple testing using the ‘multtest’ package in R. Because one of the main post hoc analyses involved comparing the 3 groups for 266 regions, multiple testing adjustments were done using the Benjamini-Hochberg (BH) method controlling for the false discovery rate (FDR). In the exploratory voxel-wise analysis, we used the SPM5 package (http://www.fil.ion.ucl.ac.uk/spm/) to compare connectivity strength maps among groups (P < .001 and P < .01, uncorrected), using all possible contrasts (supplementary table 1).
We also used principal component analysis (PCA) to evaluate how much the connectivity strength values vary among regions across individuals by extracting out the first principal component (PC; accounting for the majority of variance). We thus conceptualized the extent to which different ROIs would span a 266-dimensional space and, with PCA, could find the subspace(s) responsible for a majority of variance. This subspace also would reveal how much the different ROIs “move together” in different individuals. Connectivity strength was then investigated in relationship to the clinical and cognitive measures using Spearman Rank-order correlations, with P < .05. We also used cluster analysis to determine whether the “correlation profile” of a particular area (the correlation coefficients between a particular area and all the other areas) would be able to differentiate among groups of subjects.
Results
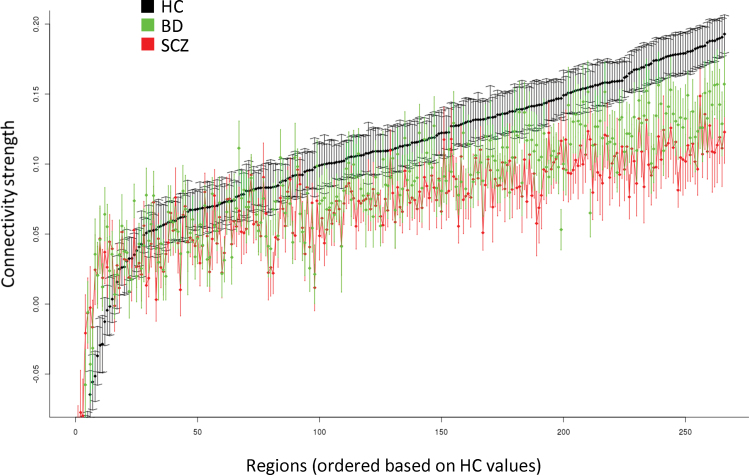
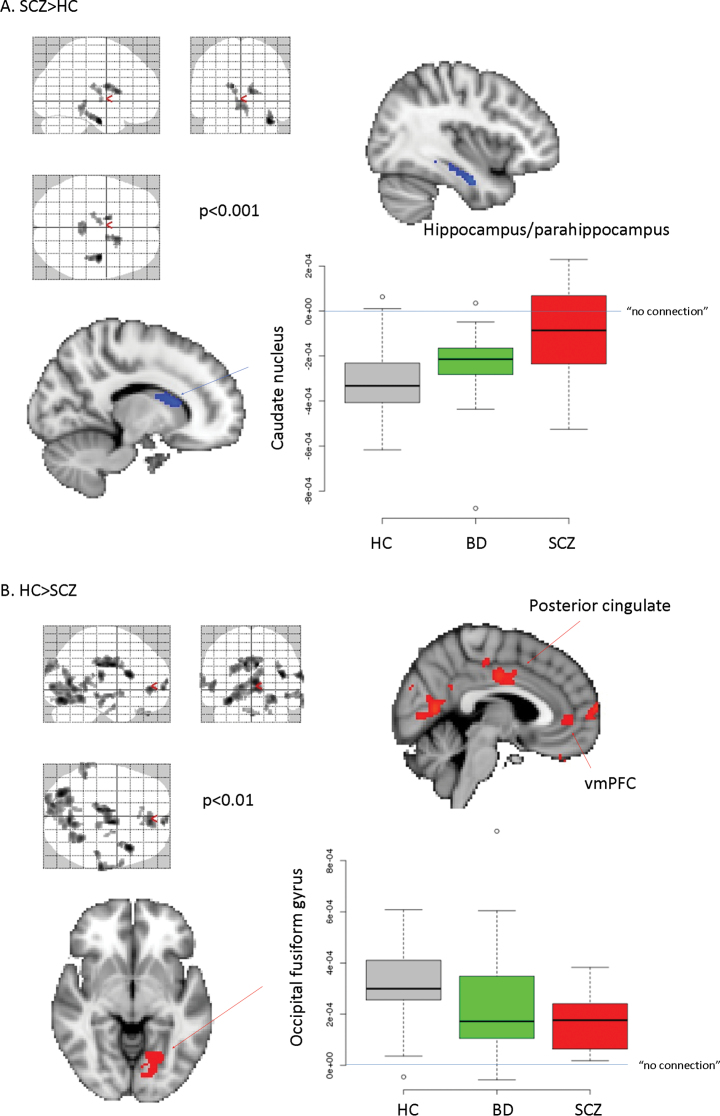
There were no significant differences among groups in distributions of age and sex (table 1). Connectivity strength across the 266 brain regions for each group in the ROI analysis is provided in figure 1. There was a significant main effect of group (F = 237.94, degrees of freedom [df] = 2, P < .0001); patients with SCZ had greater disconnectivity overall compared to HCs (P < .0001), and patients with BD had connectivity values intermediate to and significantly different from those of patients with SCZ (P < .0001) and HCs (P < .0001). The group-by-region interaction was statistically significant (F = 1.16, df = 530, P = .008) with post hoc analyses revealing that 6 of 266 unique regions survived FDR correction for multiple comparisons (BH-adjusted P value < .05) including the right (2 regions) and left thalamus, paracingulate gyrus, left caudate nucleus, and temporal occipital fusiform cortex/lingual gyrus. Subsequent analyses indicated that compared with HCs, patients with SCZ and patients with BD demonstrated significantly greater (P < .05) disconnectivity in the paracingulate gyrus and both right thalamic regions. In addition, patients with SCZ demonstrated significantly greater disconnectivity in the temporal occipital fusiform cortex, left caudate nucleus, and left thalamus compared with the HCs. No significant differences were evident between patients with BD compared with patients with SCZ in any of these 6 regions. We did not find evidence for any systematic effect of motion on the observed findings (supplementary data and supplementary figures 1–4).
Fig. 1.
Connectivity strength differences across the 266 regions of interest.
Complementary voxel-wise methods were largely consistent with the ROI approach (figure 2 and supplementary table 1). Specifically, we found that patients with SCZ had lower global connectivity in the occipital fusiform, cingulate gyrus, and cuneus. We also found differences with the opposite sign in the caudate nucleus, hippocampal/parahippocampal gyrus, thalamus, and brainstem; however, post hoc analysis indicated that in all cases, HCs had negative correlations in these latter regions, so that increased values in SCZ were closer to 0 and, thus, should be interpreted as greater disconnectivity. As evident from supplementary table 1, these regions were also identified when comparing BD patients and HCs, albeit with lower statistical strength (and mostly at an exploratory level). Findings in BD are consistent, however, with the ROI-based approach, viz, with the intermediate level of global connectivity between HCs and SCZ patients that BD patients express (see figure 2).
Fig. 2.
Voxel-wise connectivity strength.
Note: When we compared HCs and patients (either SCZ or BD), we found areas where connectivity strength was higher in patients (supplementary table 1), but post hoc analysis in these cases showed that the connectivity strength was closer to 0 (ie, less negative). In a similar manner, the opposite contrasts also revealed areas where patients’ connectivity strength was closer to 0, in very good agreement with the ROI-based calculations and figure 1. Open circles: outliers; data points outside of the quartiles ±1.5 x interquartile range (standard in boxplot with R).
PCA revealed that different brain areas had high covariance in connectivity strength (supplementary figure 5). The first PC, which has the most variance in PCA, was able to significantly differentiate between SCZ and HC (F = 3.46, df = 66, P = .037; supplementary figure 5A) and accounted for 49% of the variance. Regions that accounted for most of the variance (regions with the highest loading in the PCA analysis) included parietotemporal, medial–frontal cortex (midline), insula, and caudate nucleus bilaterally (supplementary figure 5B). This also indicates that the individual scores of the first PC account for general connectivity. The sign of PC #1 is arbitrary; in this case, more negative values represent more “normal” connectivity, whereas more positive ones indicate disconnectivity (consistent with supplementary figure 5A).
At the time of the scan, we found significantly lower antipsychotic use in BD patients compared with the SCZ patients (t = −3.09, df = 25.1, P = .005) and lower use of benztropine in the BD group than in the SCZ group (Fisher’s exact test, P = .02). As expected, lithium was used only in BD patients. We examined differences in psychotropic medication usage between high- and low-connectivity groups (table 2) but did not identify any differences. We also used divided groups based on medication status to evaluate differences in global connectivity among groups (supplementary figure 6), but no such differences were evident.
Table 2.
Medication Status for Low- and High-Connectivity Groups
| Class | High (n = 18) vs Low (n = 18) Connectivity | P Value, Fisher’s Exact Test |
|---|---|---|
| Antipsychotic | 14/18 vs 15/18 | 1.0 |
| Benzodiazepines | 2/18 vs 5/18 | .4 |
| Benztropine | 4/18 vs 4/18 | 1.0 |
| Lithium | 2/18 vs 2/18 | 1.0 |
| Antiepileptic drugs | 5/18 vs 3/18 | .7 |
Note: All patients were divided into high and low global connectivity levels to determine whether medication status influenced the results. For example, in the case of antipsychotics, 14 out of 18 patients with high-connectivity values vs 15 out of 18 patients with low-connectivity values used antipsychotics. An inverse test was also performed, where we compared global connectivity among different medication groups (see supplementary data).
Cognitive and Clinical Correlates
MCCB raw data are presented in supplementary table 2. In all cases, BD patients had significantly better neurocognitive performance compared with SCZ patients. Investigation of correlations between global connectivity (ie, PC #1 scores) and neurocognitive tasks indicated that higher PC scores (ie, greater disconnectivity) were associated with worse cognitive performance across both patient groups (table 3); however, these effects were not statistically significant in either patient group when investigated separately. Seventeen of the 19 BD and 15 of the 18 SCZ patients completed BPRS assessments. BPRS scores were significantly higher in SCZ patients compared with BD patients (t = −3.05, df = 28.84, P = .005). BPRS scores correlated significantly with global connectivity (r = .37, df = 30, P = .035) such that lower connectivity was associated with higher BPRS scores. Subgroup analysis revealed that this relationship was, however, not significant among the SCZ or BD groups when examined separately. In addition, other measures (ie, HRSD, CARS-M) more sensitive to the mood and/or manic aspects of BD did not correlate significantly with connectivity strength.
Table 3.
Cognitive Measures
| Cognitive Tests | BD vs SCZ (P Values) | P (PAT) | R (PAT) | Post Hoc | ||
|---|---|---|---|---|---|---|
| r (BD) | r (SCZ) | P (BD vs SCZ) | ||||
| MCCB (N = 30; 15 BD/15 SCZ) | ||||||
| Speed processing T score | .28 | .29 | −.20 | — | — | — |
| Attention vigilance T score | .73 | .74 | −.06 | — | — | — |
| Working memory T score | .57 | .39 | −.16 | — | — | — |
| Verbal learning T score | .11 | .07 | −.34 | −.50 | −.122 | .30 |
| Visual learning T score | .49 | .46 | −.14 | — | — | — |
| Reasoning and problem solving T score | .98 | .67 | .07 | — | — | — |
| Social cognition T score | .007* | .16 | −.26 | −.43 | .05 | .21 |
| Overall composite T score | .19 | .27 | −.21 | — | — | — |
| Verbal learning | ||||||
| HVLT (N = 32; 17 BD/15 SCZ) | ||||||
| Correct words | .02* | .01* | −.45 | −.61 | −.15 | .16 |
| Delayed | .005* | .02* | −.43 | −.54 | −.26 | .40 |
| Recognition | .005* | .08 | −.33 | −.50 | −.01 | .18 |
| Total T score | .05* | .18 | −.25 | −.29 | −.11 | .65 |
| Working memory | ||||||
| WMS-III (N = 32; 17 BD/15 SCZ) | ||||||
| Raw | .17 | .02* | −.40 | −.58 | −.14 | .18 |
| T score | .26 | .12 | −.28 | — | — | — |
| Letter–number span (N = 32; 17 BD/15 SCZ) | ||||||
| Raw | .73 | .56 | −.11 | — | — | — |
| T score | .80 | .68 | −.08 | — | — | — |
| Speed of processing | ||||||
| Trail making test A (N = 32; 17 BD/15 SCZ) | ||||||
| Trail A error | .36 | .21 | .22 | |||
| Trail A time (s) | .53 | .002* | .51 | .43 | .56 | .65 |
| Trail A T score | .74 | .04* | −.36 | −.38 | −.39 | .97 |
| BACS (N = 32; 17 BD/15 SCZ) | ||||||
| Raw | .15 | .13 | −.27 | |||
| T score | .36 | .24 | −.21 | |||
| Category fluency (N = 32; 17 BD/15 SCZ) | ||||||
| Raw | .01* | .38 | −.16 | −.04 | −.06 | .97 |
| T score | .02* | .55 | −.11 | −.03 | .03 | .90 |
| Attention task (not part of MCCB) | ||||||
| Attention network (N = 31; 18 BD/13 SCZ) | ||||||
| Alerting effect (ms) | .69 | .02* | .40 | .26 | .57 | .35 |
| Orienting effect (ms) | .91 | .69 | .07 | — | — | — |
| Conflict effect (ms) | .07 | .009* | .46 | .56 | .25 | .35 |
| Mean RT correct trials (ms) | .18 | .15 | .26 | — | — | — |
| Mean accuracy (%) | .18 | .18 | −.24 | — | — | — |
Note: BD, bipolar disorder; SCZ, schizophrenia; PAT, all patients together (BD and SCZ); MCCB, MATRICS Consensus Cognitive Battery; HVLT, Hopkins Verbal Learning Test; WMS-III, Wechsler Memory Scale—3rd edition; BACS, Brief Assessment of Cognition in Schizophrenia; RT, reaction time.
*P < .05. For all significant group differences, patients with BD performed better than patients with SCZ.
Analyses between individual regional connectivity and the clinical and neuropsychological measures are provided in supplementary table 4. These analyses indicated that greater disconnectivity in the caudate nucleus was associated with higher BPRS scores among all patients. Moreover, among all patients, greater disconnectivity in the left caudate nucleus, temporal occipital fusiform cortex/lingual gyrus, and left thalamus was associated with worse functioning on the Trailmaking Test–Part A.
Cluster Analysis
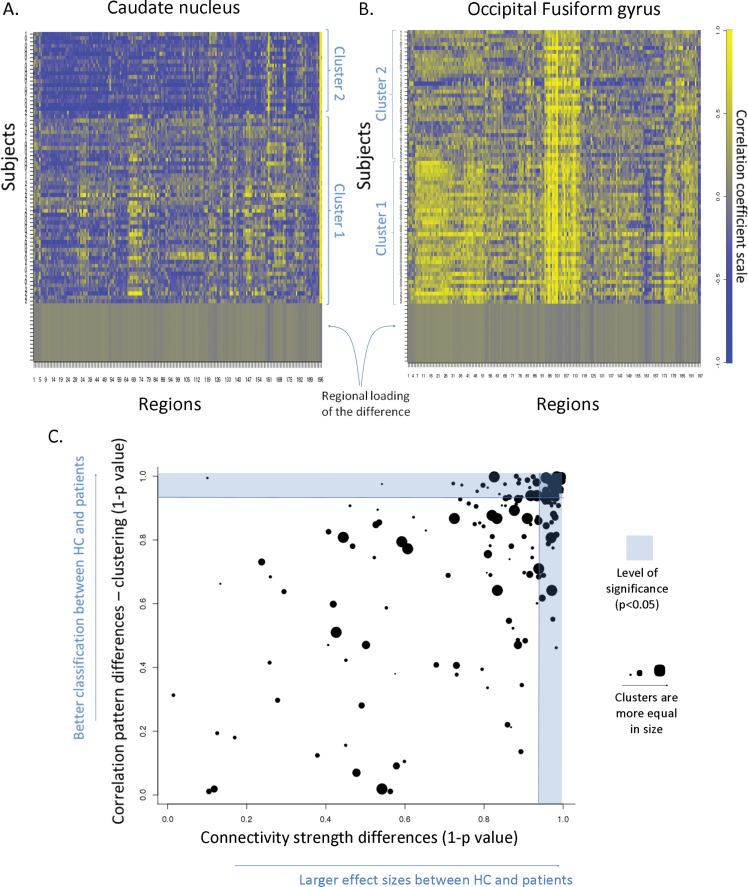
The cluster analysis indicated that regions showing group differences in their average correlation (ie, connectivity strength) were the ones that were the most useful in differentiating between patients and HCs. This data-driven approach did not differentiate between BD and SCZ, but it did distinguish between all patients in relation to HCs based on their “correlation profile” (figure 3, correlation between 2 sample t test and chi-square P values: r = .61, t = 10.64, df = 196, P < .0001; supplementary table 3).
Fig. 3.
Cluster analysis of correlation patterns.
Note: In A and B, each row corresponds to an individual and each column to a region. The color-coded values of the rows represent how well that particular region correlates with the caudate nucleus and fusiform gyrus, respectively (s upplementary table 3), which were chosen for illustration given that they demonstrated the most robust group differences in connectivity. The rows of the matrix (ie, the individuals) are already ordered according to the result of the cluster analysis. In C, the horizontal axis represents the strength that the connectivity of an area can separate HCs and patients (t test), while the vertical axis represents the statistical strength that the cluster analysis (chi-square) yields in a certain area.
Discussion
We used resting-state fMRI and connectivity measures, similar to those reported earlier in SCZ,24–26 to evaluate the disconnectivity hypothesis of SCZ38–40 and its overlap with BD. The main findings of our study indicate that patients with SCZ had lower global connectivity compared with HCs and that patients with BD had global connectivity values that were intermediate to and significantly different from SCZ patients and HCs (figure 1). Although each patient group could be distinguished from HCs in specific regions, no individual regions distinguished the patient groups. Moreover, the use of an independent voxel-based approach yielded similar results (figure 2), further supporting our findings.
It is difficult to compare our results to prior work in BD given the paucity of studies; however, our results are consistent with prior work in SCZ22,24–26,41 indicating that patients had a profound and general decrease of connectivity (figure 1). Similarly, the use of cluster analysis could not distinguish between SCZ and BD reliably, although it could differentiate between HCs and all patients (figure 3). Recently, numerous genetic and epidemiologic findings have questioned the strict dichotomy of these diseases3–12,42–46 and support the notion that they may be conceptualized as a continuum. Our findings could serve as a neurobiological framework for better clarifying this continuum and may provide some explanation regarding why SCZ typically presents with more severe cognitive symptoms and worse prognosis. Moreover, our results may provide insight into the current debate regarding the nosology of BD and SCZ and could potentially serve as an imaging biomarker in the Research Domain Criteria.47,48
Investigation of the MCCB domains30,49–51 indicated that “disconnectivity” was associated with cognitive impairment. Specifically, higher PC scores (ie, greater disconnectivity) were associated with worse cognitive performance and higher BPRS scores across both patient groups, but these relationships were not significant in either patient group. Overall, our results suggest that verbal learning, speed of processing, and attention tasks were affected by the impaired global connectivity. These domains are among the most affected cognitive functions in SCZ and BD.52–58 It is important to note, however, that all correlations were consistent with the hypothesis that more impaired connectivity was associated with worse functioning, suggesting that intact connectivity is a prerequisite of normal cognitive functioning. These findings are also comparable with our previous study demonstrating an inverse relationship between WM integrity and cognitive functions59 and with other functional connectivity findings.26
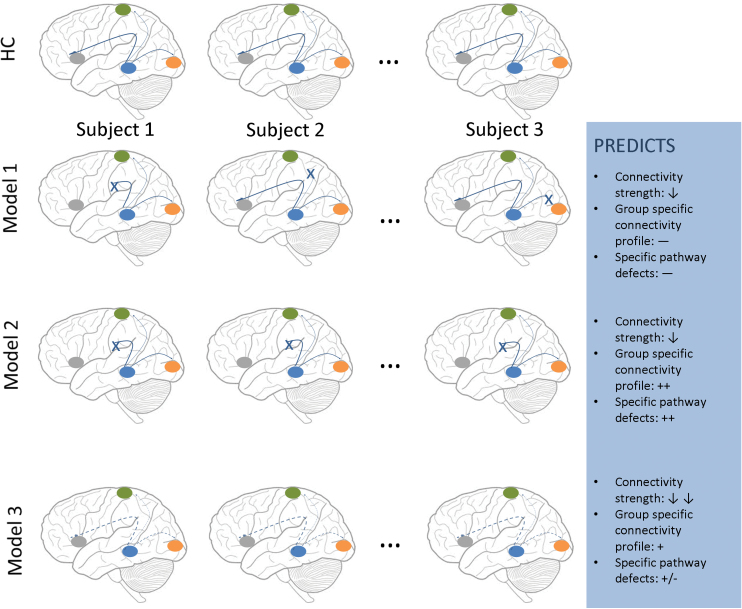
One of the overarching questions in the current connectivity literature of psychiatric illness is how to conceptualize these different disorders (figure 4). Our results are broadly consistent with model 3, where the decrease in connectivity would be predicted to “move together” across regions. This does not completely exclude combinations with model 1 or 2, but the well-characterized decrease in connectivity among multiple areas (model 3) would make our method insensitive to these (model 1 or 2) other changes. One of the implications of model 3 is that we can meaningfully describe a single individual with a generalized connectivity number, which would describe the overall change in connectivity across regions. Our results thus suggest that the first PC may be a good choice for accomplishing this aim and could be examined in relationship with cognition and clinical symptoms. We acknowledge that a further possibility (not illustrated) is that there could potentially be some degree of global reduction in connectivity with superimposed “disease” specific abnormalities in certain pathways.
Fig. 4.
Models to conceptualize disconnectivity.
There are several limitations to this study. The patient sample sizes were small and the patients were receiving antipsychotic medication, which could affect functional connectivity.60 Moreover, antipsychotic use may be related to WM abnormalities,61–64 which could conceivably lead to functional connectivity changes.23 It should also be acknowledged that patients with BD were being treated with a lower antipsychotic dosage, which may have influenced global connectivity. We do not believe this is the case, however, because other studies in first-episode SCZ patients23,25 found similar decreases in connectivity, and we did not detect significant antipsychotic medication effects in our study (table 2; supplementary figure 6). Although functional connectivity studies in high-risk and ultra-high-risk individuals have been somewhat contradictory, structural studies typically revealed decreased connectivity among preselected brain regions.23 Due to the small number of patients, the negative finding of the direct comparison between SCZ and BD patients at the ROI level should be interpreted with caution. It is conceivable that our methodology may have been less sensitive to detecting patient group differences at the ROI level, where they may be more subtle compared with the gross global connectivity measures. Finally, we acknowledge the lack of information regarding gray matter content within these specific ROIs, which could potentially further inform the functional connectivity analyses.
In summary, our results further support the disconnectivity hypothesis in SCZ and extend this to BD. These findings and their strong correlations with clinical and cognitive measures could make global connectivity measures ideal candidates for use as biomarkers in future imaging studies.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
Brain and Behavior Research Foundation (P.R.S.) and the National Institute of Mental Health (R01 MH076995 to P.S., R01 MH079800 to A.M.); the North Shore–Long Island Jewish Research Institute General Clinical Research Center (M01 RR018535); the Advanced Center for Intervention and Services Research (P30 MH090590); the Center for Intervention Development and Applied Research (P50 MH080173).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kraepelin E, Defendorf AR. Clinical Psychiatry: A Text-Book for Students and Physicians [abstracted and adapted from the 6th German ed. of Kraepelin’s “Lehrbuch der psychiatric”]. Norwood, MA: The Macmillan Company; 1904. [Google Scholar]
- 2. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR® Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 3. Herrell R, Henter ID, Mojtabai R, et al. First psychiatric hospitalizations in the US military: the National Collaborative Study of Early Psychosis and Suicide (NCSEPS). Psychol Med. 2006;36:1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maeda K, Nwulia E, Chang J, et al. Differential expression of disrupted-in-schizophrenia (DISC1) in bipolar disorder. Biol Psychiatry. 2006;60:929–935 [DOI] [PubMed] [Google Scholar]
- 7. Georgieva L, Dimitrova A, Ivanov D, et al. Support for neuregulin 1 as a susceptibility gene for bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:419–427 [DOI] [PubMed] [Google Scholar]
- 8. Prata DP, Breen G, Osborne S, Munro J, St Clair D, Collier DA. An association study of the neuregulin 1 gene, bipolar affective disorder and psychosis. Psychiatr Genet. 2009;19:113–116 [DOI] [PubMed] [Google Scholar]
- 9. Shifman S, Bronstein M, Sternfeld M, et al. COMT: a common susceptibility gene in bipolar disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;128B:61–64 [DOI] [PubMed] [Google Scholar]
- 10. Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry. 2006;60:106–114 [DOI] [PubMed] [Google Scholar]
- 11. Ripke S, Sanders AR, Kendler KS, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sklar P, Ripke S, Scott LJ, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McIntosh AM, Job DE, Moorhead TW, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–552 [DOI] [PubMed] [Google Scholar]
- 14. Janssen J, Reig S, Parellada M, et al. Regional gray matter volume deficits in adolescents with first-episode psychosis. J Am Acad Child Adolesc Psychiatry. 2008;47:1311–1320 [DOI] [PubMed] [Google Scholar]
- 15. McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377 [DOI] [PubMed] [Google Scholar]
- 16. Stanfield AC, Moorhead TW, Job DE, et al. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11:135–144 [DOI] [PubMed] [Google Scholar]
- 17. Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201 [DOI] [PubMed] [Google Scholar]
- 18. Whalley HC, Papmeyer M, Sprooten E, Lawrie SM, Sussmann JE, McIntosh AM. Review of functional magnetic resonance imaging studies comparing bipolar disorder and schizophrenia. Bipolar Disord. 2012;14:411–431 [DOI] [PubMed] [Google Scholar]
- 19. Meda SA, Gill A, Stevens MC, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van den Berg D, Gong P, Breakspear M, van Leeuwen C. Fragmentation: loss of global coherence or breakdown of modularity in functional brain architecture? Front Syst Neurosci. 2012;6:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314 [DOI] [PubMed] [Google Scholar]
- 23. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124 [DOI] [PubMed] [Google Scholar]
- 24. Alexander-Bloch AF, Gogtay N, Meunier D, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Venkataraman A, Whitford TJ, Westin CF, Golland P, Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res. 2012;139:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72:1–3 [DOI] [PubMed] [Google Scholar]
- 31. Burdick KE, Goldberg TE, Cornblatt BA, et al. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology. 2011;36:1587–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Percival D, Walden A. Wavelet Methods for Time Series Analysis (Cambridge Series in Statistical and Probabilistic Mathematics). New York, NY: Cambridge University Press; 2000. [Google Scholar]
- 36. Aldrich E Wavelets: A Package of Functions for Computing Wavelet Filters, Wavelet Transforms and Multiresolution Analyses. 2010. http://cran.r-project.org/web/packages/wavelets/index.html Accessed April 10, 2012. [Google Scholar]
- 37. Perrin JS, Merz S, Bennett DM, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125 [DOI] [PubMed] [Google Scholar]
- 39. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beaumont JG, Dimond SJ. Brain disconnection and schizophrenia. Br J Psychiatry. 1973;123:661–662 [DOI] [PubMed] [Google Scholar]
- 41. Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961 [DOI] [PubMed] [Google Scholar]
- 42. Möller H-J. Bipolar disorder and schizophrenia: distinct illnesses or a continuum? J Clin Psychiatry. 2003;64(suppl 6):23–27; discussion 28. [PubMed] [Google Scholar]
- 43. Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep. 2001;3:332–337 [DOI] [PubMed] [Google Scholar]
- 44. Laursen TM, Agerbo E, Pedersen CB. Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. J Clin Psychiatry. 2009;70:1432–1438 [DOI] [PubMed] [Google Scholar]
- 45. McIntosh AM, Job DE, Moorhead TW, Harrison LK, Lawrie SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58:254–257 [DOI] [PubMed] [Google Scholar]
- 46. McDonald C, Bullmore ET, Sham PC, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984 [DOI] [PubMed] [Google Scholar]
- 47. Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751 [DOI] [PubMed] [Google Scholar]
- 49. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213 [DOI] [PubMed] [Google Scholar]
- 50. Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220 [DOI] [PubMed] [Google Scholar]
- 51. Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39 [DOI] [PubMed] [Google Scholar]
- 52. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuro psychology. 1998;12:426–445 [DOI] [PubMed] [Google Scholar]
- 53. Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–569 [DOI] [PubMed] [Google Scholar]
- 54. Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12 [PubMed] [Google Scholar]
- 55. Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19:365–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hughes C, Kumari V, Soni W, et al. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res. 2003;59:137–146 [DOI] [PubMed] [Google Scholar]
- 57. Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149 [DOI] [PubMed] [Google Scholar]
- 58. Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103–116 [DOI] [PubMed] [Google Scholar]
- 59. Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984 [DOI] [PubMed] [Google Scholar]
- 60. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792 [DOI] [PubMed] [Google Scholar]
- 61. Friedman JI, Tang C, Carpenter D, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032 [DOI] [PubMed] [Google Scholar]
- 62. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Girgis RR, Diwadkar VA, Nutche JJ, Sweeney JA, Keshavan MS, Hardan AY. Risperidone in first-episode psychosis: a longitudinal, exploratory voxel-based morphometric study. Schizophr Res. 2006;82:89–94 [DOI] [PubMed] [Google Scholar]
- 64. Molina V, Reig S, Sanz J, et al. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.