Abstract
Background: The inflammatory hypothesis of schizophrenia is not new, but recently it has regained interest because more data suggest a role of the immune system in the pathogenesis of schizophrenia. If increased inflammation of the brain contributes to the symptoms of schizophrenia, reduction of the inflammatory status could improve the clinical picture. Lately, several trials have been conducted investigating the potential of anti-inflammatory agents to improve symptoms of schizophrenia. This study provides an update regarding the efficacy of anti-inflammatory agents on schizophrenic symptoms in clinical studies performed so far. Methods: An electronic search was performed using PubMed, Embase, the National Institutes of Health web site http://www.clinicaltrials.gov, Cochrane Schizophrenia Group entries in PsiTri, and the Cochrane Database of Systematic Reviews. Only randomized, double-blind, placebo-controlled studies that investigated clinical outcome were included. Results: Our search yielded 26 double-blind randomized controlled trials that provided information on the efficacy on symptom severity of the following components: aspirin, celecoxib, davunetide, fatty acids such as eicosapentaenoic acids and docosahexaenoic acids, estrogens, minocycline, and N-acetylcysteine (NAC). Of these components, aspirin (mean weighted effect size [ES]: 0.3, n = 270, 95% CI: 0.06–0.537, I2 = 0), estrogens (ES: 0.51, n = 262, 95% CI: 0.043–0.972, I2 = 69%), and NAC (ES: 0.45, n = 140, 95% CI: 0.112–0.779) showed significant effects. Celecoxib, minocycline, davunetide, and fatty acids showed no significant effect. Conclusion: The results of aspirin addition to antipsychotic treatment seem promising, as does the addition of NAC and estrogens. These 3 agents are all very broadly active substances, and it has to be investigated if the beneficial effects on symptom severity are indeed mediated by their anti-inflammatory aspects.
Key words: add-on antipsychotic therapy, aspirin, N-acetylcysteine, estrogens
Introduction
Forty years ago, Torrey and Peterson1 proposed that inflammatory processes play a key role in the pathophysiology of schizophrenia. Since then, different pieces of evidence have been gathered to suggest that there is an increased proinflammatory status in the brain of patients with schizophrenia. This proinflammatory status is thought to result from the interaction between genetic vulnerability and environmental factors such as infections, trauma, nutrition, and stress.2 Associated with this increased proinflammatory status is a decrease in the neurotrophic function of microglia and other supportive central nervous system (CNS) cells and enhanced production of neurotoxic proinflammatory factors such as tumor necrosis factor-α (TNF-α), free radicals, complement factors, and kynurenic acid.3,4 This shift leads to decreased proliferation of the neurons, resulting in reduced connectivity and eventually a loss of brain tissue.5 Increased proinflammatory status of the brain also interacts with glutamatergic and dopaminergic neurotransmission, which can induce or aggravate positive, negative, and cognitive symptoms of schizophrenia.6,7
Over the years, evidence has accumulated to support this theory. In recent years, some important data have been added. For example, strongest genetic association with schizophrenia is found in the major histocompatibility complex genes including loci that influence the immune response.8 Peripheral blood markers, such as C-reactive protein, have been linked to cognitive impairment in patients with schizophrenia.9–11 Positron emission tomography studies using ligands that bind to the peripheral benzodiazepine receptor, which is expressed also on astrocytes12,13 but mainly on activated microglia cells, showed increased binding capacity in the brains of patients with early-stage schizophrenia14,15 but not in chronic disease stages.16 Finally, the prevalence of autoimmune disorders, such as multiple sclerosis, type I diabetes, and systemic lupus erythematosis, is increased in patients with schizophrenia and in their first-degree relatives.17–19 The attractiveness of the immune hypothesis lies in the possibility that the shift towards a more proinflammatory status in the brain can potentially be corrected with anti-inflammatory agents. Although it is not yet proven that such agents can induce microglia or other cells to resume their neurotrophic function,5 many anti-inflammatory agents can reduce the production of toxic proinflammatory factors. In a recent meta-analysis on augmentation with nonsteroid anti-inflammatory drugs (NSAIDs), we showed modest but significant symptom improvement in patients with schizophrenia.20 However, a wide variety of components have anti-inflammatory properties and could potentially reinstate the balance between proinflammation and anti-inflammation in patients with schizophrenia. Table 1 lists the main types of medication with anti-inflammatory actions, although this summary is far from complete as many herbal and nutritional components also have anti-inflammatory actions.
Table 1.
Main Types of Medication With Anti-inflammatory Actions
| Anti-inflammatory Components | Crosses BBB | Actions in the Brain |
|---|---|---|
| Antipsychotics | ++ | Dopamine receptor blockade (D2), ↑BDNF18 |
| Aspirin | −/+ | PG↓, TNF-α↓19 |
| Celecoxib | ++ | COX-2↓, PG↓18 |
| Cytostatics | −/+ | Diverse, eg, for MTX: TNF-α↓20 |
| Davunetide | ++ | TNF-α↓18 |
| Estrogens | ++ | TNF-α↓, NO↓21 |
| Fatty acids | ++ | Zinc↑, TNF-α↓, COX-2↓, IL-1↓18 |
| Leptin | ++ | IL-4↓, IL-10↑, IFN-γ↓22 |
| Macrolides/tetracyclines | ++ | IL-1β↓, NO↓23 , 94 |
| Melatonin | ++ | NO↓, IL-1β↓, TNF-α↓, NF-κβ↓18 |
| Minocycline | ++ | Microglia inhibition, TNF-α↓24 |
| Monoclonal antibodies | −/+ | Inhibition of one specific component |
| NAC | ++ | IL-1β↓, TNF-α↓25 , 95 |
| Corticosteroids | −/+ | Inhibition of many steps in innate and specific immune response |
| Transplantation adjuncts | −/+ | IL-1/2/4↓ |
Note: ++ = excellent BBB crossing, +/− = lower CNS concentrations that in peripheral blood. BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; COX, cyclooxygenase; IFN, interferon; IL, interleukin; MTX, methotrexate; NAC, N-acetylcysteine; NF-κβ, nuclear factor-κβ; PG, prostaglandin; TNF, tumor necrosis factor.
In addition, most psychiatric medications such as antipsychotics, lithium, valproate acid, and selective serotonin reuptake inhibitors also possess some anti-inflammatory aspects.26–29 As can be viewed in table 1, most anti-inflammatory components are broadly active, with their anti-inflammatory capacity being just one of their many actions. Some of these components have been given to patients with schizophrenia in an attempt to reinstate the balance between pro- and anti-inflammation in the brain. So far, results of these studies were inconsistent. This quantitative review provides an update of the efficacy of different types of anti-inflammatory agents, given in augmentation to standard treatment, to improve symptom severity in patients with schizophrenia. We include both agents that are considered primary anti-inflammatory agents such as NSAIDs and corticosteroids, but also components that possess additional anti-inflammatory actions, such as N-acetyl cysteine (NAC), estrogens, melatonin, davunetide, and fatty acids. For obvious reasons, we will not include psychiatric medication with anti-inflammatory actions.
Methods
Literature Search
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement.30 An electronic search was performed using PubMed, Embase, the National Institutes of Health web site http://www.clinicaltrials.gov, Cochrane Schizophrenia Group entries in PsiTri, and the Cochrane Database of Systematic Reviews.
No year or language restrictions were applied. The following basic search terms were used: schizophrenia AND the specific pharmacological components: NSAIDs (aspirin, celecoxib, ibuprofen, diclofenac, naproxen), davunetide, EPA and DHA fatty acids, estrogen (and raloxifen and tamoxifen), specific antibiotics (eg, minocycline), NAC and corticosteroids (prednisone, prednisolone, hydrocortisone, methylprednisolone, dexamethasone, cortisone, triamcinolone, betamethasone), transplantation adjuncts (tacrolimus, cyclosporine, everolimus, serolimus, mycophenolate mofetil), cytostatics (bexarotene, bone marrow irradiation/transplantation, methotrexate, cyclophosphamide), and melatonin.
Inclusion
Consensus on the studies included was reached on the basis of the following criteria:
Randomized, double-blind, placebo-controlled studies regarding augmentation of antipsychotic medication with an anti-inflammatory agent listed in table 1.
Patients included had a diagnosis of a schizophrenia spectrum disorders (schizophrenia, schizophreniform disorder, or schizoaffective disorder), according to the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III, DSM-III-R, DSM-IV, and DSM-IV-TR or International Classification of Diseases, 9th or 10th Revision).
Studies reported sufficient information to compute common effect size (ES) statistics of change scores, ie, means and SDs, exact P, t, or z values31,32, or corresponding authors could supply these data upon request. Studies providing only posttreatment data were not included.
Crossover studies were not excluded in order to obtain as much information as possible. Antipsychotic, antidepressant, and mood-stabilizing agents were excluded from the search because their well-known efficacy on symptom severity would confound the results. Studies that were only published as abstracts were included after contacting the authors for more detailed information. One study that was double blind and placebo controlled was excluded because no data were given on baseline parameters, and dosing was not clear.33
Outcome Measures
The primary outcome measure was the mean change in total score on the Positive and Negative Syndrome Scale (PANSS) or the Brief Psychiatric Rating Scale. Data of the last observation carried forward analysis were used when provided. If only data of completers analyses were given, these data were used instead. Two reviewers independently extracted data from the papers, any disagreements were resolved by consensus.
Statistical Analyses
Standardized differences were calculated from the mean differences (placebo vs augmentation) of the change score (end of treatment minus baseline) means and SDs.34 When possible, change scores were used instead of pretreatment and posttreatment scores in order to avoid overestimation of the true ES because of the pre-post treatment correlations. When only exact F or P values for main effect of treatment group (augmentation or placebo) were provided, these data were used. All standardized differences were calculated twice independently from the original articles to check for errors. Standardized differences of studies were pooled in meta-analyses to obtain mean standardized differences for primary outcome measures. Hedges’s g 35 was used to quantify the mean standardized differences of combined studies using a random model. A homogeneity statistic, I 2, was calculated to test whether the studies could be taken together to share a common population ES.36 High heterogeneity (ie, I 2 ≥ 50%) indicates heterogeneity of the individual study ESs, which poses a limitation to a reliable interpretation of the results. Values of I 2 between 30% and 50% were considered moderate. Mean standardized differences with a P value smaller than .05 were considered significant. All standardized differences were computed using Comprehensive Meta-Analysis Version 2.0
Results
Search
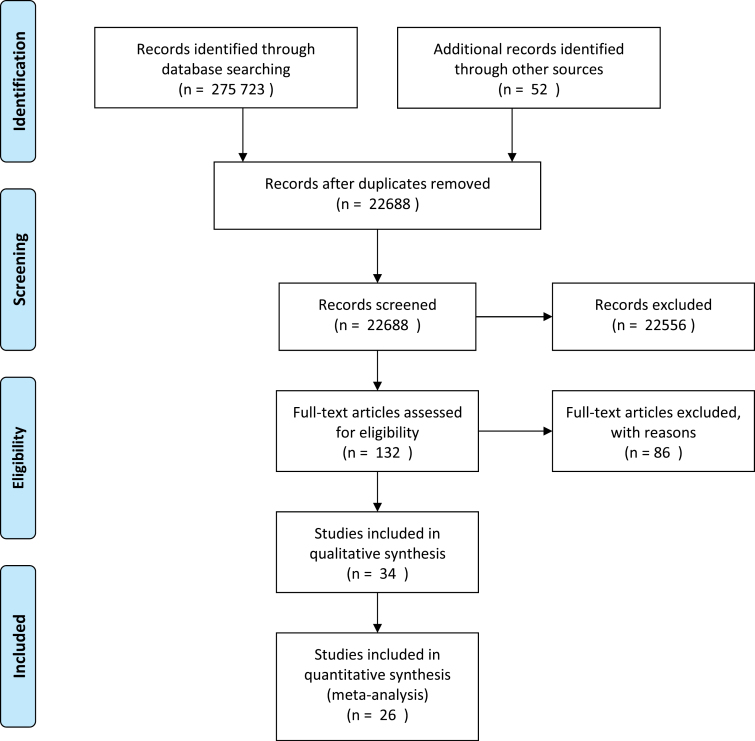
Our search yielded 26 trials that fulfilled all inclusion criteria. These studies provided information on the efficacy on symptom severity of the following components: aspirin, celecoxib, davunetide, fatty acids such as eicosapentaenoic acids (EPA) and docosahexaenoic acids (DHA), estrogens, minocycline, and NAC. The results for each component are provided in figure 1.
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses 2009 flow diagram.
Aspirin
Aspirin is a NSAID that irreversibly inhibits cyclooxygenase-1 (COX-1) and modifies activity of COX-2, thereby suppressing production of thromboxanes and prostaglandins, which are key players in the inflammatory process.37,38 Aspirin also reduces hypothalamic-pituitary-adrenal axis response.39
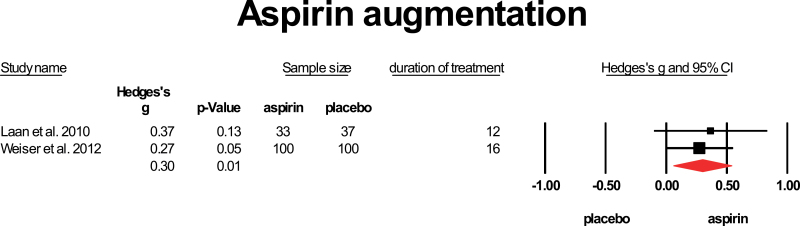
Aspirin does not readily cross the blood-brain barrier (BBB), and levels in the CNS are lower than that in peripheral blood.40 Two studies provided aspirin to patients with schizophrenia in addition to their standard treatment. Laan et al (2010)41 included patients who were ill for less than 10 years. Because the study by Weiser et al is only published as an abstract, no exact data on duration of illness are provided. Both studies applied 1000mg of aspirin daily and treatment duration was 341 and 4 months.42 The mean weighted ES was 0.3, with the 95% CI: 0.06–0.537. Heterogeneity was low (I 2 = 0%) (figure 2).
Fig. 2.
Aspirin augmentation. Black square = individual effect size of a study, diamond symbol= mean weighted effect size.
Celecoxib
Celecoxib, another NSAID, is a selective COX-2 inhibitor, which has few other actions besides its anti-inflammatory and analgesic actions. It blocks COX-2-mediated vascular permeability, thus preventing extravasation of proinflammatory cells, proteins, and enzymes, which mediate the local inflammatory response leading to edema.43 It is a small molecule that easily passes the BBB.44
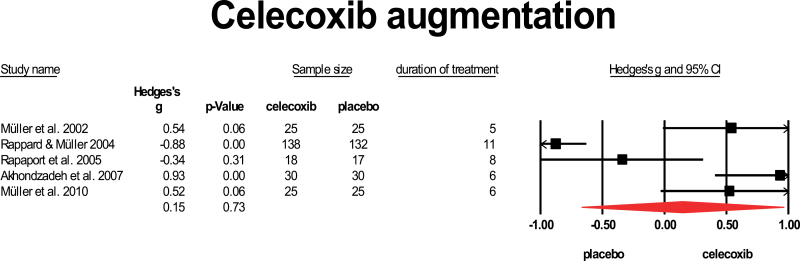
Five studies added celecoxib to standard antipsychotic treatment. Only one of them45 restricted inclusion to first-episode psychosis (FEP) patients. All these studies provided a dose of 400 mg to the patients, and duration of treatment was relatively short, varying from 5 to 11 weeks.46–49 Results were very heterogeneous, ranging from strong negative to strong positive effects. The study by Rappard and Muller (2004)47 was only published as an abstract. The mean weighted ES was 0.15, which is not significant. The 95% CI ranged from −0.669 to 0.959, and heterogeneity was high, I 2 = 93%. The only study in FEP yielded a positive result (figure 3).
Fig. 3.
Celecoxib augmentation. Black square = individual effect size of a study, diamond symbol = mean weighted effect size.
Davunetide
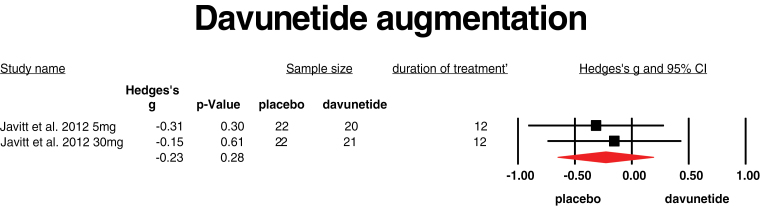
Davunetide is the smallest active part of activity-dependent neuroprotective protein, which can stabilize microtubuli and may improve neurite outgrowth.50 Davunetide also reduces the TNF-α production of activated micro glia cells. It easily passes the BBB. One study provided davunetide in addition to standard treatment to patients with chronic schizophrenia.51 Two different doses (5 and 30 mg) were provided for 3 months. Both doses did not improve symptom severity, and mean weighted ES was −0.23 (95% CI: −0.65 to 0.19, I 2 = 0) (figure 4).
Fig. 4.
Davunetide augmentation. Black square = individual effect size of a study, diamond symbol = mean weighted effect size.
EPA and DHA Fatty Acids
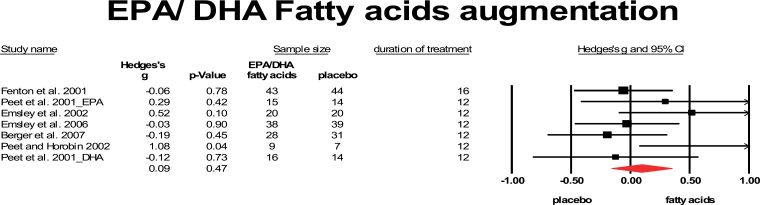
Fatty acids, especially the EPA and DHA, also have mild anti-inflammatory aspects because they decrease serum interleukin-1β (IL-1β), TNF-α, and interferon-γ levels in addition to several other actions such as neuroprotective aspects,52,53 modulation of membrane fluidity, synaptic plasticity, and effects on dopaminergic, serotonergic, and glutamatergic neurotransmission.52,54 So far, 6 studies added EPA and 1 study added DHA fatty acids to antipsychotic treatment for patients with schizophrenia.55–60 From the large study of Peet and Horribin60 only the subset on clozapine using 2 mg EPA could be included. Two of them56,59 included only FEP. The study by Fenton et al55 used 3g daily, the others studies provided 2 g/ day. The mean weighted ES was 0.09, which was not significant (95% CI: −0.16 to 0.35). Heterogeneity was low with I 2 = 26.6 (figure 5).
Fig. 5.
Eicosapentaenoic/docosahexaenoic fatty acids augmentation. Black sqaure = individual effect size of a study, diamond symbol = mean weighted effect size.
Estrogens
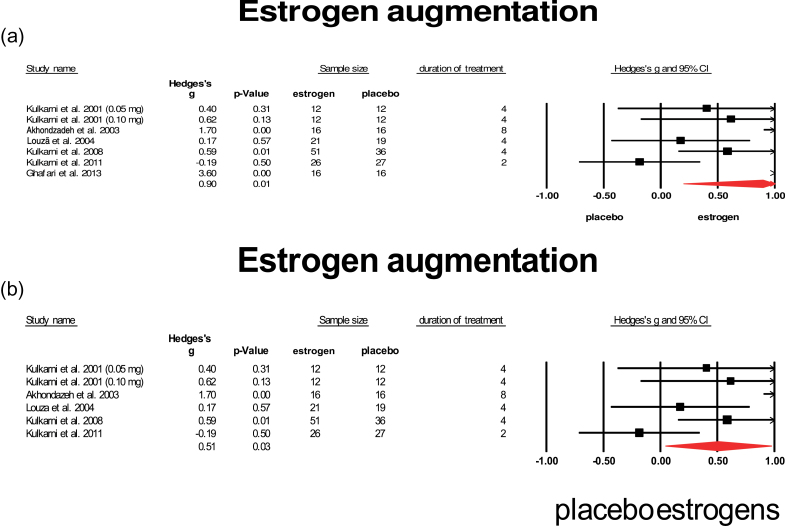
Estrogens, especially 17β-estradiol (E2), also have mild anti-inflammatory properties by ways of reducing TNF-α and NO25 in addition to many other actions such as reducing antioxidative stress, controlling energy balance and glucose homeostasis, and influencing dopaminergic neurotransmission.25 Seven studies provided estrogen in addition to standard treatment for patients with schizophrenia.61–66 Six studies included only females, and 1 study65 included only males. Six studies applied the estrogen (ethinyl)estradiol, and 1 study administered estrone.63 Estrogen doses ranged from 0.05mg/d (patch) to 2mg/d (orally). One of the studies restricted inclusion to FEP.61 One study reported a very large ES of 3.6 and was regarded as an outlier (see figure 6a).66 When this outlier was excluded (figure 6b), a mean weighted ES of 0.51 was retrieved, which was significant (95% CI: 0.043–0.972). Among these studies, the study in FEP showed the highest ES. Lowest ES was obtained in the male study and in the study applying estrone. Heterogeneity was high (I 2 = 69%) but dropped to moderate when the study in males was excluded.
Fig. 6.
Estrogen augmentation. (a) Augmentation with estrogen (including outlyer). (b) Augmentation with estrogen (outlyer removed). Black square = individual effect size of a study, diamond symbol = mean weighted effect size.
Minocycline
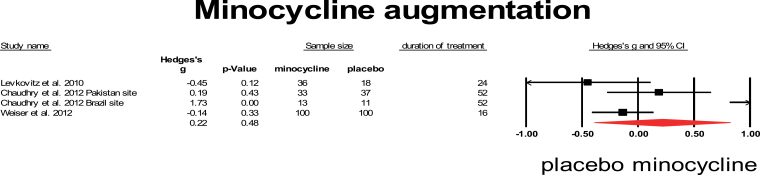
Minocycline is a broad-spectrum tetracycline antibiotic that easily crosses the BBB and has a strong inhibitory effect on microglia cells.24 Three studies assessed the effect of minocycline addition to antipsychotic treatment. All studies used a relatively high dose of 200 mg and long duration of treatment, ranging from 4 to 12 months. The studies by Levkovitz et al67 and Chaudhry et al68 included only FEP. The study by Weiser et al42 is not published yet, data are used from an abstract. The Chaudhry et al study consisted of 2 smaller trials performed in Pakistan and Brazil that are presented separately. The results of these 3 studies are very heterogeneous and, with the exception of the small Pakistan trial from Chaudhry et al, do not show a beneficiary effect on symptom severity. When combined in meta-analysis, the mean weighted effect was 0.22, which was not significant (95% CI: −0.39 to 0.82), and heterogeneity was high, I 2 = 83% (figure 7).
Fig. 7.
Minocycline augmentation. Black square = individual effect size of a study, diamond symbol = mean weighted effect size.
N-acetylcysteine
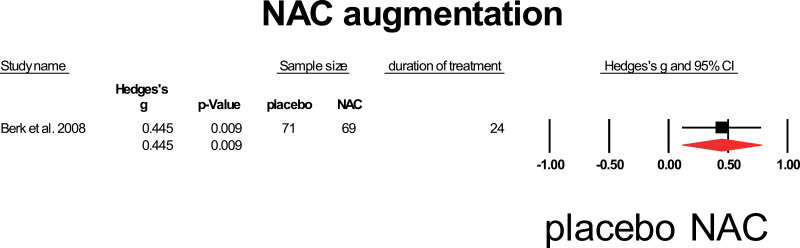
NAC has clear anti-inflammatory properties by inhibiting the inflammatory cytokines TNF-α, IL-1β, and IL-6, but is also the precursor of glutathione, the main antioxidant of the human body.69 NAC is neurotrophic and influences glutamatergic and dopaminergic neurotransmission.70 NAC is small and easily passes the BBB.71 Only one study assessed the influence of NAC addition on symptom severity in patients with schizophrenia.72 This study included chronic patients and provided 2 g of NAC for 6 months to the patients. A significant positive influence on total symptom severity was observed with a mean weighed effect of 0.45 (95% CI: 0.112–0.779) (figure 8).
Fig. 8.
N-acetylcysteine augmentation. Black square = individual effect size of a study, diamond symbol = mean weighted effect size.
Effects of Anti-inflammatory Agents on Cognitive Functioning
For aspirin, Laan et al41 found no significant effect on cognition assessed with the Rey Auditory Verbal Learning Test, the HQ Continuous Performance Test, the Purdue Pegboard Test, and the Trail Making Test. For celecoxib, no data on cognitive tests were reported in the above-mentioned studies, but a positive effect was noted on the cognition factor of the PANSS.73 For davunetide, a positive effect on skills was noted on the UCSD Performance-Based Skills Assessment, while there was no effect measured with the Matrics Consensus Cognitive Battery.51 For EPA and DHA fatty acids, only Fenton et al (2001)55 investigated cognition but found no effect using the Repeatable Battery for the Assessment of Neuropsychological Status. For estrogens, no cognitive data were assessed in the above-mentioned studies. For minocycline, 2 studies also assessed efficacy on cognitive tests, both of them using the Cambridge Neuropsychological Test Automated Battery (CANTAB), and 1 study67 found improved executive functioning with study progression (F = 1.6, P < .05), whereas the second study (Chaudhry et al68) found no difference with placebo. In the trial investigating efficacy of NAC in schizophrenia, no cognitive tests were applied.
Discussion
We quantitatively reviewed the efficacy of various anti-inflammatory agents to reduce symptom severity in patients with schizophrenia. We could include data from 26 randomized placebo-controlled double-blind studies applying 7 different agents. The results of aspirin addition to antipsychotic treatment seem promising, as does the addition of estrogens and NAC. On the other hand, addition of celecoxib, minocycline, davunetide, and EPA or DHA fatty acids did not show a beneficial effect. It is important to note that the 3 agents that significantly improved symptom severity are all very broadly active substances, and it is unsure if the beneficial effects on symptom severity are indeed mediated by their anti-inflammatory aspects.
Effects on Symptom Severity
Aspirin showed a low to moderate, but significant effect when applied for 3 or 4 months in 2 studies including 270 patients using a dose of 1000mg/d.41,42 Both studies did not limit inclusion to FEP patients.
Celecoxib, which is a more specific anti-inflammatory agent that crosses the BBB more readily, did not show overall efficacy in 465 patients, but this might be related to the relatively short duration of treatment of these trials (mostly 5–8 wk, only one study provided celecoxib for 11 wk). However, the studies with longer duration of treatment did not show better efficacy. The only study that provided celecoxib to FEP patients73 did show a positive effect. It can, therefore, not be excluded that celecoxib is effective only in FEP patients.
Two previous meta-analyses reviewed efficacy of NSAIDs for schizophrenia patients and reported modest efficacy, which reached significance in the first20 and borderline significance in the second review.74 We here show that celecoxib and aspirin may differ in their efficacy, with aspirin showing better results. However, the 2 studies on aspirin included a total of 270 patients only and the mean effect size may change when more studies become available.
The studies that used davunetide and EPA or DHA fatty acids, all 3 agents that have mild anti-inflammatory and neuroprotective actions, did not show an overall positive effect. All studies were of longer duration (12 wk and one study 16 wk). From the 2 trials that provided fatty acids to FEP patients, one showed a positive effect, whereas the other trial showed a negative effect.
Estrogen augmentation showed a significant mean effect in 6 studies including 262 patients already for relatively short duration of study (ranging from 2 to 8 wk). The single study that restricted inclusion to FEP had the largest ES. However, estrogens act on many different ways in the brain and may cause their effect by mechanisms unrelated to inflammation, eg, by affecting angiotensin and dopamine neurotransmission.75,76
Minocycline is a strong inhibitor of microglia cell activation24 and may, therefore, be expected to have potent effects on symptom severity. This component was provided in a relatively high dose of 200 mg for a relatively long time (4–12 mo), including 2 studies that restricted inclusion to FEP patients, but the results in these 348 patients were not significant although heterogeneity was high. However, it is too early to take this as evidence against the inflammation hypothesis of schizophrenia because several trials that used minocycline in patients with rheumatoid arthritis77 and amyotrophic lateral sclerosis78–80 showed a trend towards symptom aggravation rather than improvement, while these 2 diseases are clearly associated with increased proinflammatory status in the brain. Several authors report that minocycline can trigger autoimmune diseases such as lupus erythematosus, arthritis, thyroiditis, and hepatitis,81–88 suggesting that minocycline may also worsen proinflammatory and autoimmune processes.
The use of NAC for 24 weeks in a study including 140 patients showed a positive result with a moderate ES of 0.45. Because this study included more chronic patients, a study in FEP patients might show even better results. Because the positive results of NAC rely on a single randomized controlled trial, replication is needed before we can draw any conclusion.
Effects on Cognition
Only 5 of the 26 included studies provided data on cognitive test batteries and from these, 4 found no effects on cognition and 1 study observed a significant effect of minocycline. Heterogeneity of cognitive tasks employed was too great to make a quantitative review of these effects.
Side Effects
Reconsidering the 3 agents that yielded positive results in this study, it is worthwhile to compare their side effects and safety profile. To start with aspirin, it is known that application of aspirin should be combined with gastric protection because of the risk of gastrointestinal bleeding. This is not an uncommon phenomenon and, therefore, should be seriously considered and monitored during treatment. On the other hand, aspirin also has cardioprotective properties, which can be a benefit, especially in patients with metabolic syndrome.
Estrogens are not safe for longer application than 1–2 months, unless combined with progesterone. In addition, estrogens are not suitable for men. Raloxifen or tamoxifen may be safe alternatives, but efficacy of these substances remains to be determined.
NAC has very few side effects, and next to its more favorable side effect profile than aspirin, it can even be administered during pregnancy. NAC may have additional benefits in schizophrenia, such as decreasing addiction89–93 and increasing glutathione levels, thereby increasing scavenger potential.
Limitations
An important limitation is that the field of augmentation with anti-inflammatory components in schizophrenia is still in its infancy, and only few studies have been performed. Many components with strong anti-inflammatory potency, such as the glucocorticosteroids, have not been tried in patients with schizophrenia. Nor have the statins, another promising group of components, which are well tolerated and have anti-inflammatory actions on the brain in addition to their well-known ability to prevent or reduce metabolic syndrome.
Conclusion
At this point, it is too early to make conclusions on the efficacy on symptom severity of schizophrenia of augmentation with anti-inflammatory agents. Some beneficial effects of aspirin, NAC, and estrogens were observed, while addition of celecoxib, EPA/DHA fatty acids, davunetide, and minocycline did not show efficacy. The fact that aspirin, NAC, and estrogens have many other effects apart from their anti-inflammatory actions that can also improve symptoms of schizophrenia makes it even less certain that reducing the proinflammatory status of the brain improves clinical status. Efficacy on cognition is currently not supported by the data. Taken together, there is some preliminary evidence for efficacy of anti-inflammatory agents on symptom severity in patients with schizophrenia, but further trials are needed.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study. Disclosures: In the last 3 years Stefan Leucht has received honoraria for lectures, consulting or advisory boards from AstraZeneca, Alkermes, BristolMyersSquibb, EliLilly, Essex Pharma, Janssen, Johnson and Johnson, Lundbeck, Medavante, Roche and SanofiAventis. EliLilly has provided medication for a trial with Stefan Leucht as the principal investigator
References
- 1. Torrey EF, Peterson MR. Slow and latent viruses in schizophrenia. Lancet. 1973;2:22–24 [DOI] [PubMed] [Google Scholar]
- 2. Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monji A, Kato T, Kanba S. Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265 [DOI] [PubMed] [Google Scholar]
- 4. Drexhage RC, Weigelt K, van Beveren N, et al. Immune and neuroimmune alterations in mood disorders and schizophrenia. Int Rev Neurobiol. 2011;101:169–201 [DOI] [PubMed] [Google Scholar]
- 5. Chew LJ, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci. 2013;27:1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–148 [DOI] [PubMed] [Google Scholar]
- 7. Müller N, Dursun SM. Schizophrenia genes, epigenetics and psychoneuroimmunology therapeutics: all make sense now? J Psychopharmacol. 2011;25:713–714 [DOI] [PubMed] [Google Scholar]
- 8. de Jong S, van Eijk KR, Zeegers DW, et al. ; PGC Schizophrenia (GWAS) Consortium Expression QTL analysis of top loci from GWAS meta-analysis highlights additional schizophrenia candidate genes. Eur J Hum Genet. 2012;20:1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93:261–265 [DOI] [PubMed] [Google Scholar]
- 10. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is elevated in schizophrenia. Schizophr Res. 2013;143:198–202 [DOI] [PubMed] [Google Scholar]
- 11. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J Affect Disord. 2013;150:456–459 [DOI] [PubMed] [Google Scholar]
- 12. Lavisse S, Guillermier M, Hérard AS, et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. 2012;32:10809–10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative ®-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822 [DOI] [PubMed] [Google Scholar]
- 15. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807 [DOI] [PubMed] [Google Scholar]
- 16. Takano A. The application of PET technique for the development and evaluation of novel antipsychotics. Curr Pharm Des. 2010;16:371–377 [DOI] [PubMed] [Google Scholar]
- 17. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310 [DOI] [PubMed] [Google Scholar]
- 18. Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66 [DOI] [PubMed] [Google Scholar]
- 19. Eaton WW, Byrne M, Ewald H, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–528 [DOI] [PubMed] [Google Scholar]
- 20. Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73:414–419 [DOI] [PubMed] [Google Scholar]
- 21. Dodd S, Maes M, Anderson G, Dean OM, Moylan S, Berk M. Putative neuroprotective agents in neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:135–145 [DOI] [PubMed] [Google Scholar]
- 22. Pettit LK, Varsanyi C, Tadros J, Vassiliou E. Modulating the inflammatory properties of activated microglia with docosahexaenoic acid and aspirin. Lipids Health Dis. 2013;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan ES, Cronstein BN. Methotrexate–how does it really work? Nat Rev Rheumatol. 2010;6:175–178 [DOI] [PubMed] [Google Scholar]
- 24. Watabe M, Kato TA, Monji A, Horikawa H, Kanba S. Does minocycline, an antibiotic with inhibitory effects on microglial activation, sharpen a sense of trust in social interaction? Psychopharmacology (Berl). 2012;220:551–557 [DOI] [PubMed] [Google Scholar]
- 25. Liu X, Fan X, Zhao Y, et al. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-α and estrogen receptor-β in microglia. J Neurosc Res. 2005;81:653–665 [DOI] [PubMed] [Google Scholar]
- 26. Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26:469–479 [DOI] [PubMed] [Google Scholar]
- 27. Hu X, Zhou H, Zhang D, et al. Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. J Neuroimmune Pharmacol. 2012;7:187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Albayrak A, Halici Z, Polat B, et al. Protective effects of lithium: a new look at an old drug with potential antioxidative and anti-inflammatory effects in an animal model of sepsis. Int Immunopharmacol. 2013;16:35–40 [DOI] [PubMed] [Google Scholar]
- 29. Ximenes JC, de Oliveira Gonçalves D, Siqueira RM, et al. Valproic acid: an anticonvulsant drug with potent antinociceptive and anti-inflammatory properties. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:575–587 [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 31. Lipsey MW Wilson DB Practical Meta-Analysis. Thousand Oaks: , CA: Sage; 2001 [Google Scholar]
- 32. Lipsey MW, Wilson DB. The way in which intervention studies have “personality” and why it is important to meta-analysis. Eval Health Prof. 2001;24:236–254 [DOI] [PubMed] [Google Scholar]
- 33. Newcomer JW, Craft S, Askins K, et al. Glucocorticoid interactions with memory function in schizophrenia. Psychoneuroendocrinology. 1998;23:65–72 [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal R Meta-Analytic Procedures for Social Research. London, UK: Sage; 1991 [Google Scholar]
- 35. Shaddish WR, Haddock CK. Combining estimates of effect size. In: Coopers H, Hedges V, eds. The Handbook of Research Synthesis. New York, NY: Sage Publications; 1994: 226–285, 261–281 [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975;56:624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120 [DOI] [PubMed] [Google Scholar]
- 39. Nye EJ, Hockings GI, Grice JE, et al. Aspirin inhibits vasopressin-induced hypothalamic-pituitary-adrenal activity in normal humans. J Clin Endocrinol Metab. 1997;82:812–817 [DOI] [PubMed] [Google Scholar]
- 40. Vasović V, Banić B, Jakovljević V, Tomic Z, Milic-Djordjevic V. Effect of aminophylline on aspirin penetration into the central nervous system in rats. Eur J Drug Metab Pharmacokinet. 2008;33:23–30 [DOI] [PubMed] [Google Scholar]
- 41. Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527 [DOI] [PubMed] [Google Scholar]
- 42. Weiser M, Burshtein S, Fodoreanu L, et al. A randomized trial administering aspirin, minocycline or pramipexole vs placebo as add-on to antipsychotics in patients with schizophrenia or schizoaffective disorder [abstract]. Neuropsychopharmacology. 2012;38:S314–S446 [Google Scholar]
- 43. Simon LS. Role and regulation of cyclooxygenase-2 during inflammation. Am J Med. 1999;106:37S–42S [DOI] [PubMed] [Google Scholar]
- 44. Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225–242 [DOI] [PubMed] [Google Scholar]
- 45. Müller N, Krause D, Dehning S, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124 [DOI] [PubMed] [Google Scholar]
- 46. Müller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034 [DOI] [PubMed] [Google Scholar]
- 47. Rappard F, Muller N. Celecoxib add-on does not have beneficial antipsychotic effects over risperidone alone in schizophrenia [abstract]. Neuropsychopharmacology. 2004;29:S183–S241 [Google Scholar]
- 48. Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57:1594–1596 [DOI] [PubMed] [Google Scholar]
- 49. Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007; 90:179–185 [DOI] [PubMed] [Google Scholar]
- 50. Gozes I, Divinski I. NAP, a neuroprotective drug candidate in clinical trials, stimulates microtubule assembly in the living cell. Curr Alzheimer Res. 2007;4:507–509 [DOI] [PubMed] [Google Scholar]
- 51. Javitt DC, Buchanan RW, Keefe RS, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136:25–31 [DOI] [PubMed] [Google Scholar]
- 52. Calder PC. Mechanism of action of (n-3)fatty acids. J Nutr. 2012;142:592S–599S [DOI] [PubMed] [Google Scholar]
- 53. Solfrizzi V, Frisardi V, Capurso C, et al. Dietary fatty acids in dementia and predementia syndromes: epidemiological evidence and possible underlying mechanisms. Ageing Res Rev. 2010;9:184–199 [DOI] [PubMed] [Google Scholar]
- 54. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73 [DOI] [PubMed] [Google Scholar]
- 55. Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158:2071–2074 [DOI] [PubMed] [Google Scholar]
- 56. Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001;49:243–251 [DOI] [PubMed] [Google Scholar]
- 57. Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–1598 [DOI] [PubMed] [Google Scholar]
- 58. Emsley R, Niehaus DJ, Koen L, et al. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr Res. 2006;84:112–120 [DOI] [PubMed] [Google Scholar]
- 59. Berger GE, Proffitt TM, McConchie M, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68:1867–1875 [DOI] [PubMed] [Google Scholar]
- 60. Peet M, Horrobin DF; E-E Multicentre Study Group A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18 [DOI] [PubMed] [Google Scholar]
- 61. Kulkarni J, Riedel A, de Castella AR, et al. Estrogen - a potential treatment for schizophrenia. Schizophr Res. 2001;48:137–144 [DOI] [PubMed] [Google Scholar]
- 62. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1007–1012 [DOI] [PubMed] [Google Scholar]
- 63. Louzã MR, Marques AP, Elkis H, Bassitt D, Diegoli M, Gattaz WF. Conjugated estrogens as adjuvant therapy in the treatment of acute schizophrenia: a double-blind study. Schizophr Res. 2004;66:97–100 [DOI] [PubMed] [Google Scholar]
- 64. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65:955–960 [DOI] [PubMed] [Google Scholar]
- 65. Kulkarni J, de Castella A, Headey B, et al. Estrogens and men with schizophrenia: is there a case for adjunctive therapy? Schizophr Res. 2011;125:278–283 [DOI] [PubMed] [Google Scholar]
- 66. Ghafari E, Fararouie M, Shirazi HG, Farhangfar A, Ghaderi F, Mohammadi A. Combination of estrogen and antipsychotics in the treatment of women with chronic schizophrenia: a double-blind, randomized, placebo-controlled clinical trial. Clin Schizophr Relat Psychoses. 2013;6:172–176 [PubMed] [Google Scholar]
- 67. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149 [DOI] [PubMed] [Google Scholar]
- 68. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol. 2012;26:1185–1193 [DOI] [PubMed] [Google Scholar]
- 69. Palacio JR, Markert UR, Martínez P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm Res. 2011;60:695–704 [DOI] [PubMed] [Google Scholar]
- 70. Oja SS, Janáky R, Varga V, Saransaari P. Modulation of glutamate receptor functions by glutathione. Neurochem Int. 2000;37:299–306 [DOI] [PubMed] [Google Scholar]
- 71. Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183 [DOI] [PubMed] [Google Scholar]
- 72. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368 [DOI] [PubMed] [Google Scholar]
- 73. Müller N, Riedel M, Schwarz MJ, Engel RR. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255:149–151 [DOI] [PubMed] [Google Scholar]
- 74. Nitta M Kishimoto T Müller N Weiser M et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. May 29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rodriguez-Perez AI, Valenzuela R, Villar-Cheda B, Guerra MJ, Labandeira-Garcia JL. Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: role of the brain angiotensin system. Brain. 2012;135:124–138 [DOI] [PubMed] [Google Scholar]
- 76. Sánchez MG, Morissette M, Di Paolo T. Effect of a chronic treatment with 17β-estradiol on striatal dopamine neurotransmission and the Akt/GSK3 signaling pathway in the brain of ovariectomized monkeys. Psychoneuroendocrinology. 2012;37:280–291 [DOI] [PubMed] [Google Scholar]
- 77. O’Dell JR, Haire CE, Palmer W, et al. Treatment of early rheumatoid arthritis with minocycline or placebo: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1997;40:842–848 [DOI] [PubMed] [Google Scholar]
- 78. Gordon PH, Moore DH, Miller RG, et al. ; Western ALS Study Group Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053 [DOI] [PubMed] [Google Scholar]
- 79. Leigh PN, Meininger V, Bensimon G, Cudkowicz M, Robberecht W. Minocycline for patients with ALS. Lancet Neurol. 2008;7:119–120; author reply 120. [DOI] [PubMed] [Google Scholar]
- 80. Manev H, Manev R. Interactions with GluR1 AMPA receptors could influence the therapeutic usefulness of minocycline in ALS. Amyotroph Lateral Scler. 2009;10:416–417 [DOI] [PubMed] [Google Scholar]
- 81. Mannargudi B, McNally D, Reynolds W, Uetrecht J. Bioactivation of minocycline to reactive intermediates by myeloperoxidase, horseradish peroxidase, and hepatic microsomes: implications for minocycline-induced lupus and hepatitis. Drug Metab Dispos. 2009;37:1806–1818 [DOI] [PubMed] [Google Scholar]
- 82. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787–790 [DOI] [PubMed] [Google Scholar]
- 83. Ahmed F, Kelsey PR, Shariff N. Lupus syndrome with neutropenia following minocycline therapy - a case report. Int J Lab Hematol. 2008;30:543–545 [DOI] [PubMed] [Google Scholar]
- 84. Tehrani R, Nash-Goelitz A, Adams E, Dahiya M, Eilers D. Minocycline-induced cutaneous polyarteritis nodosa. J Clin Rheumatol. 2007;13:146–149 [DOI] [PubMed] [Google Scholar]
- 85. Benjamin RW, Calikoglu AS. Hyperthyroidism and lupus-like syndrome in an adolescent treated with minocycline for acne vulgaris. Pediatr Dermatol. 2007;24:246–249 [DOI] [PubMed] [Google Scholar]
- 86. Leydet H, Armingeat T, Pham T, Lafforgue P. [Minocycline-induced lupus-like disease]. Rev Med Interne. 2006; 27:72–75 [DOI] [PubMed] [Google Scholar]
- 87. van Steensel MA. Why minocycline can cause systemic lupus - a hypothesis and suggestions for therapeutic interventions based on it. Med Hypotheses. 2004;63:31–34 [DOI] [PubMed] [Google Scholar]
- 88. Bachmeyer C, Cadranel JF. Minocycline-induced lupus and autoimmune hepatitis: family autoimmune disorders as possible risk factors. Dermatology. 2002;205:185–186 [DOI] [PubMed] [Google Scholar]
- 89. Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Somaini L, Donnini C, Raggi MA, et al. Promising medications for cocaine dependence treatment. Recent Pat CNS Drug Discov. 2011;6:146–160 [DOI] [PubMed] [Google Scholar]
- 91. Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gray KM, Watson NL, Carpenter MJ, Larowe SD. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19:187–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. LaRowe SD, Myrick H, Hedden S, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117 [DOI] [PubMed] [Google Scholar]
- 94. Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ferreira AP, Pasin JS, Saraiva AL, et al. N-acetylcysteine prevents baker’s-yeast-induced inflammation and fever. Inflamm Res. 2012;61:103–112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.