Abstract
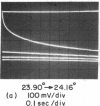
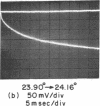
Aqueous dispersions of synthetic dimyristoyl L-α-lecithin undergo a sharp decrease in turbidity in the temperature range where the crystalline-liquid crystalline phase transition occurs. Equilibrium transition curves, monitored by the absorbancy change at 300 nm, reproduce all the important features of a calorimetric melting curve [Hinz & Sturtevant (1972) J. Biol. Chem. 247, 6071]. A rapid temperature-jump of the dispersion, measured by the same absorbancy change, has detected several different molecular processes depending on the magnitude of the perturbation. These processes include a phase transition, permeation of ions and water across the membrane, and repture, annealing, or fusion of the bilayer structures. When the temperature-jump is limited to 1 degree, only reactions associated with the phase transition are detectable and no signal is generated after a temperature-jump except for those which end within or extend beyond the transition zones.
At least two relaxation times are resolved for the phase transition reactions. The faster one in the millisecond time range is strongly temperature-dependent and has an activation energy close to one million calories per mole. The second one, in the 100-msec time range, appears to have a much smaller activation energy. These observations indicate that strongly cooperative nucleation processes and energy-dependent fast propagation steps occur during the phase transition. Since the kinetics of the transition are complex, intermediate state or transient structures must exist in the transition regions of the bilayer dispersions. These thermal fluctuations of the bilayer structures may have important effects on the lateral diffusion and permeation of molecules and ions in the membrane structure.
Keywords: lipid bilayer, temperature-jump
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barratt M. D., Green D. K., Chapman D. E.S.R. studies of nitroxide probes in lecithin-water systems. Chem Phys Lipids. 1969 Apr;3(2):140–144. doi: 10.1016/0009-3084(69)90004-8. [DOI] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Elson E. L. A sequential model of nucleation-dependent protein folding: kinetic studies of ribonuclease A. Analysis of the steady-state approximation for the sequential model. J Mol Biol. 1972 Feb 14;63(3):469–475. doi: 10.1016/0022-2836(72)90441-x. [DOI] [PubMed] [Google Scholar]
- Elson E. L. Simple sequential model for the kinetics of conformational transitions of oligomeric helices and proteins. Biopolymers. 1972;11(7):1499–1520. doi: 10.1002/bip.1972.360110713. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Hsu M. C., Chan S. I. Nuclear magnetic resonance studies of the interaction of valinomycin with unsonicated lecithin bilayers. Biochemistry. 1973 Sep 25;12(20):3872–3876. doi: 10.1021/bi00744a012. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Spin-label studies of the excitable membranes of nerve and muscle. Proc Natl Acad Sci U S A. 1968 Sep;61(1):12–16. doi: 10.1073/pnas.61.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Morowitz H. J., Sturtevant J. M., Tsong T. Y. Characterization of the plasma membrane of Mycoplasma laidlawii. VII. Phase transitions of membrane lipids. Biochim Biophys Acta. 1970;219(1):114–122. doi: 10.1016/0005-2736(70)90066-0. [DOI] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Bennion B. C., Holmes L. P., Eyring E. M., Berg M. W., Lords J. L. Temperature jump relaxations in aqueous saline suspensions of human erythrocytes. Biochim Biophys Acta. 1970 Mar 17;203(1):77–82. doi: 10.1016/0005-2736(70)90037-4. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Hemmes P., Eyring E. M. Light scattering temperature jump relaxations in mixed solvent suspensions of phosphatidylcholine vesicles. Biochim Biophys Acta. 1970 Dec 1;219(2):276–282. doi: 10.1016/0005-2736(70)90206-3. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Reinert J. C., Steim J. M. Calorimetric detection of a membrane-lipid phase transition in living cells. Science. 1970 Jun 26;168(3939):1580–1582. doi: 10.1126/science.168.3939.1580. [DOI] [PubMed] [Google Scholar]
- SCHWARZ G. ON THE KINETICS OF THE HELIX-COIL TRANSITION OF POLYPEPTIDES IN SOLUTION. J Mol Biol. 1965 Jan;11:64–77. doi: 10.1016/s0022-2836(65)80171-1. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Träuble H., Galla H. J., Overath P. Lateral diffusion, protein mobility, and phase transitions in Escherichia coli membranes. A spin label study. Biochemistry. 1973 Dec 18;12(26):5360–5369. doi: 10.1021/bi00750a020. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Träuble H. Phasenumwandlungen in Lipiden. Mögliche Schaltprozesse in biologischen Membranen. Naturwissenschaften. 1971 Jun;58(6):277–284. doi: 10.1007/BF00624732. [DOI] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Wu S. H., McConnell H. M. Lateral phase separations and perpendicular transport in membranes. Biochem Biophys Res Commun. 1973 Nov 16;55(2):484–491. doi: 10.1016/0006-291x(73)91112-1. [DOI] [PubMed] [Google Scholar]