Abstract
Zinc is an allosteric modulator of glycine receptor function, enhancing the effects of glycine at nM to low μM concentrations, and inhibiting its effects at higher concentrations. Because of zinc’s high potency at the glycine receptor, there exists a possibility that effects attributed solely to exogenously-applied glycine in fact contain an undetected contribution of zinc acting as an allosteric modulator. We found that glycine solutions made up in standard buffers and using deionized distilled water produced effects that could be decreased by the zinc chelator tricine. This phenomenon was observed in three different vials tested and persisted even if vials were extensively washed, suggesting the zinc was probably present in the buffer constituents. In addition, polystyrene, but not glass, pipets bore a contaminant that enhanced glycine receptor function and that could also be antagonized by tricine. Our findings suggest that without checking for this effect using a chelator such as tricine, one cannot assume that responses elicited by glycine applied alone are not necessarily also partially due to some level of allosteric modulation by zinc.
Keywords: Zinc, Tricine, Allosteric modulation, Glycine receptor, electrophysiology, Xenopus oocytes
1. Introduction
The glycine receptor (GlyR) is a member of the cys-loop superfamily of ligand-gated ion channels. GlyR are pentameric in structure and can be either composed solely of α subunits homomeric, or heteromerically in the form of α & β subunits arranged around a central chloride-conducting pore. To date, three α and one β subunits have been described in humans (Baer et al., 2009). Responsible for the majority of fast inhibitory neurotransmission in the brainstem and spinal, GlyR are also found in many higher brain regions including the hippocampus, nucleus accumbens, and prefrontal cortex (Baer et al., 2009; Jonsson et al., 2012, 2009; Lynch, 2004). GlyR function is modulated by inhaled anesthetic, alcohols and inhalants, making it a possible target for the development of therapeutics for the treatment of alcoholism (Beckstead et al., 2000; Mascia et al., 1996; Tipps et al., 2010).
Zinc is a biphasic allosteric modulator of GlyR function, enhancing GlyR currents at concentrations below 10 μM while inhibiting receptor function at higher concentrations (Harvey et al., 1999; Laube et al., 2000, 1995). Recent studies have shown that zinc is a contaminant present at low nM concentrations in physiological solutions and in commonly-used labware (Kay, 2004; McCracken et al., 2010). Due to an increasing concern that some of the results of our studies may have been due to an effect of glycine in conjunction with zinc, rather than glycine itself, we utilized an electrophysiological approach to examine the effects of background contaminating zinc levels and to distinguish among several possible sources of zinc contamination in reagents and labware used in the preparation of solutions for electrophysiological recordings.
2. Materials & Methods
2.1 - Reagents
Except NaOH [Cat. No. SS255] obtained from Fisher (Pittsburgh, PA), all other chemicals were purchased from Sigma-Aldrich (St Louis, MO) including NaCl [Cat. No. S9625], CaCl2 [C7902], KCl [P5405], MgSO4 · 7H2O [2030391], Ca(NO3)2 · 4H2O [C1396], HEPES [H3375], NaHCO3 [S3817], tricine [T5816] and glycine [G7126]. Distilled water was purified further using a Barnstead E-Pure Ultrapure D4641 Water Purification System.
2.2 – Oocyte isolation and cDNA injection
Xenopus laevis were obtained from Nasco (Fort Atkinson, WI) and housed at room temperature on a 12-hour light/dark cycle. Oocytes were obtained via surgery, performed in accordance with AAALAC regulations, and placed in isolation media containing 108 mM NaCl, 1 mM EDTA, 2 mM KCl, and 10 mM HEPES. Forceps were used to manually remove the thecal and epithelial layers from stage V and VI oocytes followed by removal of the follicular layer using a 10 minute incubation in 0.5 mg/mL Sigma type 1A collagenase in buffer containing 83 mM NaCl, 2 mM MgCl2, and 5 mM HEPES. Oocytes were injected through their animal poles with 30 nL of α1β glycine receptor subunit cDNA (at a 1:20 α1:β ratio) in a modified pBK-cytomegalovirus vector (Mihic et al., 1997), using a micropipette (10–15 μm tip size) attached to an electronically-activated microdispenser. Oocytes were stored in the dark at room temperature for 24 hours followed by subsequent storage in the dark at 19 °C for up to 5 days post-injection in 96-well plates containing modified Barth’s saline (MBS) [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4•7H2O, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2 at pH 7.5] supplemented with 2 mM sodium pyruvate, 0.5 mM theophylline, 10 U/ml penicillin, 10 mg/l streptomycin and 50 mg/l gentamicin, and sterilized by passage through a 0.22 μm filter.
2.3 – Two-electrode voltage-clamp electrophysiology
Oocytes expressed heteromeric GlyR within 48h, and all electrophysiological recordings were made within 5 days of cDNA injection. Oocytes were placed in a 100 μL bath with the animal poles facing upwards and impaled with two high-resistance (0.5–10 MΩ) glass electrodes filled with 3M KCl. Cells were voltage-clamped at –70mV using an OC-725C oocyte clamp (Warner Instruments, Hamden, CT) and perfused with MBS at a rate of 2mL/min. using a Masterflex USA peristaltic pump (Cole Parmer Instrument Co., Vernon Hills, IL) through 18-gauge polyethylene tubing. All glycine solutions were prepared in MBS or MBS + 2.0 or 2.5 mM tricine. When maximally-effective concentrations of glycine were applied, applications lasted for 15s and were followed by 10 minute washouts with MBS to allow for complete receptor resensitization. For experiments using submaximal concentrations of glycine, concentrations that yielded 5 percent of the maximally-effective glycine response (EC5) were applied for 45 s followed by 3 minute washouts with MBS to allow for complete receptor resensitization. Data were acquired at a rate of 1kHz using a Powerlab 4/30 digitizer using LabChart version 7 software (ADInstruments, Bella Vista, NSW, Australia).
2.4.4 – Cadmium and Zinc Concentration Determination
Zinc and cadmium concentrations were determined in MBS and distilled water using a quadrupole-based Agilent 7500ce inductively-coupled plasma mass spectrometer (ICP-MS) at the Jackson School of Geosciences Isotope Geochemistry Facility at the University of Texas at Austin. Solutions were diluted as necessary in 2% HNO3 before analysis.
2.5 – Data Analysis
Peak currents were measured and used in data analysis. Currents generated under the various experimental conditions were normalized against currents generated by the indicated control applications and expressed as mean ± S.E.M. of the percent of control generated current (sections 3.1 and 3.2) or percent change from control generated current (section 3.3). Staistically significant differences among experimental conditions were determined using one-way or three-way ANOVAs and post hoc tests, as indicated. SigmaPlot version 11.0 (Systat Software, San Jose, CA) was used for statistical testing.
3. Results
3.1 – Type of vial containing glycine does not affect degree of contaminating zinc-mediated GlyR enhancement
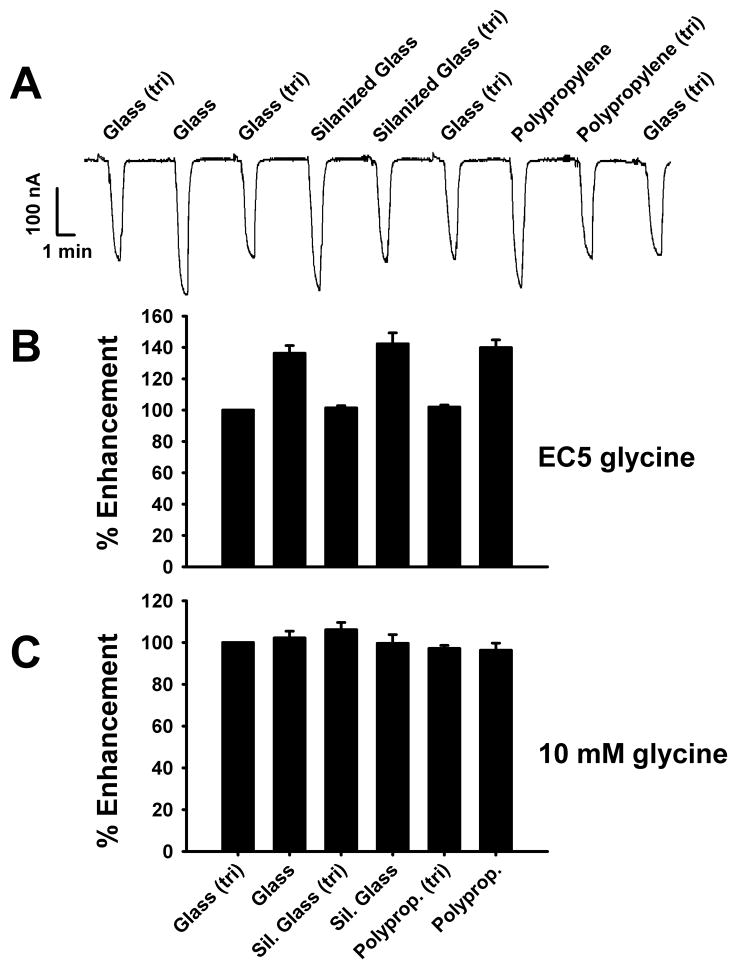
To determine if various vials commonly used for the preparation of agonist solutions contain different amounts of contaminating zinc sufficient to affect GlyR currents, glycine solutions were prepared in three different vials in the presence of the zinc-chelating agent tricine or without: Fisher glass screw-thread vials (Cat. No. 03-339-22J), National Scientific silanized glass vials (Cat. No. B7999-S3), or BD Falcon™ polypropylene tubes (Cat. No. 352096). Low concentrations of zinc (below 10 μM) left-shift glycine concentration-response curves of α-containing GlyR, with the greatest enhancing effects of zinc seen at low concentrations of glycine (Laube. et al. 1995). We therefore tested, in different types of vials, concentrations of glycine that elicited 5 percent of maximally-effective glycine responses (EC5).
EC5 concentrations of glycine in buffer containing 2.5 mM tricine were determined in Fisher glass vials and used as controls against which all other experimental conditions were normalized (Figs. 1 A,B). Fig. 1A shows a sample tracing of successive 45 s applications of EC5 glycine from solutions made up in glass, silanized glass or polypropylene vials, with or without 2.5 mM tricine. Control applications of EC5 glycine + 2.5 mM tricine in glass were interspersed throughout to account for any drift in EC5 glycine-mediated responses. For all vials, GlyR currents mediated by EC5 glycine in MBS without tricine were consistently higher than currents generated by EC5 glycine in MBS + 2.5 mM tricine [F(4,19) = 21.77, p < 0.001] (Fig. 1B). However, Student-Newman-Keuls posthoc tests revealed no significant differences in currents generated by EC5 glycine in MBS among the different vials used, in either the absence or presence of tricine (Fig. 1B). As previously shown by McCracken et al. (2010), chelation of zinc by tricine does not affect currents mediated by saturating concentrations of glycine (Fig. 1C). Further, vial choice does not significantly affect currents mediated by saturating (10 mM) concentrations of glycine [F(4, 8) = 1.77, p > 0.19] (Fig. 1C).
Fig 1.
Contaminating zinc-mediated enhancement of GlyR function is not affected by the type of vial in which glycine solutions are prepared. A) Sample tracing showing submaximal α1β GlyR currents elicited by glycine solutions prepared in glass, silanized glass, and polypropylene vials in the absence or presence of 2.5 mM tricine (tri). B) Summary data of experiments depicted in panel A. EC5 glycine was determined in the presence of 2.5 mM tricine in glass vials and its effect was used as the control against which all other conditions were normalized in each oocyte. Control applications of EC5 glycine + tricine solutions in glass vials were tested throughout each experiment to account for possible drift in GlyR responses over time. For all vials, EC5 glycine-mediated currents were consistently higher for solutions prepared in the absence of 2.5 mM tricine but, among vial types, there were no significant differences in currents generated by EC5 glycine solutions prepared in either the presence of absence of 2.5 mM tricine. C) Summary data showing that choice of vial type does not affect GlyR currents mediated by a saturating (10 mM) concentration of glycine. Data are presented as the mean ± S.E.M. of 3 – 5 oocytes.
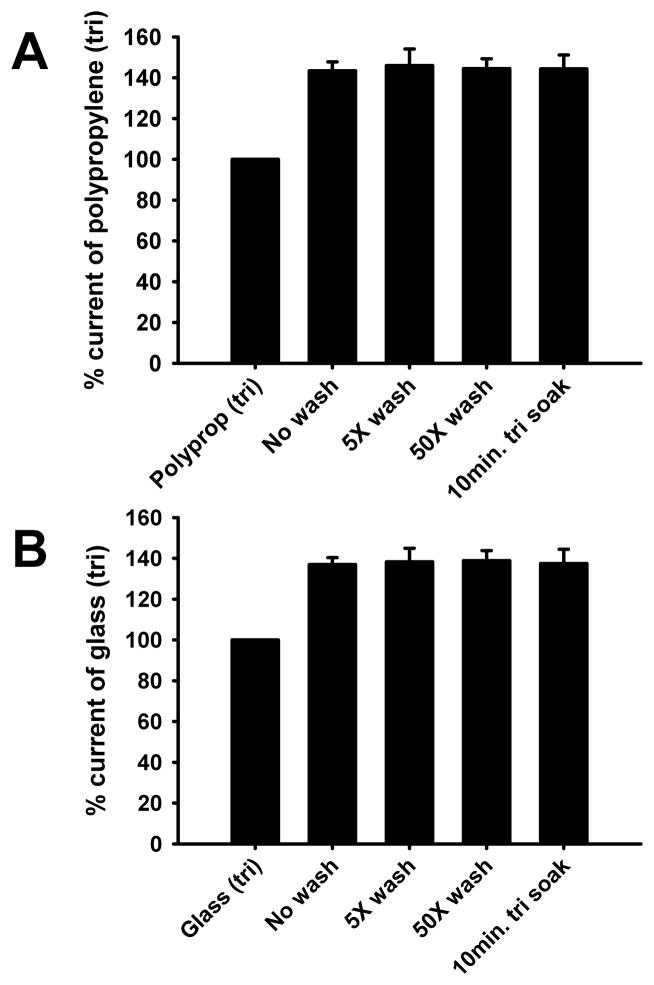
3.2 – Washing agonist-solution vials does not affect the degree of contaminating zinc-mediated GlyR enhancement
We next wished to determine whether zinc was present on the surfaces of vials and whether washing them prior to the preparation of EC5 glycine solutions would decrease contaminating zinc-mediated GlyR enhancement. Glass and polypropylene vials were either: (1) not washed, (2) washed 5 times with de- ionized H2O (diH2O), (3) washed fifty times with diH2O, or (4) soaked in MBS + 2.5 mM tricine for 10 minutes before immediate drying and preparation of EC5 glycine. GlyR responses elicited by EC5 glycine prepared in these were compared with EC5 glycine solutions prepared in corresponding unwashed vials with MBS + 2.5 mM tricine. The various washing procedures did not affect the degree of contaminating zinc-mediated GlyR enhancement for polypropylene [F(3,19) = 0.03, p > 0.99] or glass [F(3,11) = 0.02, p > 0.99] vials (Figs. 2A,B).
Fig 2.
Washing vials does not affect the degree of contaminating zinc-mediated GlyR enhancement. EC5 glycine was determined in the presence of 2.5 mM tricine in polypropylene or glass vials and used for solutions prepared in the absence of tricine in vials that were either not washed, washed five times with diH2O, washed 50 times with diH2O H2O, or submitted to a 10 min. soak in MBS + 2.5 mM tricine prior to drying and use. Data are reported as percent current of that elicited by EC5 glycine in the presence 2.5 mM tricine. A) Summary data for currents elicited by EC5 glycine solutions prepared in polypropylene tubes under the various wash conditions. B) Summary data for currents elicited by EC5 glycine solutions prepared in glass vials under the various wash conditions. Data are presented as the mean ± S.E.M. of 3 – 5 oocytes.
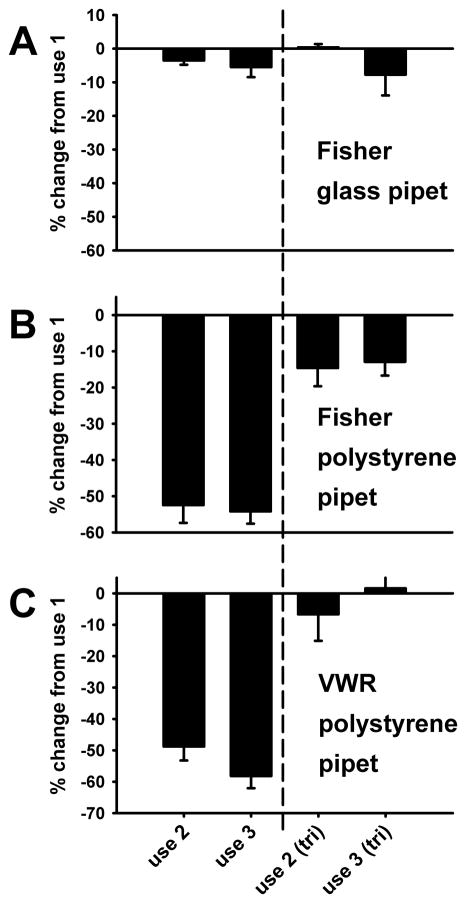
3.3 – Polystyrene but not glass serological pipets contain contaminating zinc that significantly affects EC5 glycine-mediated GlyR currents
During the course of conducting these studies we observed that the first EC5 glycine solution made using a polystyrene serological pipet to transfer the MBS buffer to vials seemed to produce larger GlyR currents than subsequent EC5 glycine solutions made using the same pipet to transfer MBS. Further, it appeared that this phenomenon did not occur when glass pipets were used for the transfer of MBS. To quantify this, experiments were conducted in which three successive EC5 glycine solutions were prepared using the same serological pipet to transfer MBS to the working-solution vial (either Fisher glass [Cat No. 13-678-31J], Fisher polystyrene (Cat. No. 13-678-12E], or VWR polystyrene [Cat. No. 89130-898]). GlyR currents generated by solutions prepared with the second and third use of each pipet were compared to currents generated by solutions made with the first use of a given pipet. Subsequently, an amount of tricine equal to a final concentration of 2 mM was added to each of those vials and the experiment was repeated to determine if zinc contamination was responsible for the observed effects. All data shown in Fig. 3 obtained with the second and third uses of pipets were normalized against the responses observed during the first use of the pipet, in either the absence or presence of tricine. Tricine always decreased absolute currents as expected. Fig. 3 shows the results for glycine solutions prepared using glass as well as two different polystyrene serological pipets. A three-way ANOVA illustrates a significant effect of the type of pipet used [F(2,59) = 48.85, p < 0.001] and an effect of tricine [F(1,59) = 128.53, p < 0.001] but no difference between the second and third repeated uses of each pipet [F(1,59) = 0.48, p > 0.49].
Fig 3.
Polystyrene, but not glass serological pipets contain zinc contamination sufficient to affect submaximal GlyR currents. EC5 glycine solutions were prepared in glass vials with either Fisher glass, Fisher polystyrene, or VWR polystyrene serological pipets. Each pipet was used to prepare 3 identical EC5 glycine solutions. The first application was the control and the results of the subsequent two applications were normalized against the first and presented as percent change relative to the first. After application of all three solutions, a final concentration of 2mM tricine was added to each vial and the solutions are reapplied. The vertical dashed line separates responses observed in the absence (left of line) and presence (right of line) of tricine. A) Summary data for currents elicited by EC5 glycine solutions prepared with Fisher glass pipets. B) Summary data for currents elicited by EC5 glycine solutions prepared with Fisher polystyrene pipets. C) Summary data for currents elicited by EC5 glycine solutions prepared with VWR polystyrene pipets. Data are presented as the mean S.E.M. of 4 – 6 oocytes.
3.4 – ICP-MS determination of zinc and cadmium concentrations
Distilled water and MBS samples were submitted for determination of zinc and cadmium levels via ICP-MS. Cadmium was below the detection limit in all water samples tested but was detected at a concentration of 7.5 ± 0.1 nM (n=3) in MBS. The zinc concentration in distilled water was 8.0 ± 1.7 nM (n=4) , increasing to 44.4 ± 2.2 nM (n=13) in MBS. Water initially dispensed from a Fisher polystyrene pipet had a zinc concentration of 129.7 ± 15.2 nM (n=4), decreasing to 28.9 ± 5.4 nM (n=4) the second time it was used, indicative of zinc washout from the pipet.
4. Discussion
Due to its enhancing effects on glycine receptor function in the low nM concentration range, zinc may complicate experimental measurements that are presumed to be due solely to an effect of exogenously-applied glycine. Our previous studies (Kirson et al., 2013; McCracken et al., 2010) showed that tricine decreases the magnitudes of currents elicited by low but not maximally-effective concentrations of glycine, as well as all concentrations of the partial agonist taurine. The most parsimonious explanation of these findings is that, by chelating zinc, tricine allows one to measure the true effects of agonists in isolation on the GlyR. This has implications for previously published studies in which it was tacitly assumed that background contaminating levels of zinc were insufficient to affect experimental results. This appears not to be the case and in this study we attempted to determine the sources of zinc in our electrophysiological assays.
The choice of type of vial in which experimental solutions are made before application to oocytes does not appear to play a major role in zinc contamination (Fig. 1), since currents elicited by solutions made up in all three vials were similar to one another. In all three cases, addition of tricine decreased currents elicited by low but not high concentrations of glycine, most likely via chelation of this allosteric modulator of the GlyR. These data suggest that either all three types of vials have similar contaminating levels of zinc on their surfaces or, more likely, that the zinc contamination is coming from either the deionized water or buffer constituents. We ruled out the former possibility by washing vials repeatedly, or even soaking them for 10 min in the presence of tricine, before using them in experiments and still saw greater effects of glycine, compared to those seen when tricine was added to vials just before use (Fig. 2). However, even though there appears to be no surface contamination of vials with zinc, this is not necessarily the case with pipets used to make solutions. Both Fisher and VWR polystyrene pipets appear to be contaminated with zinc since the first use of each pipet led to solutions that elicited markedly higher currents than subsequent uses of each pipet (Fig. 3); the presence of zinc in the Fisher polystyrene pipets was confirmed by ICP-MS measurements. This was not true of the Fisher glass pipets tested. As in previous studies, the presence of tricine prevented contaminants from having modulatory effects. This last study also argues against the remote, but admittedly not disproved, possibility that all of these findings are simply due to an allosteric effect that tricine itself has on the GlyR; i.e., that tricine acts as an inverse allosteric modulator. This seems unlikely since we found that another zinc chelator, N,N,N',N'-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN), also produces the same decreases in glycine-mediated currents as tricine, and that tricine has no significant effects if applied with TPEN (data not shown). In addition, tricine does not act as an allosteric modulator at GABAA receptors (Wilkins and Smart, 2002).
Kay (2004) used the zinc-sensitive fluorimetric probe FluoZin-3 with a detection threshold of 50 pM to measure zinc contamination in commonly used labware. He found it in a wide range of materials, ranging from several plastic labware sources such as transfer pipettes, Tygon tubing, stainless steel and glass. Even transient contact with contaminated labware could lead to concentrations in the ~100 nM concentration range, at which half maximal enhancement of GlyR function can be observed using an EC5 concentration of glycine (Miller et al., 2005). In our studies, zinc contamination appeared to come from a variety of sources including the distilled water, solutes used to make the MBS buffer, as well as the polystyrene pipets.
Cadmium is a transition metal falling just below zinc on the element periodic table and could conceivably be thought responsible for some of the effects we observed. Kay (2004) reported that FluoZin-3 cannot distinguish between Cd2+ and Zn2+. We found cadmium in the MBS at a concentration of about 7 nM. It is unlikely that cadium is the responsible agent in our studies since cadmium inhibits rather than enhances GlyR-mediated currents in dissociated rat hippocampal neurons and has low potency, with an IC50 of 1.27 mM (Wang et al., 2006). It was also found to act at a site distinct from that mediating zinc actions.
In conclusion, our results suggest that zinc is found as a contaminant in laboratory buffers and some labware in amounts sufficient to enhance glycine receptor function. Without checking for this effect using a chelator such as tricine, one cannot assume that responses elicited by the application of glycine alone are not necessarily also partially due to some level of allosteric modulation by zinc. This issue may be especially important to those attempting to glean detailed mechanistic information of glyine receptor function, such as in single channel electrophysiology studies.
Highlights.
Zinc is a contaminant in common electrophysiological buffers and labware.
Contaminating zinc levels enhance glycine receptor function.
Different vial types do not affect zinc mediated glycine receptor enhancement.
Washing vials did not affect zinc mediated glycine receptor enhancement.
Use of polystyrene serological pipets can lead to zinc contamination of solutions.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse & Alcoholism grant 5P01AA020683 and Bruce Jones & Frayne predoctoral fellowships. The zinc and cadmium quantitation performed by Dr. Nathan R. Miller of the Laser Ablation and ICP-MS Facility at the University of Texas at Austin is greatly appreciated.
Abbreviations
- EC5
effective concentration producing 5% of maximal effect
- GlyR
glycine receptor
- ICP-MS
inductively-coupled plasma mass spectrometer
- MBS
Modified Barth’s Saline; N, N,N',N'-tetrakis(2-pyridylmethyl)ethane-1,2-diamine,
- TPEN
tri; tricine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baer K, Waldvogel HJ, Faull RLM, Rees MI. Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci. 2009;2:25. doi: 10.3389/neuro.02.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha1 subunit. J Physiol. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytiä P, Ericson M, Söderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res. 2009;1305:S27–S36. doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Söderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- Kirson D, Cornelison GL, Philpo AE, Todorovic J, Mihic SJ. Physiological concentrations of zinc reduce taurine-activated GlyR responses to drugs of abuse. Neuropharmacol. 2013 doi: 10.1016/j.neuropharm.2013.07.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiol. 2004;4:4. doi: 10.1186/1472-6793-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522(Pt 2):215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Rundström N, Kirsch J, Schmieden V, Betz H. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol. 1995;483:613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye JH. Glycine-activated chloride currents of neurons freshly isolated from the prefrontal cortex of young rats. Brain Res. 2011;1393:17–22. doi: 10.1016/j.brainres.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. Zinc enhances ethanol modulation of the alpha1 glycine receptor. Neuropharmacology. 2010;58:676–681. doi: 10.1016/j.neuropharm.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Lawshe JE, Ellington AD, Mihic SJ. Identification of novel specific allosteric modulators of the glycine receptor using phage display. J Biol Chem. 2010;285:22840–22845. doi: 10.1074/jbc.M110.130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gu Y, Wang HL, Li XM, Wang M, Sun LG, Ruan DY. Inhibitory effect of Cd2+ on glycine-induced chloride current in rat hippocampal neurons. Brain Res Bull. 2006;69:680–686. doi: 10.1016/j.brainresbull.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Wilkins ME, Smart TG. Redox modulation of GABAA receptors obscured by Zn2+ complexation. Neuropharmacol. 2002;43:938–944. doi: 10.1016/s0028-3908(02)00238-1. [DOI] [PubMed] [Google Scholar]