Abstract
Background
We previously reported renal arterial periarteritis after implantation of a continuous-flow left ventricular assist device in calves. The purpose of the present study was to investigate whether the same periarteritis changes occur in the intrapulmonary arteries after implantation of a continuous-flow right ventricular assist device (CFRVAD) in calves and to determine the mechanism of those histological changes.
Methods
Ten calves were implanted with a CFRVAD for 29 ± 7 days, and we compared pulmonary artery samples and hemodynamic data pre- and post-CFRVAD implantation prospectively.
Results
Post implantation, the pulsatility index (pulmonary arterial pulse pressure/pulmonary arterial mean pressure) significantly decreased (pre 0.88 ± 0.40 vs. post 0.51 ± 0.22; p < 0.05), with severe periarteritis of the intrapulmonary arteries in all cases. Periarterial pathology included hyperplasia and inflammatory cell infiltration. The number of inflammatory cells positive for the angiotensin II type 1 receptor was significantly higher after implantation (pre-CFRVAD 7.8 ± 6.5 vs. at autopsy 313.2 ± 145.2; p < 0.01). Serum angiotensin-converting enzyme activity significantly decreased after implantation (pre-CFRVAD 100%, week 1 49.7 ± 17.7%; p = 0.01). Tissue levels of angiotensin-converting enzyme also demonstrated a significant reduction (pre 0.381 ± 0.232 vs. at autopsy 0.123 ± 0.096; p = 0.043).
Conclusions
Periarteritis occurred in intrapulmonary arteries of calves after CFRVAD implantation. The local renin–angiotensin system (not the angiotensin-converting enzyme pathway) plays an important role in such changes.
Keywords: Artery/arteries (includes any peripheral arteries); Circulatory support devices (LVAD, RVAD, BVAD, TAH); Pulmonary arteries/veins (includes normal and diseased); Pathology (Lung)
Introduction
Whether pulsatility is essential to blood circulation has long been a subject of lively discussion. Within the last decade, continuous-flow left ventricular assist devices (CFLVADs) have replaced pulsatile left ventricular assist devices (LVADs) as the device treatment of choice for end-stage heart failure patients; CFLVAD patients now routinely live with their implanted devices for years. It is expected that, over the next decade, the number of patients with CFLVADs as “destination therapy” will exceed the current number of “bridge-to-transplant” LVAD patients. The much smaller CFLVADs have proven to be more reliable, to have a lower incidence of infection, and to be subject to at least an equivalent, very low incidence of thromboembolic events compared with the HeartMate I pulsatile LVAD [1]. With the increased duration of support provided by continuous-flow pumps, the long-term pathophysiologic effects of continuous-flow blood pumps takes on more importance. We now propose a closer look at the pulmonary and systemic arterial histological changes seen in our laboratory in animals with continuous-flow pumps.
We have reported that periarteritis in the kidney occurred in calves after CFLVAD implantation and that angiotensin (Ang) II type 1 receptor (AT1R) was positive in the renal periarterial mononuclear inflammatory cells infiltrate [2]. The purpose of the present study was to investigate whether the same histological changes occur in intrapulmonary arteries (IPAs) after implantation of a continuous-flow right ventricular assist device (CFRVAD). We compared hemodynamic data and pulmonary histological changes before and after CFRVAD implantation and used immunohistochemical methods to investigate the mechanisms of the histological changes in the reduced pulsatile pulmonary circulation.
Material and Methods
Animals, Device Description, and Implantation Procedure
This study was approved by Cleveland Clinic’s Institutional Animal Care and Use Committee, and all animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised 1996.
Our current CFRVAD, Cleveland Clinic’s DexAide blood pump [3], was implanted in 10 male, Holstein calves (110.1 ± 21.6 kg) for 29 ± 7 days (21–41 days). Anesthesia was induced with ketamine 10 mg/kg i.m. and 5.0% isoflurane via mask inhalation. The animal was then intubated, and anesthesia was maintained with isoflurane (0.5–2.5%) and oxygen. Through a thoracotomy, the outlet cannula was anastomosed to the main pulmonary artery (PA). After systemic heparinization (300 U/kg), a right ventriculotomy was made in the outflow portion of the right ventricle for inlet cannulation while the animal remained on cardiopulmonary bypass (CPB) support. The CFRVAD was connected to the inflow cannula and outflow graft. Upon discontinuation of CPB, the CRFVAD was immediately initiated.
Intraoperative Hemodynamic Data
An ultrasonic perivascular flow probe (Transonic Systems Inc., Ithaca, NY) was placed around the PA or the ascending aorta for stroke volume measurement. A fluid-filled pressure line was inserted into the PA to continuously monitor PA pressure (PAP). Before starting CPB, baseline hemodynamic data were recorded without CFRVAD support. After CFRVAD implantation and weaning from CPB support, hemodynamic data were again recorded in fixed-speed mode at 2,400 rpm. The pulsatility of the PAP was quantified by PA pulse pressure (PA systolic pressure – PA diastolic pressure) and by the pulsatility index (PA pulse pressure/mean PAP).
Autopsy
Following device implantation, no anticoagulants or antiplatelet medications were administered to the calves for the duration of their post-operative recovery and chronic care. At the completion of each study, the animal was sacrificed after full heparinization (500 U/kg bolus injection) with an overdose of sodium pentobarbital (5,000 mg) and potassium chloride (240 mEq), and a thorough autopsy was performed.
Pathological Study
In surgery before CFRVAD implantation and at autopsy, PA tissue specimens were obtained and fixed in 10% formaldehyde. Sections of 4-μm thickness were stained with hematoxylin-eosin for light microscopy evaluation. To quantify morphological changes, hematoxylin-eosin-stained sections were scanned at 100x magnification with a light microscope equipped with a Retiga EXi Fast 1394 camera (QImaging, Burnaby, British Columbia, Canada). The arterial wall diameter, wall thickness, ratio of wall thickness/wall diameter, wall cross-sectional area, and ratio of smooth muscle layer cross-sectional area/number of smooth muscle cells were measured using Image-Pro Plus (Media Cybernetics, Bethesda, MD). Data from an average of 20 to 30 arteries were reported for each animal. The arterial smooth muscle layer cross-sectional area was determined by ascertaining area 1 (calculated from the minimal diameter of the external elastic lamina) and subtracting area 2 (calculated from the minimal diameter of the internal elastic lamina). We used this straightforward method because the measurements are least affected by the sectioning angle.
Immunohistochemical Study
Four-micrometer-thick sections, cut from paraffin blocks of the lung tissues, were immunostained for AT1R by rabbit anti-AT1R antibody (ab18801, Abcam, Cambridge, UK). Tissue sections were deparaffinized in xylene and descending grades of alcohol. Retrieval of the antigen was accomplished by treating the sections in 10 mM citrate buffer, pH 6.0, in a 120 °C autoclave for 5 min. After blocking the endogenous peroxidase activity, the primary and then secondary antibodies were added to tissue sections and incubated for 1 hour each at room temperature. Antibody binding was visualized by adding diaminobenzidine as a chromogen and hematoxylin for counterstaining. To quantify the number of inflammatory cells present, AT1R-positive cells were measured on 20 different sections of the endothelial layer at a final magnification of 200:1 by using Image-Pro Plus Image. The average number of positive cells found in each section was reported for each animal.
Angiotensin-Converting Enzyme Assay
Blood samples were obtained weekly after CFRAVD implantation, and the standard angiotensin-converting enzyme (ACE) assay [4], which measures ACE cleavage of the Ang I analog Hip-His-Leu, was performed by incubating serum.
Western Blot
Tissue samples were homogenized in lysis buffer (0.5% Triton X100, 150 mM NaCl, 20 mM HEPES, pH 7.4, 1.5 mM MgCl2, 2 mM DTT, 2 mM EGTA, 10 mM NaF, 12.5 mM β-glycerophosphate, 1 mM Na3VO4, and protease inhibitor). Samples were centrifuged at 14,000 rpm at 4 °C for 15 min, and the supernatants were used for analysis. Protein samples (50 μg) were separated by 8% SDS-PAGE gel. These protein samples were transferred onto a polyvinylidene fluoride membrane was blocked with 5% dry milk in Tris-buffered saline with 0.05% Tween 20, then incubated with goat anti-ACE antibody at 4 °C for 16 h [5]. The membrane was incubated with HRP-labeled anti-goat antibody for 1 hour at room temperature. Bands were visualized by enhanced chemiluminescence (ECL western blotting detection system, Amersham Biosciences, Amersham, UK). The intensity of both bands was quantified using NIH Image J software.
Statistical Analysis
All data were converted for evaluation in Microsoft Excel XP (Microsoft, Redmond, WA), and data are expressed as mean ± standard deviation. Paired t tests were performed with a commercially available software program (StatView 5.0, SAS Institute, Inc., Cary, NC). A probability value of <0.05 was considered significant.
Results
Intraoperative Hemodynamic Data
The intraoperative hemodynamic data are summarized in Table 1. The pulsatility index of post-CFRVAD implantation was significantly smaller than that of the pre-CFRVAD implantation (pre 0.88 ± 0.40 vs. post 0.51 ± 0.22, p < 0.05). The average flow of the CFRVAD during entire implantation was 5.6 ± 1.2 L/min.
Table 1.
Hemodynamic Data
| Variables | Pre Implantation | Post Implantation |
|---|---|---|
| PA Pressure (mm Hg) | ||
| Systole | 30.1 ± 7.4 | 36.8 ± 12.3 |
| Mean | 21.5 ± 5.5 | 29.3 ± 10.5† |
| Diastole | 11.5 ± 5.8 | 22.7 ± 10.1 † |
| PA Pulse Pressure (mm Hg) | 18.6 ± 8.5 | 14.1 ± 4.9 |
| PA Pulsatility Index | 0.88 ± 0.40 | 0.51 ± 0.22† |
| PA Flow (ml/mim) | 12.5 ± 2.9 | 11.1 ± 2.6 |
| PVR | 99.3 ± 51.7 | 183.4 ± 141.5 |
| Ao Pressure (mm Hg) | ||
| Systole | 127.9± 23.8 | 116.5 ± 28.6 |
| Mean | 105.0 ± 17.2 | 94.6 ± 23.9 |
| Diastole | 88.7 ± 14.9 | 77.5 ± 20.8 |
| Ao Pulse Pressure (mm Hg) | 39.1 ± 16.6 | 39.0 ± 16.5 |
| Ao Pressure Pulsatility Index | 0.37 ± 0.12 | 0.42 ± 0.15 |
| Stroke Volume (ml) | 188.4 ± 191.6 | 184.8 ± 195.6 |
| SVR | 705.7 ± 209.5 | 732.2 ± 292.1 |
PA, pulmonary artery; PVR, pulmonary vascular resistance: Ao, aortic; SVR systemic vascular resistance.
p < 0.05 post vs. pre implantation status.
Pathological Changes After CFRVAD Implantation
All 10 calves demonstrated diffuse periarteritis in the IPAs, characterized by hyperplasia and an inflammatory response (Fig 1A). The major pathological findings were these: The medium-sized IPAs showed wall thickening, with abundant mononuclear cells infiltrating the periarterial areas and endothelium. The artery structure was relatively conserved with extensive hyperplasia of the smooth muscle layer of the vascular wall media and few inflammatory cells present. These findings were not observed in pre-CFRVAD implantation lung histology (Fig 1B) or in kidney histology after CFRVAD implantation (Fig 1C).
Fig. 1. Hematoxylin-eosin (HE) staining of the lung and kidney (magnification x50).
(A) Lung specimen obtained at autopsy after CFRVAD implantation, in which extensive hyperplasia of the media and adventitia of the arteries is evident, along with mononuclear inflammatory cell infiltrates. (B) Lung specimen taken prior to CFRVAD implant shows no pathologic morphology or inflammatory cell infiltrates. (C) Kidney specimen taken at autopsy after CFRVAD implantation from the same animal shows no pathologic morphology or inflammatory cell infiltrates.
Morphometry of IPA
Compared with pre-CFRVAD implantation histological findings, those at autopsy exhibited significantly greater IPA wall diameter, thickness and cross-sectional area (Table 2). There was no increase in the smooth muscle layer cross-sectional area/smooth muscle cell count ratio, suggesting that the increased wall thickness was due to hyperplasia, not hypertrophy.
Table 2.
Morphometric data
| Variables | Pre-CFRVAD implantation | Autopsy | P Values |
|---|---|---|---|
| Wall diameter (μm) | 163.7 ± 86.3 | 382.8 ± 87.9 | 0.0001 |
| Wall thickness (μm) | 52.7 ± 33.1 | 159.7 ± 47.1 | 0.0001 |
| Wall thickness/diameter | 0.30 ± 0.02 | 0.38 ± 0.09 | 0.060 |
| SML-CSA (mm2) | 0.035 ± 0.038 | 0.217 ± 0.116 | 0.001 |
| SML-CSA/nSMC (μm2/cell) | 148.9 ± 53.9 | 186.5 ± 34.6 | 0.124 |
CFRVAD, continuous-flow right ventricular assist device; SML-CSA, cross-sectional area of the smooth muscle layer; nSMC, number of smooth muscle cells.
Immunohistochemical Findings
AT1R was observed in the endothelial and inflammatory cells that infiltrated the periarterial interstitial areas of all animals at autopsy (Fig 2). The average number of inflammatory cells in the periarterial areas was significantly higher at autopsy than pre-CFRVAD (pre-CFRVAD 28.2 ± 14.6 vs. at autopsy 394.2 ± 178.4; p < 0.01) (Fig 3). The average number of AT1R-positive cells in periarterial areas was significantly higher at autopsy than pre-CFRVAD (pre-CFRVAD 7.8 ± 6.5 vs. at autopsy 313.2 ± 145.2; p < 0.01) (Fig 3).
Fig. 2. Immunohistochemical staining for angiotensin II type 1 receptor (AT1R).
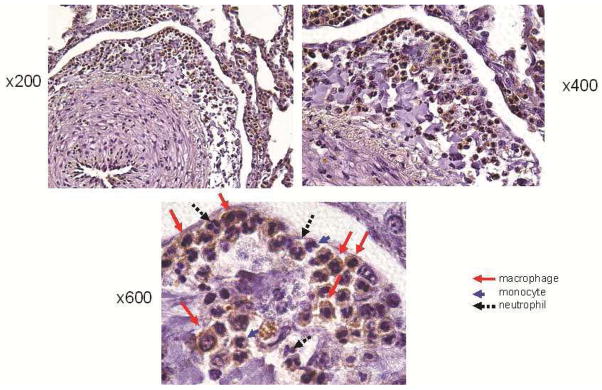
AT1R was observed at autopsy in inflammatory cells that had infiltrated the periarterial areas of all animals (stained brown; magnification x200 and x400).
Fig. 3. Numbers of inflammatory cells and AT1R positive cells.
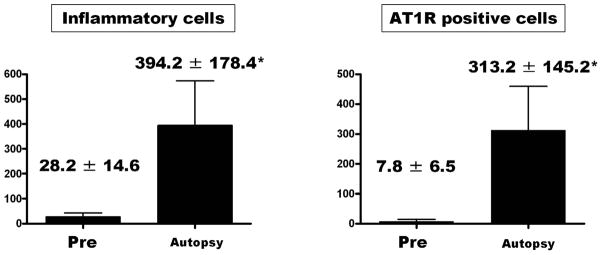
The average number of inflammatory cells in periarterial areas was significantly higher at autopsy than pre-CFRVAD. *p < 0.01 vs. Pre.
Serum ACE Assay and Tissue ACE Expression Level by Western Blot
Serum ACE enzymatic activity significantly decreased after implantation (pre-CFRVAD 100% vs. week 1, 49.7 ± 17.7%; p = 0.01) (Fig 4a). Tissue ACE level significantly decreased after implantation (pre-CFRVAD 0.381 ± 0.232 vs. at autopsy 0.123 ± 0.096; p = 0.043) (Fig 4b).
Fig. 4.
Serum ACE and tissue ACE levels.
Comment
In this study, we observed changes in calf lungs indicating periarteritis at 1 month after CFRVAD implantation. Macrophages and monocyte cells had infiltrated around the IPAs. Levels of AT1R were upregulated in those mononuclear inflammatory cells around the IPAs after implantation of the CFRVAD. The pulsatility index of the PA dropped significantly after CFRVAD implantation. In comparison, CFLVAD support has been reported to cause renal cortical artery hypertrophy and inflammatory cell infiltration in the renal cortex [6]. We previously investigated histological changes indicating periarteritis in the kidneys of calves after CFLVAD implantation, and there were no histological changes in other organs, including IPAs [2]. In the present study, there were no histological changes in the renal arteries after CFRVAD implantation (Fig 1C) in contrast to our reported pulmonary arterial histological findings. One group has reported that at moderate levels of CFLVAD support (25%–40% of cardiac output), the amount of blood flow distal to the outflow graft anastomosis decreased by approximately 25% because of increased regurgitated blood flow to the aorta [7]. Those investigators postulated that the reduced renal blood flow under CFLVAD increased the levels of systemic renin-angiotensin and caused renal arterial hyperplasia. Our data does not support this, however, as per the following summary of our findings: 1) the pulsatility of PA dropped significantly under CFRVAD flow; 2) changes indicating periarteritis occurred in IPAs after CFRVAD implantation; and 3) serum and tissue ACE levels significantly decreased after CFRVAD implantation The following summary of the findings in this study mirror those for the renal arteries in our CFLVAD study [2]: 1) the pulsatility of PA dropped significantly under CFRVAD flow; 2) changes indicating periarteritis occurred in IPAs after CFRVAD implantation; and 3) serum and tissue ACE levels significantly decreased after CFRVAD implantation. These results supported the hypothesis that continuous-flow devices induce these histological changes in the vasculature of organs peripheral to the respective devices.
Our morphologic findings in terms of the size of pulmonary arteries demonstrating inflammatory responses (164 ± 86 μm) parallels findings in primary pulmonary hypertension (PPH) patients with decreased and increased biomechanical stimuli to the endothelium being the common thread. Eunhee et al. [8] reported that pulmonary arterial pathology of PPH patients (n=15) showed a pattern of predominantly small-arterial involvement using computer-assisted image analysis and immunohistochemical methods. The increase of % intimal thickness was observed most prominently in small arteries and arterioles (external diameters of 50 to 200 μm). The PPH patients also showed a significant increase in % intimal thickness within medium-sized muscular arteries (201 to 400 μm) but not in large arteries of > 400 μm external diameters as compared with their normal human controls. One could postulate that endothelial response to biomechanical forces is mediated primarily within pulmonary arteries in the range of 50 to 400 μm and invokes a similar inflammatory response to hypertension and arterial depulsation. These potential relationships certainly need much more scientific study and would be inappropriate to include in any detail within this manuscript.
We detected AT1R in mononuclear cells in the IPAs after exposure to reduced pulsatile circulation, just as we had previously detected AT1R in mononuclear cells in the renal arteries after CFLVAD implantation [1]. In addition to its effect on blood pressure, Ang II is a factor in a variety of nonhemodynamic actions, including pro-inflammatory and cell growth through an AT1R [9], and Ang II also modulates the proliferation of vascular smooth muscle cells and causes marked thickening of the vascular wall [10–12]. Several reports have indicated the possible role of Ang II in inducing chemoattractants for monocyte/macrophage infiltration in a rabbit model of early accelerated arteriosclerosis and in a rat model [13,14]. Klotz and colleagues [15,16] reported that CFLVADs induce reverse structural remodeling, such as a leftward shift of the end-diastolic pressure-volume relationship, and reduced LV mass with increases in total and cross-linked collagen. They also reported that after CFLVAD implantation, myocardial Ang II levels increased and myocardial collagen crosslinking and myocardial stiffness were reversed by ACE inhibitor therapy [15,16].
We found that serum ACE enzymatic activity and tissue ACE levels also decreased after CFRVAD implantation. These findings are contrary to the findings of Klotz et al. [15], which indicated ACE-I therapy reserve the CFLVADs histological change. We hypothesized that the local renin-angiotensin system is activated under conditions of continuous flow, and our results suggest that Ang II is synthesized not by systemic ACE in serum or local ACE in pulmonary tissue, but by other ACE-independent pathways, such as the chymase pathway. The positive effects of treatment with ACE inhibitors or AT1R blockers (ARBs) for heart failure patients have been demonstrated. Most patients who have received a ventricular assist device take these medications as part of a standard therapeutic regimen to suppress the progression of heart failure. If reduced pulsatility induces an ACE-independent local renin-angiotensin system, ARBs may be clinically superior in protecting against periarteritis. ARBs target Ang II synthesized by both ACE-dependent and -independent pathways; however, ACE inhibitors target only ACE-dependent Ang II formation. Further research will be needed to determine the effects of ACE inhibitors or ARBs on the mechanisms of these pathologic changes.
This study’s main limitation is that the number of animals is small (n = 10). However, to induce reduced systemic pulsatility without flow reduction is possible only when a ventricular assist device or total artificial heart is implanted, and ideally the experimental animal should be about the size of a human, and to assess histological changes, the model animal should be observed over the course of at least 1 month.
Another limitation is that this study did not have a specific control group in which a pulsatile RVAD was implanted; however, a review of necropsy records and histology reports from our pulsatile total artificial heart experience showed no reports of intrapulmonary vascular pathology. Also, we saw no histological changes in the organs of the opposing pulsatile circulation with either our CFLVAD or CFRVAD implants. The periarteritis occurred exclusively in the arteries of the depulsed circulation. Since the pumps used for the CFLVAD and CFRVAD were of the same type and materials, any postoperative inflammation, antigenic stimulation, or drug effects on the animals would presumably be the same in both studies, except at the site of implantation. It is also expected that any reaction to operative intervention or medications or an immune response to the implant materials would have affected all organs perfused by both the pulmonary and systemic circulation. From those results, we suspect that it was the continuous-flow condition, brought about by these assist devices, that induced those histological changes. Another possible cause for these histological changes is shear stress related very short acting as yet unknown substances. Further research to quantify a dose response over multiple levels of flow pulsatility will be needed to better characterize low pulsatile flow effects
In conclusion, periarteritis occurred in the IPAs with a CFRVAD in calves. AT1R levels were upregulated in mononuclear inflammatory cells in the IPAs. The local renin-angiotensin system (not the ACE pathway) may play an important role in such changes.
Acknowledgments
This work was supported by Bioengineering Research Partnerships grant 5R01HL074896 (to K.F.) from the National Heart, Lung, and Blood Institute/NIH.
List of abbreviations
- ACE
Angiotensin-converting enzyme
- Ang
Angiotensin
- ARB
Angiotensin II type 1 receptor blocker
- AT1R
Angiotensin II type 1 receptor
- CFLVAD
Continuous-flow left ventricular assist device
- CFRVAD
Continuous-flow right ventricular assist device
- CPB
Cardiopulmonary bypass
- IPAs
Intrapulmonary arteries
- LVAD
Left ventricular assist device
- PA
Pulmonary artery
- PAP
Pulmonary arterial pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LW, Pagani FD, Russell SD, et al. HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Ootaki C, Yamashita M, Ootaki Y, et al. Reduced pulsatility induces periarteritis in kidney: role of the local renin-angiotensin system. J Thorac Cardiovasc Surg. 2008;136:150–8. doi: 10.1016/j.jtcvs.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukamachi K, Saeed D, Massiello AL, et al. Development of DexAide right ventricular assist device: Update II. ASAIO J. 2008;54:589–93. doi: 10.1097/MAT.0b013e31818a30f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler SP, Gomos JB, Scheidemantel TS, Rowe TM, Smith HL, Sen GC. The germinal isozyme of angiotensin-converting enzyme can substitute for the somatic isozyme in maintaining normal renal structure and functions. J Biol Chem. 2002;277:4271–6. doi: 10.1074/jbc.M109474200. [DOI] [PubMed] [Google Scholar]
- 5.Chattopadhyay S, Santhamma KR, Sengupta S, et al. Calmodulin binds to the cytoplasmic domain of angiotensin-converting enzyme and regulates its phosphorylation and cleavage secretion. J Biol Chem. 2005;280:33847–55. doi: 10.1074/jbc.M501718200. [DOI] [PubMed] [Google Scholar]
- 6.Kihara S, Litwak KN, Nichols L, et al. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous-flow left ventricular assist. Ann Thorac Surg. 2003;75:178–83. doi: 10.1016/s0003-4975(02)04087-0. [DOI] [PubMed] [Google Scholar]
- 7.Litwak KN, Kihara S, Kameneva MV, et al. Effects of continuous-flow left ventricular assist device support on skin tissue microcirculation and aortic hemodynamics. ASAIO J. 2003;49:103–7. doi: 10.1097/00002480-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Yi ES, Kim H, Ahn H, et al. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med. 2000;162:1577–86. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 9.Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Curr Protein Pept Sci. 2004;5:267–73. doi: 10.2174/1389203043379693. [DOI] [PubMed] [Google Scholar]
- 10.Powell JS, Clozel JP, Müller RK, et al. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989;245:186–8. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- 11.Campbell-Boswell M, Robertson AL., Jr Effects of angiotensin II and vasopressin on human smooth muscle cells in vitro. Exp Mol Pathol. 1981;35:265–76. doi: 10.1016/0014-4800(81)90066-6. [DOI] [PubMed] [Google Scholar]
- 12.Kessler SP, deS Senanayake P, Scheidemantel TS, Gomos JB, Rowe TM, Sen GC. Maintenance of normal blood pressure and renal functions are independent effects of angiotensin-converting enzyme. J Biol Chem. 2003;278:21105–12. doi: 10.1074/jbc.M3023472000. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Presa M, Bustos C, Ortego M, et al. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–41. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Ortega M, Bustos C, Hernández-Presa MA, Lorenzo O, Plaza JJ, Egido J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–9. [PubMed] [Google Scholar]
- 15.Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AH. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J. 2009;7:805–12. doi: 10.1093/eurheartj/ehp012. [DOI] [PubMed] [Google Scholar]
- 16.Klotz S, Danser AH, Foronjy RF, et al. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–74. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]




