Abstract
Objective
Follistatin-like protein 1 (FSTL-1) is a secreted glycoprotein overexpressed in certain inflammatory diseases. Our objective was to correlate FSTL-1 levels with gene expression, known biomarkers, and measures of disease activity in systemic juvenile idiopathic arthritis (sJIA), including macrophage activation syndrome (MAS).
Methods
FSTL-1 serum levels were measured by ELISA in 28 patients with sJIA, including 7 patients who developed MAS, and 30 healthy controls. Levels were correlated with erythrocyte sedimentation rate (ESR), ferritin, and soluble interleukin-2 receptor-α (sIL-2Rα). Gene expression based on FSTL-1 levels was analyzed in peripheral blood mononuclear cells (PBMC).
Results
Serum levels of FSTL-1 were elevated at time of presentation of sJIA (mean 200.7 ng/ml) and decreased to normal (mean 133.7 ng/ml) over 24 months (p < 0.01). FSTL-1 levels were markedly elevated during acute MAS (mean 279.8 ng/ml) and decreased to normal following treatment (p < 0.001). FSTL-1 levels correlated with serum markers of inflammation, including sIL-2Rα and ferritin. Ferritin/ESR ratio was superior to ferritin, sIL-2Rα, and FSTL-1 in discriminating MAS from new-onset sJIA. PBMC from patients with FSTL-1 levels > 200 ng/ml showed altered expression of genes related to innate immunity, erythropoiesis, and natural killer cell dysfunction. Two patients with the highest FSTL-1 levels at disease onset (> 300 ng/ml) ultimately developed MAS.
Conclusion
Elevated pretreatment serum FSTL-1 levels in sJIA are associated with dysregulated gene expression suggestive of occult MAS, and may have utility in predicting progression to overt MAS. Ferritin/ESR ratio may be superior to ferritin alone in discriminating overt MAS from new-onset sJIA. (First Release May 15 2013; J Rheumatol 2013;40:1191–9; doi:10.3899/jrheum.121131)
Key Indexing Terms: SYSTEMIC JUVENILE IDIOPATHIC ARTHRITIS, SENSITIVITY, SPECIFICITY, MACROPHAGE ACTIVATION SYNDROME, FOLLISTATIN-LIKE PROTEIN 1, BIOMARKERS
Systemic juvenile idiopathic arthritis (sJIA) is a subtype of juvenile idiopathic arthritis (JIA), clinically manifested by high spiking quotidian fevers, evanescent characteristic “salmon-patch” rash, and arthritis, as well as systemic inflammation1. Additionally, sJIA carries the risk of development of macrophage activation syndrome (MAS), a potentially fatal complication occurring in about 10% of patients. MAS is characterized by excessive activation of macrophages and T cells, leading to an overwhelming inflammatory response2. Clinical observations indicate that sJIA is a heterogeneous entity. A subset of about 50%–60% of patients have a persistent disease course, which generally requires aggressive therapy and carries a high burden of morbidity and disability, while another 40% have monocyclic disease3,4,5,6. Similar molecular and genetic differences among patients with sJIA have been described, with a subset of patients displaying abnormal laboratory features such as occult hemophagocytosis, elevated soluble CD163, and elevated soluble interleukin 2 receptor-α (sIL-2Rα). It has been suggested that these patients may represent a subclinical or occult MAS, in that they demonstrate some laboratory features of inflammation reminiscent of active MAS without developing overt MAS7. Bone marrow findings showing frequent occult hemophagocytosis in sJIA patients without overt MAS suggest that subclinical MAS may be common in patients with sJIA8. Gene expression signature distinguishing these patients with subclinical MAS was reflective of peripheral expansion of immature monomyelocytoid precursors9. In some cases patients identified as having subclinical MAS developed subsequent overt MAS10. Diagnosis of MAS in the setting of sJIA is challenging. A previous study analyzed the sensitivity and specificity of certain markers, especially ferritin, in the diagnosis of MAS complicating sJIA11, but did not address the role of subclinical MAS in their analysis.
Follistatin-like protein 1 (FSTL-1) was initially discovered to play a role in inflammatory arthritis when it was found that the protein was highly overexpressed in mouse paws in early stages of collagen-induced arthritis12. FSTL-1 was subsequently shown to upregulate the proinflammatory cytokines IL-1β, tumor necrosis factor-α (TNF-α), and IL-613. In a recent study, we measured serum and synovial fluid FSTL-1 levels in children with various forms of JIA, including oligoarticular, polyarticular, and sJIA. Only patients with sJIA whose disease was active had elevated serum FSTL-1, suggesting that FSTL-1 might be a marker of active disease in sJIA14. However, the number of children with active sJIA was small (n = 5), making it difficult to draw firm conclusions. In that study, FSTL-1 was discovered to be produced in the joint space by cells of the mesenchymal lineage and to be induced by proinflammatory cytokines, especially IL-1β. We recently reported that serum FSTL-1 is also elevated in Kawasaki disease, another pediatric systemic illness characterized by elevated serum IL-1β and IL-6 and with reported cases of associated MAS15.
Our present study was designed to expand on our preliminary observations to further characterize the relationship between serum FSTL-1 levels and disease activity in sJIA. We also investigated the role of other biomarkers in discriminating MAS from new-onset sJIA.
MATERIALS AND METHODS
Patient samples
Serum samples were obtained from 28 individual patients with sJIA over a 24-month period, including 7 who developed MAS, and 30 healthy controls. Controls were of similar age, sex, and race distribution (Table 1). Four patients had paired samples before and after treatment for MAS. Most JIA specimens and data as well as control serum samples were obtained from the biorepository at Cincinnati Children’s Hospital Medical Center (CCHMC), with additional samples and data from the pediatric rheumatology clinics at Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center. Samples obtained from CCHMC were initially obtained as part of a multicenter study of gene expression profiles in childhood arthritis7. Patient samples were obtained at time of initial presentation (pretreatment) and roughly 6, 12, and 24 months after initiation of treatment. Some patients began treatment up to 2 months after initial presentation. Pretreatment samples were obtained an average of 3 months after reported onset of disease and prior to treatment. Patients were in remission after 24 months according to chart review. American College of Rheumatology response data were not collected. A total of 107 patient samples were obtained. The International League of Associations for Rheumatology diagnostic criteria10 were used to diagnose sJIA. MAS was diagnosed based on hyperferritinemia, relative cytopenias, and elevated soluble CD163 and sIL-2Rα. Available detail regarding MAS episodes is reviewed in Table 2. Treatment included nonsteroidal antiinflammatory drugs (NSAID), methotrexate, anakinra, etanercept, and cyclosporine, and combinations of these medications. A majority of patients received glucocorticoids at some point in their course. Use of samples was approved by the institutional review boards of the University of Pittsburgh and CCHMC.
Table 1.
Patient demographic data.
| Patient Group (no. patients) | Age, yrs (SD) | Sex | Race |
|---|---|---|---|
| Systemic JIA (28) | 7.0* (4.3) | 10 female 18 male |
23 white 2 black 3 other |
| Controls (30) | 8.0 (2.8) | 10 female 20 male |
27 white 3 black |
| p | 0.33† | 1.0†† | 0.19†† |
Age at pretreatment sample.
Student t test.
Fisher exact test. JIA: juvenile idiopathic arthritis.
Table 2.
Laboratory measures and medication course for patients with macrophage activation syndrome (MAS).
| Patient | FSTL-1 at Onset of sJIA, ng/ml | FSTL-1 at MAS, ng/ml | ESR, mm/h | Ferritin, ng/ml | sIL2-Rα, pg/ml | Medication Course |
|---|---|---|---|---|---|---|
| 1* | NA | 117 | 16 | 10,000 | 4369.6 | MPSL pulses |
| 2* | NA | 215 | 19 | 1740 | 5985.6 | MPSL pulses |
| 3* | NA | 185 | 22 | NA | 6027.3 | MPSL pulses, cyclosporine |
| 4 | 311 | 289 | 30 | 2416 | 16670 | MPSL pulses, cyclosporine |
| 5 | 175 | 190 | 59 | 8420 | 9063.3 | Anakinra, prednisone oral |
| 6 | † | 389 | 20 | 5424 | 5890 | MPSL pulses |
| 7 | † | 251 | 16 | 2352 | 8349 | MPSL pulses, anakinra |
| 8 | 307 | NA | NA | NA | NA | MPSL pulses, cyclosporine |
| 9 | NA | NA | NA | NA | NA | MPSL pulses, cyclosporine |
Three separate episodes of MAS separated by periods of resolution.
Patient’s initial presentation was with MAS. FSTL-1: Follistatin-like protein 1; ESR: erythrocyte sedimentation rate; sIL-2Rα: soluble interleukin 2 receptor-α; MPSL: methylprednisolone; NA: not available; sJIA: systemic juvenile idiopathic arthritis.
Measurement of standard inflammatory markers
Erythrocyte sedimentation rate (ESR) was derived from the clinical record. Serum ferritin levels were measured at the Children’s Hospital of Pittsburgh Clinical Laboratory using standard techniques.
ELISA
For detection of serum FSTL-1, Nunc Immunomodule MaxiSorp F8 Framed ELISA plates (Nalgene) were coated with 5 μg/ml polyclonal anti-FSTL1 (AF1694; R&D Systems) in phosphate buffered saline (PBS) and incubated at 4°C overnight. Plates were then washed with PBS/0.05% Tween 20 and blocked for 1 h with bovine serum albumin (BSA) buffer (1% BSA and 5% sucrose in PBS). Plates were washed again, and sera diluted 1:10 in serum dilution buffer (20 mM Tris, 150 mM NaCL, 0.1% BSA, 0.05% Tween 20; pH 7.3) were added and incubated 1 h. After washing, 2.5 μg/ml biotinylated monoclonal anti-FSTL1 (MAB1694; R&D Systems) was added for 1 h. Plates were washed again and incubated with streptavidin–horseradish peroxidase (Invitrogen) and developed with TMB peroxidase substrate (BD Biosciences). The absorbance of the plates was read at 450 nm on a microplate reader. A titration of purified FSTL-1 was used to generate a standard curve from which serum concentration of samples was calculated. Serum levels of sIL-2Rα were measured using a Quantikine ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Gene expression analysis
Blood samples were processed and preserved as quickly as possible to reduce processing-induced technical artifacts16. RNA was prepared from peripheral blood mononuclear cells (PBMC) and quality was assessed using a Bioanalyzer (Agilent) prior to labeling. For microarray analysis, labeled complementary DNA (cDNA) was synthesized from total RNA using the Ovation Biotin RNA Amplification and Labeling System (NuGen). Labeled cDNA was hybridized to the Affymetrix U133 plus 2.0 GeneChip. Data quality was assessed using standard metrics, which include assessment of positive and negative control features on the arrays. Expression values were derived using the robust multiarray average preprocessing method as implemented in GeneSpring GX 7.3 (Agilent). The gene expression data discussed here have been deposited in the NCBI Gene Expression Omnibus (Website: www.ncbi.nlm.nih.gov/geo/; accession no. GSE77537). Each probe set was normalized to median expression of that probe set in the control samples. In the Affymetrix U133 plus 2.0 GeneChip, a large number of genes are represented by > 1 oligonucleotide probes set.
Statistical analysis
Statistical analysis was performed using Stata version 11. All reported p values are 2-sided and considered significant at p < 0.05. Non-normal data distribution was transformed by log transformation. Correlation between FSTL-1 and other variables (ESR, ferritin, and sIL2-Rα) was conducted using a linear regression approach with adjustment for potential correlation using generalized estimating equations.
Significance of differential gene expression was identified by Welch’s Student t test. A 1.7-fold change cutoff was used to account for multiple comparisons because traditional multiple testing correction could not be applied with this limited number of samples. Hierarchical clustering using Pearson correlation and complete linkage was used to group genes and samples by expression pattern. Analysis of sensitivity and specificity was performed using receiver-operating characteristic (ROC) analysis.
RESULTS
FSTL-1 serum levels are elevated in early sJIA and decrease during treatment
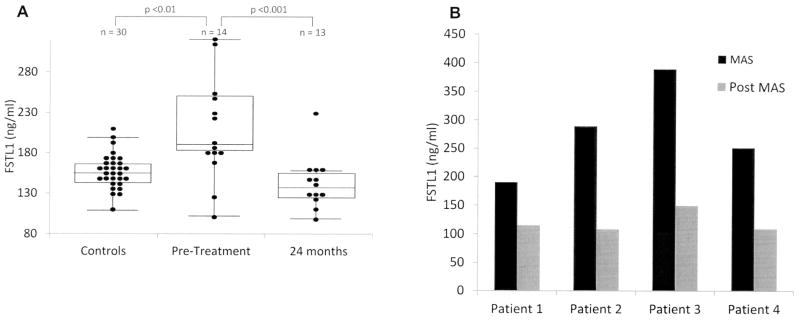
To evaluate how FSTL-1 levels change during the course of treatment, serum FSTL-1 levels were measured from 28 patients with sJIA pretreatment (initial evaluation), and at about 6, 12, and 24 months after initiation of treatment. In addition, serum FSTL-1 was measured in 30 controls. Not all patients had samples available from each timepoint. Fourteen patients had pretreatment serum available. As shown in Figure 1A, mean FSTL-1 levels near time of diagnosis were elevated compared to those of controls (200.7 ng/ml vs 146.7 ng/ml, respectively; p = 0.009). Mean FSTL-1 levels were 152.0 ng/ml by 6 months into treatment and 169.1 ng/ml at 12 months into treatment, and then decreased further to 133.7 ng/ml by 24 months after initiation of treatment (p = 0.0002 vs pretreatment). Notably, while some patients had elevated FSTL-1 levels at onset, a subset had levels that were not elevated.
Figure 1.
A. Serum FSTL-1 levels during the course of sJIA. Sera were assayed by ELISA from sJIA patients pretreatment and 24 months after initiation of therapy and compared to matched controls. The interior line represents the median; upper and lower boundaries of the box represent the 75th and 25th percentiles; top and bottom whiskers represent the 95th and 5th percentiles. Dots represent individual values for patients’ FSTL-1. Two patients whose disease onset was with MAS were not included. B. Paired sera collected from 4 patients during and after an episode of MAS were assayed (p = 0.0096). FSTL-1: Follistatin-like protein 1; sJIA: systemic juvenile idiopathic arthritis; MAS: macrophage activation syndrome.
FSTL-1 serum levels are markedly elevated during MAS and decline following treatment
We next evaluated whether FSTL-1 values varied with presence or absence of MAS. Seven patients had onset of MAS during the 24-month period, with 1 patient having 3 separate instances of MAS. Of these 7 patients, 4 had samples drawn both before and after treatment for MAS. As shown in Figure 1B, all 4 patients presenting with MAS had very high levels of FSTL-1 (mean 279.7 ng/ml). In all cases, FSTL-1 levels returned to normal following treatment (mean 120.3 ng/ml; p = 0.0096).
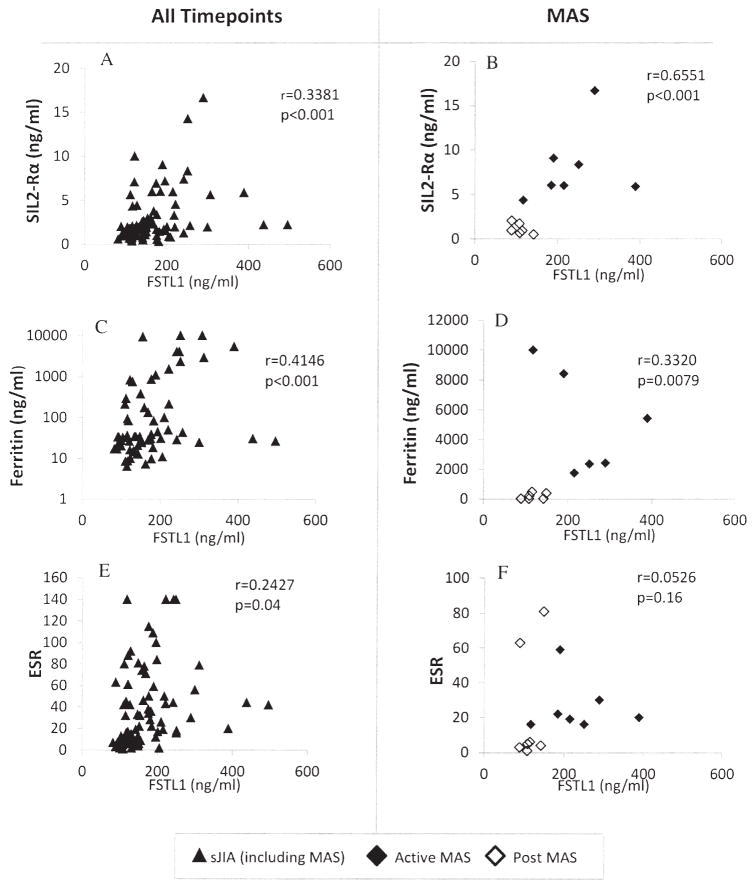
Correlation of serum FSTL-1 with inflammatory markers in patients with MAS
To determine whether FSTL-1 might improve the sensitivity of diagnosis of MAS, FSTL-1 levels were compared to known markers. Serum FSTL-1 levels were compared with ferritin, sIL-2Rα, and ESR in all patients at all timepoints of disease as well as in patients at onset and resolution of MAS (Figure 2). Serum FSTL-1 was highly correlated with both sIL-2Rα (Figure 2A, 2B) and with ferritin (Figure 2C, 2D), but correlated least well with ESR (Figure 2E, 2F). Of the 7 patients with active MAS, 6 showed an ESR of 30 or less despite markedly elevated FSTL-1 levels. The mean ESR in the MAS group was 26 mm/h, while the mean ESR in the pretreatment (sJIA presentation) group was 90 mm/h. Low ESR at onset of MAS is a well-established laboratory finding in sJIA, due to fibrinogen consumption and decreased production of fibrinogen2.
Figure 2.
Correlation of FSTL-1 with serum markers of inflammation in patients with sJIA, including sIL-2Rα (A, B), ferritin (C, D), and ESR (E, F). Left-side panels represent all patient samples at all timepoints (including MAS); right-side panels represent only the MAS subset. R values are uncorrected for presence of correlation between dependent data; p values were corrected through generalized estimating equations. FSTL-1: Follistatin-like protein 1; sJIA: systemic juvenile idiopathic arthritis; MAS: macrophage activation syndrome; ESR: erythrocyte sedimentation rate; sIL-2Rα: soluble interleukin-2 receptor-α.
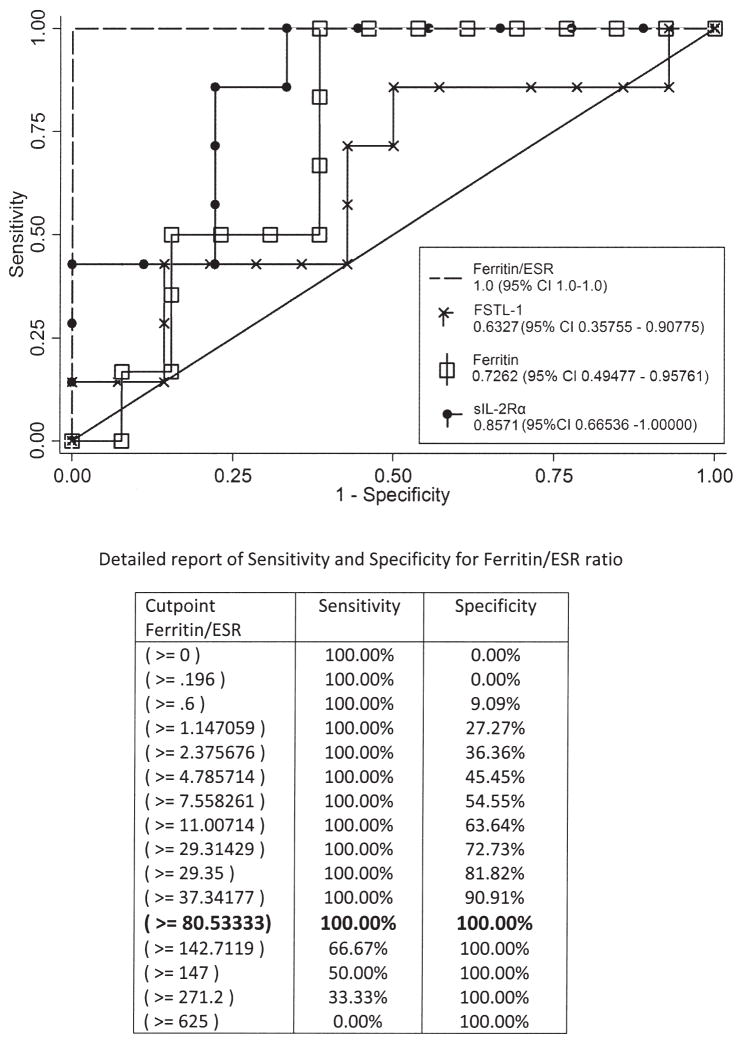
The ratio of ferritin to ESR provides a strong sensitivity and specificity for differentiation of overt MAS from pretreatment sJIA
To evaluate the sensitivity and specificity of FSTL-1 for differentiating overt MAS from new-onset sJIA, we analyzed the levels of FSTL-1, sIL-2Rα, and ferritin in our patients. Because many patients with new-onset (pretreatmemt) sJIA have elevated FSTL-1, ferritin, and sIL-2Rα, but nearly all patients with MAS had decreased ESR, we compared the ratio of FSTL-1, sIL-2Rα, and ferritin to ESR for patients with overt MAS and pretreatment sJIA. We performed ROC analysis for all of these and found that both ferritin/ESR ratio and sIL-2Rα/ESR ratio were strongest in discriminating active MAS from new-onset sJIA (Figure 3; sIL-2Rα/ESR is not shown, but was equivalent in area under the ROC curve to ferritin/ESR). A ferritin/ESR ratio > 80 provided statistically optimal sensitivity and specificity (100%) in our sample.
Figure 3.
Receiver-operating characteristics (ROC) curve shows an optimal sensitivity and specificity for MAS, obtained by use of ferritin/ESR ratio. Comparison of ROC curves with other biomarkers is shown; table gives details of ferritin/ESR sensitivity and specificity. A cutpoint of ferritin/ESR ratio of 80 provides optimum sensitivity and specificity for MAS, as shown in the detailed analysis. MAS: macrophage activation syndrome; ESR: erythrocyte sedimentation rate; FSTL-1: Follistatin-like protein 1; sIL-2Rα: soluble interleukin-2 receptor-α.
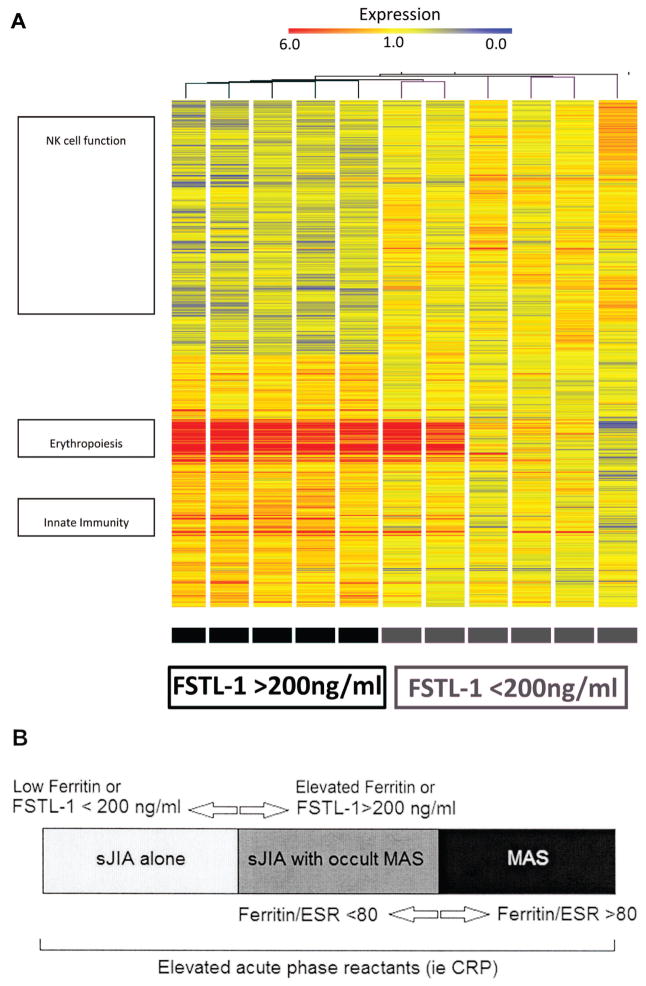
sJIA patients with high pretreatment levels of FSTL-1 show differential expression of genes related to erythrocyte precursors, innate immune function, and natural killer (NK) cell function
To determine whether markedly elevated serum FSTL-1 at disease onset might represent a subset of patients with a unique gene expression profile, PBMC gene expression was compared between patients with pretreatment FSTL-1 levels > 200 ng/ml versus < 200 ng/ml. FSTL-1 level of 200 ng/ml was selected as a division point for this analysis because it is midway between the median disease-onset FSTL-1 values for patients without MAS and the median FSTL-1 values at MAS presentation. Statistical analysis (p < 0.05 by Welch’s t test) yielded a total of 2660 significant genes, with 318 genes upregulated > 1.7-fold and 135 genes that were downregulated > 2-fold. Three clusters of differentially expressed genes are particularly notable (Figure 4): the first includes increased expression of genes related to erythropoiesis, such as rare and fetal hemoglobin variants; the second includes increased expression of a cluster of genes related to innate immune activity, such as resistin, peptidoglycan recognition protein 1, and adrenomedullin, as well as a subgroup of innate immune genes that appear to be related to neutrophil and other immune cell migration/chemotaxis, including matrix metallopeptidase 9 (MMP9), lipocalin 2 (LCN2), and interleukin 8/chemokine CXC motif 8 (IL8/CXCL8); finally, a third cluster includes decreased expression of a number of genes encoding various NK cell receptors and effectors of lytic granule function, including sialiac acid binding Ig-like lectin (SIGLECP3), killer cell immunoglobulin-like receptor (KIR3DL1), and synaptotagmin-like 3 (SYTL3).
Figure 4.
A. Gene expression profiles from pretreatment sJIA PBMC. RNA was collected from PBMC and assayed for differentially expressed genes based on FSTL-1 serum level above or below 200 ng/ml. Gene clusters are represented on the left with approximate locations of genes. B. Schema for utility of FSTL-1 and ferritin/ESR ratio as biomarkers in sJIA. FSTL-1 and ferritin are useful as markers of patients with altered gene expression suggestive of MAS, and of active MAS. Ferritin/ESR ratio can discriminate between active MAS and subclinical MAS or sJIA alone. PBMC: peripheral blood mononuclear cells; sJIA: systemic juvenile idiopathic arthritis; FSTL-1: Follistatin-like protein 1; ESR: erythrocyte sedimentation rate; MAS: macrophage activation syndrome; CRP: C-reactive protein.
FSTL-1 and ferritin levels at time of presentation may have predictive value in determining disease course and risk of developing MAS
The above data suggest that patients with the highest levels of FSTL-1 prior to treatment have more severe immune dysregulation. To determine whether this correlated with disease course, we compared pretreatment FSTL-1 levels with subsequent medication requirement. We compared 3 groups of patients: those requiring NSAID alone (4 patients), those requiring methotrexate and/or biologic (anakinra or etanercept; 8 patients), and those requiring cyclosporine (2 patients). As seen in Table 3, the 2 patients who were treated with cyclosporine for MAS had the highest levels of pretreatment serum FSTL-1. That these 2 patients were treated with cyclosporine, while a single patient with pretreatment FSTL-1 < 200 ng/ml, who developed MAS at a later point, did not require as aggressive treatment, may suggest that these 2 patients had a more severe MAS. Cyclosporine therapy may serve as a proxy for severe MAS, because in patients with systemic sJIA cyclosporine is the standard therapy for MAS, while “aggressive” treatment for persistently active sJIA includes anti-IL-1 or anti-IL-6 agents. Additionally, the 3 patients who developed MAS had the highest FSTL-1 and/or ferritin levels of the cohort. Small sample size limited the ability to determine whether there was a statistical association with higher initial FSTL-1 and ferritin levels in patients requiring methotrexate or biologic therapy, compared to NSAID alone (Table 3). No correlation with other laboratory markers or clinical markers such as cumulative joint count was noted, and all patients with pretreatment sJIA had fever and evanescent rash.
Table 3.
Pretreatment laboratory and clinical measures in sJIA. All patients presented with quotidian fever and rash.
| FSTL-1, ng/ml | Age, yrs | Cumulative Joint Count | WBC, 109/l | Hgb, g/dl | Platelet Count, 109/l | ESR, mm/h | CRP, mg/dl | sIL2-Rα, pg/ml | Ferritin, ng/ml | Medication Course† |
|---|---|---|---|---|---|---|---|---|---|---|
| 94 | 11 | 13 | 23.4 | 9 | 693 | 98 | 14.4 | 929.6 | 34 | 1 |
| 118 | 4 | 33 | 5.4 | 9.1 | 670 | 140 | NR | NR | 84 | 2 |
| 159 | 3 | 10 | 20.6 | 10.5 | 427 | 74 | NR | 2684 | 176 | 2 |
| 175 | 4 | 32 | 24.6 | NR | 314 | > 100 | 5.6 | 6934 | 9770 | 2†† |
| 176 | 14 | 13 | 27.4 | 11.2 | 521 | 115 | NR | 3385.6 | 869 | 2 |
| 176 | 3.5 | 3 | 8.3 | 10.6 | 519 | 34 | NR | 1020 | 39 | 1 |
| 177 | 2 | 4 | 27.6 | 10.1 | 631 | 50 | 1.4 | 587 | 10 | 1 |
| 187 | 5 | 13 | 18.5 | 9.4 | 677 | 109 | NR | 969.6 | 1102 | 2 |
| 218 | 3 | 5 | 12.9 | NR | 975 | 50 | NR | 3314 | NR | 2 |
| 222 | 4.5 | 2 | 19.9 | 7.6 | 364 | 140 | 18.9 | NR | 1541 | 1 |
| 242 | 4.5 | 9 | 44.5 | 10.7 | 924 | 140 | 20.5 | 7413 | 4109 | 2 |
| 248 | 11 | 13 | 21.1 | 9.6 | 539 | 140 | NR | NR | 4104 | 2 |
| 307 | 16 | 1 | 21.2 | NR | 325 | NR | NR | 5644 | 10200 | 3†† |
| 311 | 14 | 16 | 8.6 | 7.5 | 400 | 79 | 16.3 | NR | 2950 | 3†† |
| Average FSTL < 200 | 5.8 | 15.1 | 19.5 | 10.0 | 556.5 | 88.6 | 7.1 | 2358.6 | 1510.5 | — |
| Average FSTL > 200 | 8.8 | 7.7 | 21.4 | 8.9 | 587.8 | 109.8 | 18.6 | 5457.1 | 4580.8 | — |
| p value, FSTL > 200 vs < 200 | 0.297 | 0.144 | 0.756 | 0.250 | 0.814 | 0.398 | 0.085 | 0.098 | 0.141 | — |
Medication course: 1: NSAID; 2: methotrexate/biologic; 3: cyclosporine.
Developed MAS later in course. MAS: macrophage activation syndrome; NSAID: nonsteroidal antiinflammatory drugs; sJIA: systemic juvenile idiopathic arthritis; FSTL-1: Follistatin-like protein 1; WBC: white blood cells; Hgb: hemoglobin; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; sIL-2Rα: soluble interleukin-2 receptor-α; NR: not recorded.
DISCUSSION
Our study demonstrates that serum levels of FSTL-1 are elevated at the onset of sJIA and decline over the course of treatment. This is also, to our knowledge, the first reported association of MAS with a protein produced by cells of the mesenchymal lineage. These results support our earlier studies suggesting that FSTL-1 is a mediator of the inflammatory mechanisms that underlie arthritis, and specifically may be related to the IL-1β and innate immune pathways in sJIA12,13,14. We have also demonstrated for the first time that FSTL-1 is elevated in MAS and reverts to normal following treatment of MAS. Notably, a subset of patients with sJIA did not have elevated FSTL-1 levels at disease onset, while elevated FSTL-1 was seen both with overt MAS and with genetic dysregulation in another subset of sJIA patients, suggesting that this subset of patients may represent a group with subclinical MAS because they share some physiologic features with overt MAS.
A strong correlation was noted between FSTL-1 and both ferritin and sIL-2Rα. Ferritin is known to be elevated, along with CD163 and sIL-2Rα, in patients with MAS2,11. The strong correlation of FSTL-1 with sIL-2Rα is of particular interest, as this protein is known to be a strong diagnostic marker for hemophagocytic lymphohistiocytosis (HLH), a condition with a pathogenesis similar to that of MAS17. There are currently no well-defined biomarkers for MAS complicating sJIA. Our data suggest that other biomarkers such as ferritin and sIL-2Rα may, like FSTL-1, serve to identify those patients who have both overt MAS and new-onset sJIA with genetic dysregulation suggestive of subclinical MAS. Statistical analysis of sensitivity and specificity of these biomarkers in discriminating overt MAS from new-onset sJIA supports this hypothesis. Nevertheless, the ability of these biomarkers to discriminate patients with a greater degree of genetic dysregulation is important, given that our data suggest that these patients with the highest levels of FSTL-1 and ferritin may go on to develop MAS at a later stage. A comparison of our data with similar genetic expression profile data10 for ferritin suggests that FSTL-1 may be equivalent to ferritin as a marker of genetic dysregulation. The potential value of FSTL-1 as a marker that could be used in conjunction with other markers to aid in predicting patients at risk for MAS is suggested in Table 3, in which 1 patient with markedly elevated ferritin (9770 pg/ml) but a lower level of FSTL-1 (175 ng/ml) did go on to develop MAS, while another patient with elevated FSTL-1 (311 ng/ml) but much lower ferritin (2950 ng/ml) also developed MAS. Because no single marker is entirely specific, evaluating multiple markers associated with altered gene expression suggestive of occult MAS may help to identify a patient at risk.
Additionally, we found that patients with sJIA who have higher levels of FSTL-1 at initial presentation (> 200 ng/ml) have altered expression of a number of genes. Gene expression profiling demonstrated upregulation of genes related to processes of hemoglobin synthesis and oxygen transport and genes for rare hemoglobins, which can represent immature (nucleated) red blood cells copurifying with PBMC. Similar expression profiles were previously demonstrated in patients with sJIA and elevated serum ferritin9,10. The upregulation of genes related to erythropoiesis may represent a response to occult hemophagocytosis, while upregulation of genes related to innate immune activity and the Toll-like receptor/IL-1R pathway suggests that this group of patients has a stronger innate immune activity promoting more active disease. Additionally, certain of these proteins, such as CXCL8, have been found to be associated with overexpression of FSTL-118, indicating a link between FSTL-1 production and over-expression of these genes. Finally, we also found that patients with pretreatment FSTL-1 levels > 200 ng/ml had decreased expression of NK cell-related proteins, including NK cell receptors and some effectors of lytic granule function, such as SYTL319. The dysregulation of genes associated with these 3 clusters — erythropoiesis, innate immunity, and NK cell function — suggests a pattern of gene expression similar to MAS. Elevated pretreatment FSTL-1 level is a novel marker of this dysregulation of gene expression in sJIA, which is difficult to discern through traditional inflammatory markers and other clinical and laboratory markers.
A second notable finding in our data was that ferritin/ESR ratio was a more robust marker of active MAS than ferritin, sIL-2Rα, or FSTL-1 alone. ESR is known to be an inverse marker of MAS because it often falls during an episode of MAS2. This can give the false impression of improvement when in fact the patient is entering a dangerous clinical state. Using our data and these concepts, we were thus able to calculate a strong sensitivity and specificity with the ratio of ferritin to ESR for the differentiation of overt MAS from pretreatment sJIA. This method could be used to differentiate flares of sJIA from overt MAS. The method of using the ratio of these markers enhances specificity between MAS and pretreatment sJIA, a point at which a sizeable minority of patients will have elevated FSTL-1, ferritin, and sIL-2Rα associated with immune dysregulation, probably indicative of subclinical MAS. This is the first report that we know of that demonstrates strength of tests for ferritin, FSTL-1, and sIL-2Rα for development of MAS, and the first use of the ferritin/ESR ratio as a marker. While our results show a high sensitivity, specificity, and CI for the ferritin/ESR ratio, additional patient samples in a future study would be appropriate to validate this. The definition of MAS used for these patients may or may not reflect MAS as described by others using either the HLH criteria or criteria suggested by Ravelli, et al11.
Our study is the first, to our knowledge, to demonstrate a marker for sJIA whose level prior to initiation of treatment may be predictive of subsequent need for more aggressive therapy. The observation that patients with very high levels of FSTL-1 (> 300 ng/ml) were treated with more aggressive medical therapy is supportive of this, although the retrospective design of our study and the small sample size limit a definitive conclusion. Recent changes in the understanding of sJIA as a heterogeneous syndrome rather than a single disease have been suggested20. The heterogeneity in genetic expression and treatment course in patients with elevated FSTL-1 may suggest that these patients represent a subgroup of sJIA, and that patients with sJIA present in at least 2 subgroups — patients with and those without subclinical MAS. We present a schema for this heterogeneity in Figure 4B.
A caveat to our study is that FSTL-1 is not specific for sJIA and FSTL-1 is not intended as a specific marker for sJIA. FSTL-1 is elevated in sepsis (data not shown), and has also been found to be elevated in patients with Kawasaki disease who developed coronary aneurysms15, and in patients with neonatal onset multisystem inflammatory disease with increased arthropathy (unpublished data). In this regard, FSTL-1 appears to be a nonspecific, highly sensitive marker of severity in inflammatory disease. Like many other markers and mediators of disease, such as rheumatoid factor, FSTL-1 can be elevated in multiple conditions including infection, and levels must be interpreted in the correct clinical setting. We did not attempt to use FSTL-1 levels to differentiate MAS from sepsis, because severe sepsis and systemic inflammatory response syndrome share many laboratory and biochemical features with MAS, including elevated ferritin, sIL-2Rα, hemophagocytosis, and NK cell dysfunction, and may be phenotypes of a similar inflammatory process21. A second caveat to our study is the condition of the data we reviewed. Our data were collected as part of a study from a series of patients from several hospitals, and FSTL-1, ferritin, and sIL-2Rα were measured from stored serum. At some timepoints serum was not available, and complete medical records from each patient were not available at all timepoints. Finally, definitive conclusions regarding the use of these markers are limited by small sample size, and our results need to be confirmed in larger prospective studies.
Acknowledgments
Supported in part by US National Institutes of Health grants R01-AI-073556, R01-AR-056959, and T32-AR-052282 to Dr. Hirsch; P01-AR048929 and P30 AR047363 to Dr. Thompson; and the Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center.
References
- 1.Vastert SJ, Kuis W, Grom AA. Systemic JIA: New developments in the understanding of the pathophysiology and therapy. Best Pract Res Clin Rheumatol. 2009;23:655–64. doi: 10.1016/j.berh.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol. 2010;22:561–6. doi: 10.1097/01.bor.0000381996.69261.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. 2011;7:416–26. doi: 10.1038/nrrheum.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramanan AV, Grom AA. Does systemic-onset juvenile idiopathic arthritis belong under juvenile idiopathic arthritis? Rheumatology. 2005;44:1350–3. doi: 10.1093/rheumatology/keh710. [DOI] [PubMed] [Google Scholar]
- 5.Singh-Grewal D, Schneider R, Bayer N, Feldman BM. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 2006;54:1595–601. doi: 10.1002/art.21774. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel LR, Schneider R, Lang BA, Birdi N, Silverman ED, Laxer RM, et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402–9. doi: 10.1002/1529-0131(200011)43:11<2402::AID-ANR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–71. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 8.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–8. [PubMed] [Google Scholar]
- 9.Hinze CH, Fall N, Thornton S, Mo JQ, Aronow BJ, Layh-Schmitt G, et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res Ther. 2010;12:R123. doi: 10.1186/ar3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 11.Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598–604. doi: 10.1016/j.jpeds.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Thornton S, Sowders D, Aronow B, Witte DP, Brunner HI, Giannini EH, et al. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin Immunol. 2002;105:155–68. doi: 10.1006/clim.2002.5227. [DOI] [PubMed] [Google Scholar]
- 13.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, et al. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunology. 2006;177:4758–62. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 14.Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–6. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorelik M, Wilson DC, Cloonan YK, Shulman ST, Hirsch R. Plasma follistatin-like protein 1 is elevated in Kawasaki disease and may predict coronary artery aneurysm formation. J Pediatr. 2012;161:116–9. doi: 10.1016/j.jpeds.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes MG, Grom AA, Griffin TA, Colbert RA, Thompson SD. Gene expression profiles from peripheral blood mononuclear cells are sensitive to short processing delays. Biopreserv Biobank. 2010;8:153–62. doi: 10.1089/bio.2010.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–52. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–8. doi: 10.1002/art.33422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, et al. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119:3879–89. doi: 10.1182/blood-2011-09-382556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martini A. It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann Rheum Dis. 2012;71:1437–9. doi: 10.1136/annrheumdis-2012-201388. [DOI] [PubMed] [Google Scholar]
- 21.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387–92. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]