Abstract
7,12-Dimethylbenz[a]anthracene (DMBA) destroys ovarian follicles at all stages of development. This study investigated DMBA-induced DNA double strand break (DSB) formation with subsequent activation of the ovarian DNA repair response in models of pre-antral or pre-ovulatory follicle loss. Postnatal day (PND) 4 Fisher 344 (F344) rat ovaries were cultured for 4 days followed by single exposures of vehicle control (1% DMSO) or DMBA (12.5 nM or 75 nM) and maintained in culture for 4 or 8 days. Alternately, PND4 F344 rat ovaries were exposed to 1 µM DMBA at the start of culture for 2 days. Total RNA or protein was isolated, followed by qPCR or Western blotting to quantify mRNA or protein level, respectively. γH2AX and phosphorylated ATM were localized and quantified using immunofluorescence staining. DMBA exposure increased caspase 3 and γH2AX protein. Additionally, DMBA (12.5 nM and 1 µM) increased levels of mRNA encoding Atm, Xrcc6, Brca1 and Rad51. In contrast, Parp1 mRNA was decreased on d4 and increased on d8 of DMBA exposure, while PARP1 protein increased after 8 days of DMBA exposure. Total ATM increased in a concentration-dependent temporal pattern (75 nMd4; 12.5 nMd8), while pATM was localized in large primary and secondary follicles and increased after 8 days of 75 nM DMBA exposure compared to both control and 12.5 nM DMBA. These findings support that, despite some concentration effects, DMBA induces ovarian DNA damage and that DNA repair mechanisms are induced as a potential mechanism to prevent follicle loss.
Keywords: 7,12-Dimethylbenz[a]anthracene; Ovotoxicity; DNA damage; DNA double strand break repair
Introduction
The ovary is the ovum-producing female reproductive organ composed of follicles at different stages of development. The oocytes encased in primordial follicles are maintained in a dormant state until activation into the growing follicular pool where they subsequently go through a series of follicular developmental stages (McGee and Hsueh, 2000). Approximately 99% of ovarian follicles die by a process known as atresia (Borman et al., 2000), and ovarian senescence (menopause) occurs when the finite pool of primordial follicles has become exhausted (Broekmans et al., 2007; Hansen et al., 2008; Hoyer and Sipes, 1996). Since primordial follicles cannot be regenerated (Hirshfield, 1991), their depletion leads to infertility and premature ovarian failure (POF). A number of chemical classes can deplete follicles causing ovotoxicity, including the polycyclic aromatic hydrocarbon, 7,12-dimethylbenz[a]anthracene (DMBA) (Hoyer et al., 2009; Igawa et al., 2009; Mattison and Schulman, 1980).
DMBA causes destruction of all follicle types leading to POF in mice and rats (Mattison and Schulman, 1980). Sources of environmental DMBA exposure come from burning of organic matter, including cigarette smoke, charred foods and car exhaust fumes (Gelboin, 1980). DMBA causes apoptosis in granulosa and theca cells of pre-ovulatory follicles through increased expression of pro-apoptotic BAX and activation of the executioner protein caspase 3 (Tsai-Turton et al., 2007). Cigarette smoke exposure induces early menopause in female smokers compared to their non-smoking counterparts (Harlow and Signorello, 2000; Jick, 1979; Mattison et al., 1983). Also, the offspring of female smokers, are born with a decreased number of oocytes, potentially leading to abnormal ovarian function, decreased fertility, and/or early menopause, even if smoking was halted during pregnancy (Jurisicova et al., 2007). Chronic low dose DMBA exposure (0.35 mg/kg to 7.0 mg/kg interperitoneal (i.p.) daily for 15 days), mimicking human DMBA exposure, resulted in more follicle loss compared to a single high dose (80 mg/kg i.p.) in rats (Borman et al., 2000). Use of a neonatal ovary culture system has determined that a single DMBA exposure depleted large primary follicles at concentrations of 12.5 and 75 nM, while secondary follicles were destroyed at the 12.5 nM concentration only (Madden et al., under review), further suggesting that single low dose DMBA exposures are of equal concern as high dose exposures for ovarian function.
DMBA can cause dose-dependent DNA damage in extra-ovarian tissues (peripheral lymphocytes, liver and skin cells) when exposed along with physical stress in rats (Muqbil et al., 2006). Double-strand breaks (DSB) in DNA are the most cytotoxic lesions, generated by ionizing radiation, man-made chemicals (van Gent et al., 2001) and chemotherapeutic drugs (Helleday et al., 2008). These DSBs pose a serious threat to genome stability if either unrepaired or repaired incorrectly, and could potentially lead to permanent damage that could be a negative consequence for gamete health (Petrillo et al., 2011; Summers et al., 2011). DSBs can be sensed by ataxia telangiectasia mutated (ATM) protein, a phosphatidylinositol-3 kinase (PI3K) family member, with subsequent activation of cellular DNA damage responses (DDR) leading to cell cycle arrest, DNA damage repair, and subsequent cell cycle resumption (Giunta et al., 2010; Norbury and Hickson, 2001; Shiloh, 2003; Yang et al., 2003). One event downstream of ATM activation is phosphorylation of histone H2AX (termed γH2AX) which leads to recruitment of DNA repair molecules to the site of damage (Svetlova et al., 2010).
There are two major pathways that repair DNA DSBs: Homologous Recombination (HR) and Non-Homologous End Joining (NHEJ). HR requires regions of extensive DNA homology and is most active in the S and G2 phases of the cell cycle in dividing cells (Chiruvella et al., 2012). During HR, breast cancer type 1 (BRCA1) is phosphorylated by ATM and co-localizes with RAD51 at the site of DNA damage to induce DSB repair (Scully et al., 1997). NHEJ, active at all phases of the cell cycle, can rejoin a DSB with or without processing of the ends (Chiruvella et al., 2012). Key signaling molecules involved in NHEJ repair include the KU70/80 heterodimer (XRCC6 and XRCC5) which recognize and bind to the DSB, recruiting DNA dependent-protein kinase (DNA-PKs) to the DSB ends (Calsou et al., 2003). In addition, Artemis, X-ray repair complementing defective repair in Chinese hamster cells 4 (XRCC4), DNA ligase IV, and XRCC4-like factor (XLF) are activated during NHEJ repair (Dobbs et al., 2010).
Little is known about whether DNA damage is induced in the ovary during DMBA exposure, and if so, which DNA repair pathway predominates. The objective of this study was therefore to confirm that cell death was occurring after 8 days of DMBA exposure through quantification of the pro-apoptotic protein, caspase 3, and to subsequently investigate the DNA repair response induced by DMBA at an earlier time point (day 4) in postnatal day (PND) 4 cultured rat ovaries at concentrations previously established to induce pre-antral (1 µM DMBA; Igawa et al., 2009) and pre-ovulatory (12.5 and 75 nM DMBA; Madden et al., under review) follicle depletion. These concentrations were chosen based on previous studies in which depletion of all stage follicles was observed in the 1 µM DMBA exposure after 4 days (Igawa et al., 2009) and differential depletion of large primary and secondary follicles by 12.5 nM (large primary and secondary follicles targeted) and 75 nM (large primary follicles only targeted) (Madden et al., under review). Primordial and small primary follicles were grouped as pre-antral follicles, while large primary and secondary follicles were grouped as pre-ovulatory follicles.
Methods and materials
Reagents
7,12-Dimethylbenz[a]anthracene (DMBA; CAS # 57-97-6), bovine serum albumin (BSA), ascorbic acid, transferrin, 2-β-mercaptoethanol, 30% acrylamide/0.8% bisacrylamide, ammonium persulphate, glycerol, N′N′N′N′-tetramethylethylenediamine (TEMED), Tris base, Tris HCL, sodium chloride, and Tween-20 were purchased from Sigma-Aldrich Inc. (St Louis, MO). Dulbecco's Modified Eagle Medium:nutrient mixture F-12 (Ham) 1× (DMEM/Ham's F12), Albumax, penicillin (5000 U/ml), and Hanks' Balanced Salt Solution (without CaCl2, MgCl2 or MgSO4) were from Invitrogen Co. (Carlsbad, CA). Millicell-CM filter inserts and 48 well cell culture plates were obtained from Millipore (Bedford, MA) and Corning Inc. (Corning, NY) respectively. RNeasy Mini kit, QIA shredder kit, RNeasy Min Elute kit, and Quantitect TM SYBR Green PCR kit were purchased from Qiagen Inc. (Valencia, CA). All primers were purchased from the Iowa State University DNA facility. All primary antibodies were purchased from Abcam (Cambridge, MA). RNA later was obtained from Ambion Inc. (Austin, TX). Goat anti-mouse, goat anti-rabbit and rabbit anti-goat secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Ponceau S was from Fisher Scientific. ECL plus chemical luminescence detection kit was obtained from GE Healthcare, Amersham (Buckinghamshire, UK).
Ovary culture
Ovaries were collected from PND4 female F344 rats and cultured as described previously (Devine et al., 2002). Briefly, ovaries were removed, trimmed of oviduct and other excess tissues and placed onto membrane floating on 250 µl of DMEM/Ham's F12 medium containing 1 mg/ml BSA, 1 mg/ml Albumax, 50 µg/ml ascorbic acid, 5 U/ml penicillin and 27.5 µg/ml transferrin per well in a 48 well plate that had previously been equilibrated to 37 °C. A drop of medium was placed on top of each ovary to prevent it from drying. Ovaries were cultured in control medium for four days to allow development of large primary and secondary follicles, and were then treated once with medium containing vehicle control (1% DMSO) ± DMBA (12.5 nM or 75 nM) and the culture was maintained for four or eight days (as described below) at 37 °C and 5% CO2. This exposure induces pre-ovulatory follicle depletion after 8 days (Madden et al., under review). In addition, ovaries were exposed to vehicle control (1% DMSO) ± DMBA (1 µM) on alternate days from the start of culture (PND4) for 2 days. At this concentration, pre-antral follicle loss occurs from four days onward (Igawa et al., 2009). The medium was replaced every two days. One ovary per animal was placed in control medium, while the contralateral ovary was exposed to the experimental treatment. All animal procedures were approved by the Institutional Animal Care and Use Committee at Iowa State University.
RNA isolation and quantitative qPCR
RNA was isolated using an RNeasy Mini kit (Qiagen) and the concentration was determined using an ND-1000 Spectrophotometer (λ = 260/280 nm; NanoDrop technologies, Inc., Wilmington, DE) (n = 3; 10 ovaries per pool). Total RNA (200 ng) was reverse transcribed to cDNA utilizing the Superscript III One-Step qPCR (Qiagen). cDNA was diluted (1:20) in RNase-free water. Diluted cDNA (2 µl) was amplified on an Eppendorf PCR Master cycler using Quantitect SYBR Green PCR kit (Qiagen). Primers for Atm, Brca1, Parp1, Rad51, Xrcc6 and Gapdh were designed by Primer 3 Input Version (0.4.0) and are listed in Table 1. The regular cycling program consisted of a 15-min hold at 95 °C and 45 cycles of denaturing at 95 °C for 15 s, annealing at 58 °C for 15 s, and extension at 72 °C for 20 s at which point data were acquired. There was no difference in Gapdh mRNA expression between treatments, thus each sample was normalized to Gapdh before quantification. Quantification of fold-change in gene expression was performed using the 2− ΔΔCt method (Livak and Schmittgen, 2001; Pfaffl, 2001).
Table 1.
Primer sequence used for qPCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Atm | cgtgggtgtgaccttcagta | actctgccactcccactgtt |
| Brca1 | gagctggagatgaaggcaag | ctgcatccagtctgtcctca |
| Parp1 | ttaatgacacctgcctgctg | actccagcgcaacctatctg |
| Prkdc | tcctagaccttcaccgatgg | gtgtgggtatgtgtgggtga |
| Rad51 | ctccagttgcatgcttgttc | agagcacaggaacgatacgg |
| Xrcc6 | gatctgacactgcccaaggt | tgcttcttcggtccactctt |
| Gapdh | gtggacctcatggcctacat | ggatggaattgtgagggaga |
Protein isolation and Western blotting
Protein was isolated from cultured ovaries (n = 3; 10 ovaries per pool). Homogenates were prepared from cultured ovaries via homogenization in tissue lysis buffer containing protease and phosphatase inhibitors as previously described (Thompson et al., 2005). Briefly, homogenized samples were placed on ice for 30 min, followed by two rounds of centrifugation at 10,000 rpm for 15 min and protein concentration was measured using a BCA protocol. Protein was stored at − 80 °C until further use. SDS-PAGE was used to separate protein homogenates which were then transferred to a nitrocellulose membrane. Membranes were blocked for 1 h in 5% milk in Tris-buffered saline containing Tween 20, followed by incubation with anti-PARP1 antibody (1:200), anti-ATM antibody (1:100), anti-RAD51 antibody (1:500), anti-γH2AX (1:200) or anti-caspase 3 antibody (1:50) for 36 h at 4 °C. Following three washes in TTBS (1×), membranes were incubated with species-specific secondary antibodies (1:2000) for 1 h at room temperature. Membranes were washed 3× in TTBS and incubated in chemiluminescence detection substrate (ECL plus) for 5 min followed by X-ray film exposure. Densitometry of the appropriate bands was performed using ImageJ software (NCBI). Equal protein loading was confirmed by Ponceau S staining of membranes and protein level was normalized to Ponceau S densitometry values.
Immunofluorescence staining
Ovaries were fixed in 4% paraformaldehyde for 2 h, transferred to 70% ethanol, embedded in paraffin, and serially sectioned (5 µM thick), and every 10th section was mounted. Slides were deparaffinized in xylene and rehydrated with subsequent washes in ethanol. Antigen retrieval was carried out by microwaving sections for 7 min in sodium citrate buffer (1 M, pH 6.1). Sections were then blocked in 5% BSA for 1 h at room temperature. Sections were incubated with primary antibodies directed against pATM (1:100) or γH2AX (1:50) overnight at 4 °C. After washing in 1% PBS, sections were incubated with the appropriate goat anti-mouse IgG-FITC or donkey anti-rabbit IgG-FITC secondary antibodies for 1 h. Slides were then counterstained with 4-6-diamidino-2-phenylindole (DAPI) or Hoechst for 5 min. Images were taken using a Leica fluorescent microscope and the number of follicles with foci for pATM and γH2AX was analyzed using ImageJ software (NCBI). 5 large primary and 3 secondary follicles were quantified per slide (n = 3 ovaries; 15 large primary and 9 secondary follicles quantified per ovary).
Statistical analysis
Raw data were analyzed by paired t-tests comparing treatment with control using Graphpad Prism 5.04 software. Values are expressed as mean ± SE; n = 3 (10 ovaries per pool). Statistical significance was defined as * = P < 0.05.
Results
Effect of DMBA on caspase-3 protein level
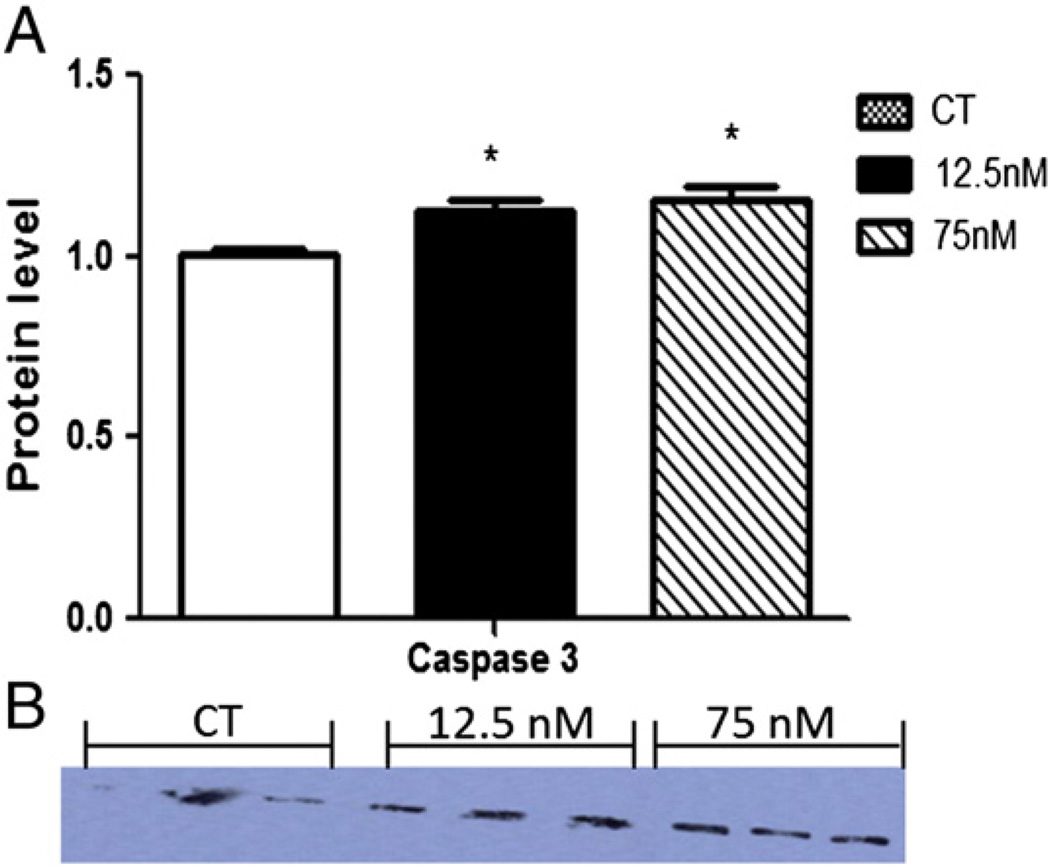
The effect of DMBA on the protein level of an apoptosis marker, caspase-3, was determined. Caspase-3 protein level (17 kDa) was increased (P < 0.05) by both DMBA treatments (CT – 1.0 ± 0.02; 12.5 nM – 1.12 ± 0.03; 75 nM – 1.15 ± 0.03), after 8 days confirming that apoptosis was induced by DMBA exposure (Fig. 1).
Fig. 1.
Effect of DMBA exposure on caspase-3 protein expression. Following 8 days of culture, total protein was isolated from PND4 rat ovaries exposed to control (CT), 12.5 or 75 nM DMBA. Caspase-3 protein was measured by Western blotting. (A) Densitometry data was normalized to Ponceau S and expressed as mean raw data ± SE; n = 3 (10 ovaries per pool). Statistical significance was defined as * = P < 0.05. (B) Western blot of caspase-3.
Localization and quantification of DMBA-induced markers of DNA damage
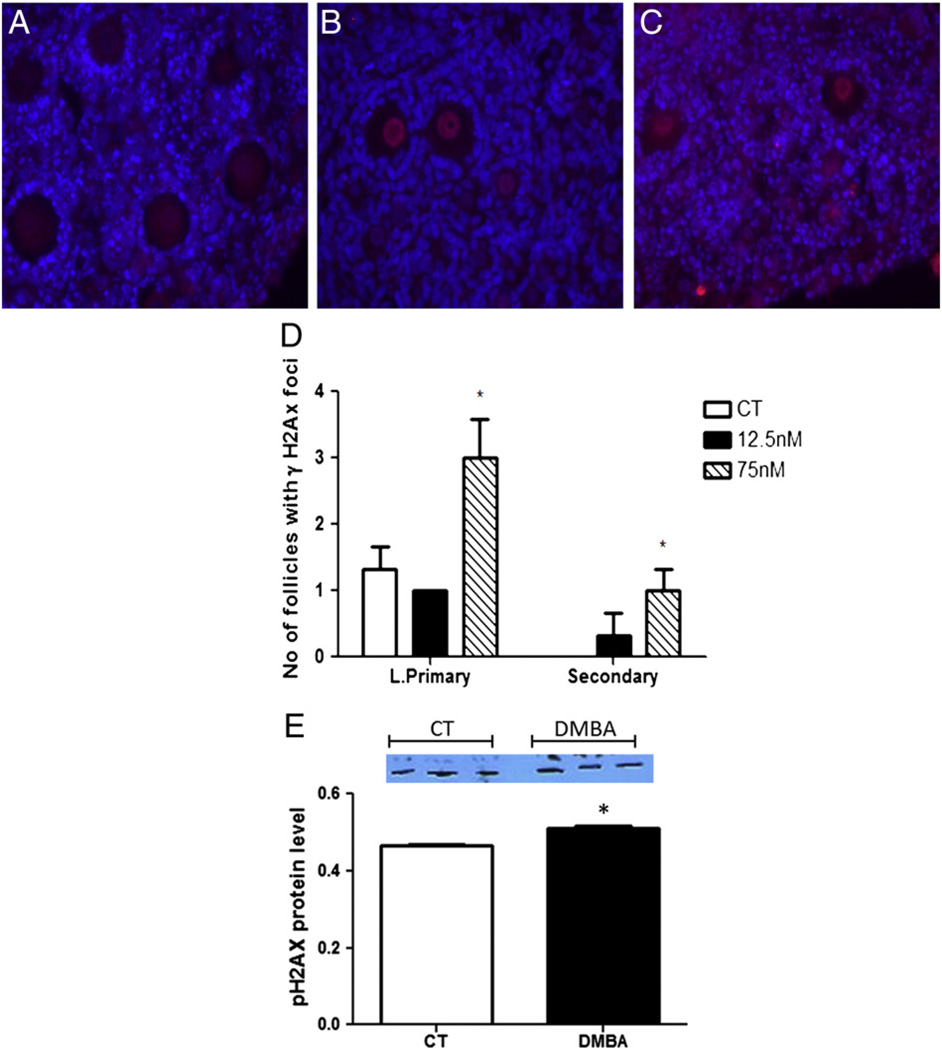
Immunofluorescence staining was used to determine localization and staining intensity for pATM and γH2AX proteins. γH2AX protein was localized in granulosa cells of CT ovaries at both time points, but increased in the oocyte nucleus of pre-ovulatory (large primary and secondary follicles) following DMBA treatment (Figs. 2A–C). Furthermore, Western blotting demonstrated that γH2AX protein level was also increased (P < 0.05) after 2 days of 1 µM DMBA exposure (Fig. 2D).
Fig. 2.
Localization and quantification of γH2AX protein. Paraffin embedded ovarian sections from PND4 rat ovaries exposed to (A) control (CT), (B) 12.5 or (C) 75 nM DMBA were used to perform immunohistochemistry to determine localization of γH2AX protein after 4 days. (D) Quantification of γH2AX loci in large primary and secondary follicles; data is expressed as number of follicles positive for γH2AX ± SE; n = 3; Statistical significance was defined as * = P < 0.05. (E) Western blot (D2) to detect γH2AX in vehicle control (C) or 1 µM DMBA (D) treated ovaries and expressed as mean raw data ± SE; n = 3 (10 ovaries per pool). Statistical significance was defined as * = P < 0.05.
Ovarian DMBA exposure alters expression of genes involved in DNA repair
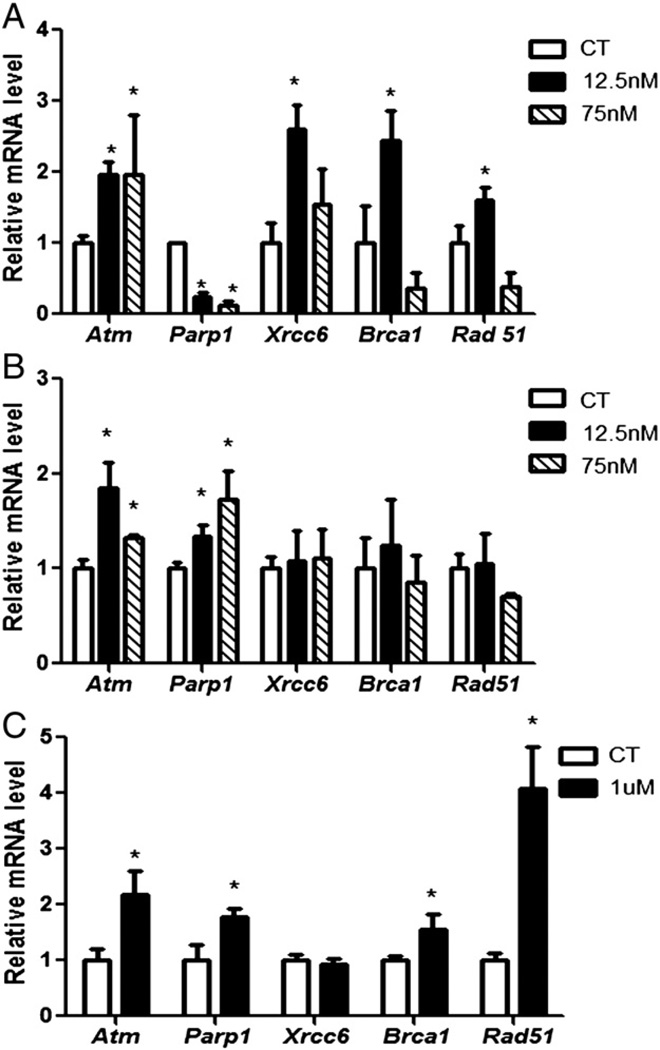
After exposure to DMBA, RNA was isolated and qPCR was performed to determine mRNA expression of DNA repair genes. Relative to control treated ovaries, Atm (0.9-fold ± 0.1), Xrcc6 (1.6-fold ± 0.3), Brca1 (1.4-fold ± 0.4) and Rad51 (0.6-fold ± 0.1) mRNA were increased (P < 0.05) by the 12.5 nM DMBA treatment after 4 days of exposure. In contrast, Rad51 (0.7-fold ± 0.2) mRNA was decreased (P < 0.05) only by treatment with 75 nM DMBA. Parp1 mRNA expression was decreased (P < 0.05) by both 12.5 (0.75-fold ± 0.04) and 75 nM (0.89-fold ± 0.06) DMBA concentrations after 4 days (Fig. 3A).
Fig. 3.
Effect of DMBA exposure on DNA repair gene mRNA expression. Following (A) 4 or (B) 8 days of culture, RNA was isolated from PND4 rat ovaries exposed to control (CT), 12.5 or 75 nM DMBA and qPCR performed. Additionally, RNA was isolated from ovaries exposed to (C) control (CT) or 1 µM DMBA for qPCR. Values are expressed as mean fold change ± SE; n = 3 (10 ovaries per pool). Statistical significance was defined as * = P < 0.05.
After 8 days of both DMBA treatments, mRNA encoding Atm (12.5 nM: 0.84-fold ± 0.2; 75 nM: 0.3-fold ± 0.03) and Parp1 (12.5 nM: 0.3-fold ± 0.1; 75 nM: 0.7-fold ± 0.3) were increased (P < 0.05) compared to control treated ovaries (Fig. 3B).
To further confirm that the DNA repair response is activated prior to follicle depletion, F344 female rat PND4 pup ovaries were cultured in medium containing vehicle control (DMSO) or DMBA (1 µM) for 2 days. At this concentration, DMBA induces primordial and small primary follicle loss from 4 days of exposure onwards (Igawa et al., 2009). Relative to control treated ovaries, Atm (1.18-fold ± 0.4), Parp1 (0.78-fold ± 0.1), Brca1 (0.55-fold ± 0.2) and Rad51 (3.07-fold ± 0.7) mRNA were increased (P < 0.05) by 1 µM DMBA (Fig. 3C).
Ovarian DMBA exposure alters DNA repair protein level
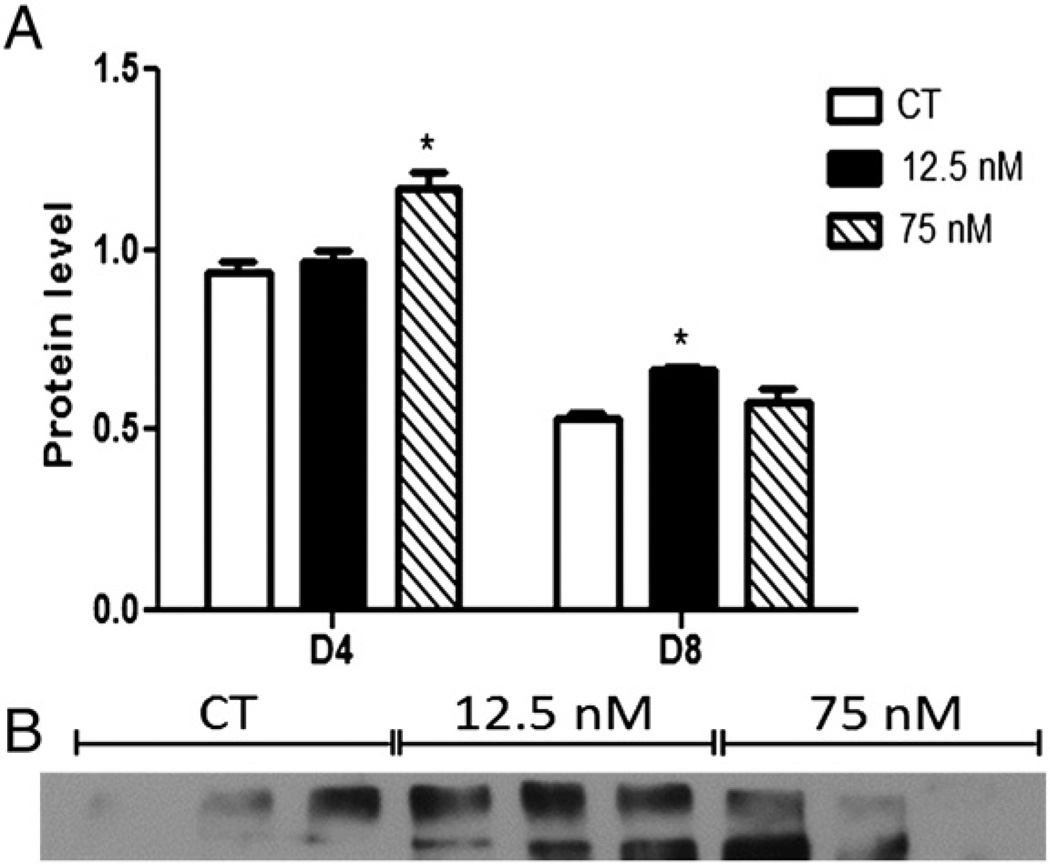
Total ATM protein was measured by Western blotting and was increased (P < 0.05) after 4 days in ovaries treated with 75 nM DMBA (CT – 0.93 ± 0.03; 12.5 nM – 0.96 ± 0.03; 75 nM – 1.17 ± 0.04; Fig. 4A), and after 8 days in ovaries exposed to 12.5 nM DMBA (CT – 0.53 ± 0.01; 12.5 nM – 0.66 ± 0.01; 75 nM – 0.57 ± 0.03; Fig. 4A).
Fig. 4.
Effect of DMBA exposure on total ATM protein level. Following 4 or 8 days of culture, total protein was isolated from PND4 rat ovaries exposed to control (CT), 12.5 or 75 nM DMBA. ATM protein was measured by Western blotting and a representative blot fromd8 of exposure is presented. (A) Results were normalized to Ponceau S and expressed as mean raw data ± SE; n = 3 (10 ovaries per pool). Statistical significance was defined as * = P < 0.05. (B) Representative Western blot for ATM.
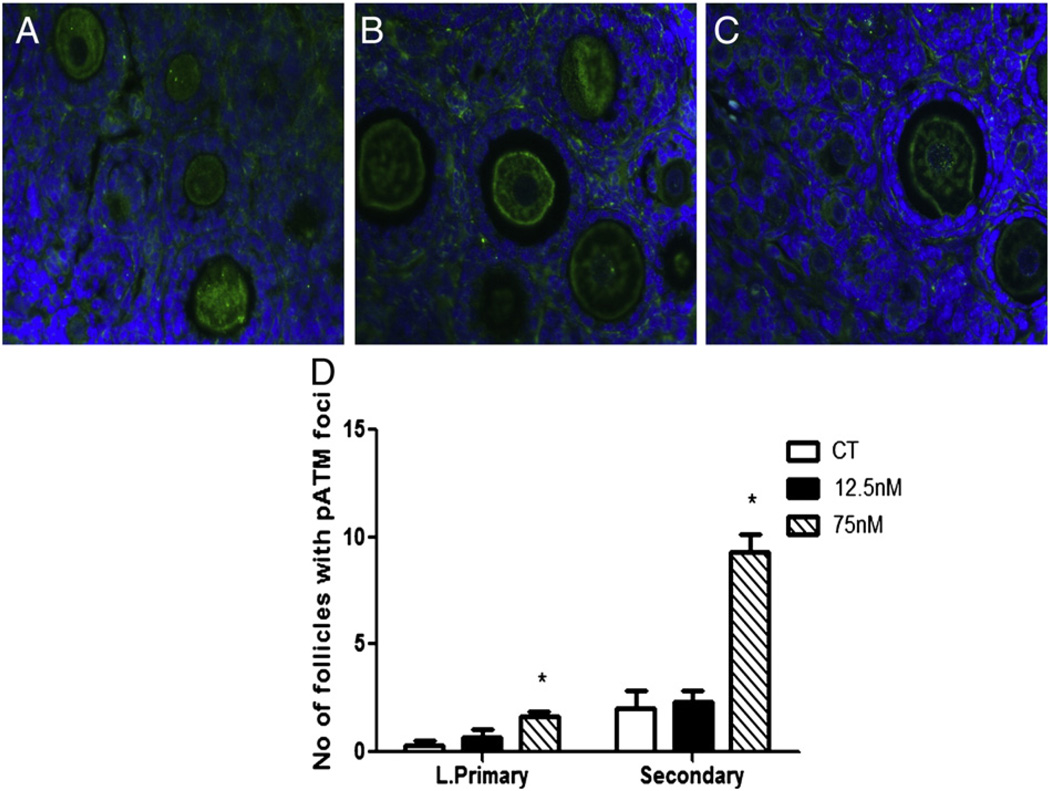
pATM protein was observed in the nucleus and cytoplasm of oocytes and in granulosa cells of all types of follicles (Figs. 5A–C). There was no effect of 12.5 nM DMBA on pATM protein staining, relative to control. However, pATM was increased (P < 0.05) by 75 nM DMBA in pre-ovulatory follicles; large primary (CT – 0.333 ± 0.33; 75 nM – 1.67 ± 0.33) and secondary follicles (CT – 2 ± 1.5; 75 nM – 9.33 ± 1.3) (Fig. 5D).
Fig. 5.
Localization and quantification of pATM protein. Following 8 days of culture, paraffin embedded ovarian sections from PND4 rat ovaries exposed to (A) control (CT), (B) 12.5 or (C) 75 nM DMBA were used to perform immunohistochemistry to determine localization and (D) quantification of pATM protein. Data is expressed as number of follicles positive for pATM ± SE; n = 3; statistical significance was defined as * = P < 0.05.
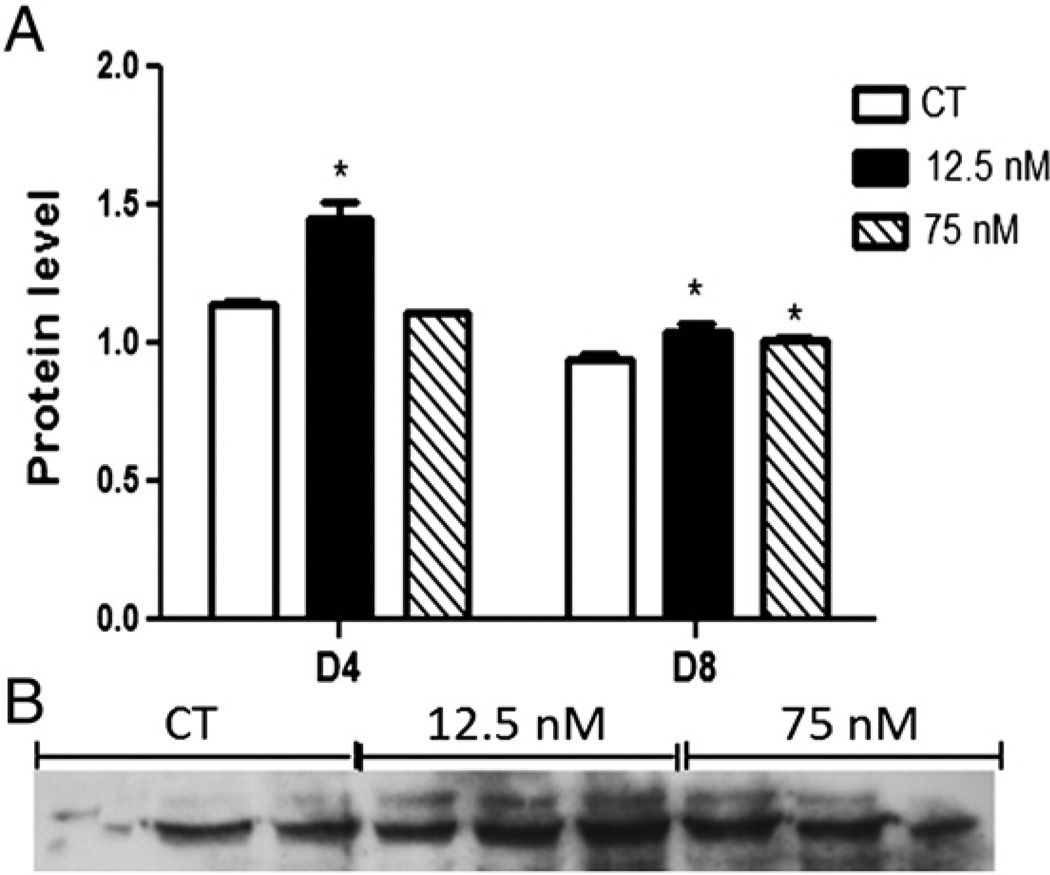
Western blotting demonstrated that PARP1 protein level increased (P < 0.05) after 4 days when exposed to 12.5 nM DMBA (CT – 1.14 ± 0.01; 12.5 nM – 1.44 ± 0.07; 75 nM – 1.10 ± 0.002; Fig. 6A), and was increased (P < 0.05) by both DMBA exposures after 8 days (CT – 0.94 ± 0.02; 12.5 nM – 1.03 ± 0.03; 75 nM – 1.01 ± 0.01; Fig. 6A).
Fig. 6.
Effect of DMBA exposure on PARP1 protein level. Following 4 or 8 days of culture, total protein was isolated from PND4 rat ovaries exposed to control (CT), 12.5 or 75 nM DMBA. PARP1 protein was measured by Western blotting and a representative blot from d8 of exposure is presented. (A) Results were normalized to Ponceau S and expressed as mean raw data ± SE; n = 3 (10 ovaries per pool). Statistical significance was defined as * = P < 0.05. (B) Representative Western blot for PARP1.
Discussion
DMBA causes depletion of follicles at all stages of development (Igawa et al., 2009; Rajapaksa et al., 2007). Our previous work established that a single exposure to 12.5 or 75 nM DMBA caused loss of large primary and/or secondary follicles (Madden et al., under review), and thereby established a model to study pre-ovulatory follicle loss. In the current study, we first confirmed that DMBA induced apoptosis at these concentrations by evaluating the level of caspase-3 in the ovary, which increased during DMBA exposure. Having established that atresia is ongoing in the ovary 8 days after DMBA exposure, we determined the impact of DMBA on DNA repair proteins as a proxy for DNA damage.
DMBA directly or indirectly causes DNA damage in extra-ovarian tissues (Baskaran et al., 2011), which can induce different cell fates such as DNA repair, cell cycle arrest and apoptosis (Norbury and Hickson, 2001). While DMBA-induced DNA damage has been characterized in many cell types (Bolognesi et al., 1991; Muqbil et al., 2006; Baskaran et al., 2011), the ovarian mechanisms are not well understood. DNA DSBs are highly cytotoxic lesions, generated by DMBA (Muqbil et al., 2006) and DSBs are one of the mechanisms that cause phosphoramide mustard-induced follicle loss in ovaries (Petrillo et al., 2011). We localized γH2AX protein to the nucleus and granulosa cells of large primary and secondary follicles after both DMBA exposures however this staining was negligible in the oocyte nucleus of control-treated ovaries. Additionally, we demonstrated that γH2AX protein is increased in ovaries exposed for two days to a concentration of DMBA that causes primordial and small primary depletion after 4 days. Since, detection of γH2AX is considered the gold standard for localization of DNA DSBs (Petrillo et al., 2011; Svetlova et al., 2010) these data indicate that DNA damage is occurring in ovaries exposed to DMBA.
The PI3K family member ATM is described as a critical sensor to initiate the cellular genotoxic response after the occurrence of DSBs (Gao et al., 2008; Shiloh, 2003; Yang et al., 2003). In this study, we determined an increase in the level of mRNA encoding Atm by exposure to both DMBA treatments. Total ATM protein was also increased by DMBA exposure, thus potentially also serving as a sensor of DNA DSB occurrence in the ovary. Interestingly, there was a temporal pattern of ATM protein induction, with the 75 nM DMBA concentration demonstrating induction of ATM after 4 days, while the 12.5 nM DMBA exposure took a longer time for ATM induction to occur. Whether the 12.5 nM exposure caused DNA damage prior to the induction of ATM cannot be confirmed from the data herein but is possible. These data are consistent with a previous study that demonstrated increased ATM in WT female mice spleen cells exposed to 50 mg/kg DMBA (Gao et al., 2008). ATM has been localized to the cytoplasm of growing follicles in the mouse ovary (Barlow et al., 1998). We also observed localization of pATM to the oocyte cytoplasm and nucleus of large primary and secondary follicles further supporting that DMBA induces DNA damage in the rat ovary. The ATM pathway regulates cell survival by either inducing apoptosis or preventing cell progression and activating DNA repair (Lavin and Kozlov, 2007). ATM can initiate the action of the HR or the NHEJ DNA repair machinery components in the mice embryo (Chiruvella et al., 2012) and in MCF7 cells after exposure to ionizing radiation (Paull et al., 2000; Summers et al., 2011), respectively. In this study, Xrrc6, Brca1 and Rad51 mRNA were increased by exposure to the concentration of DMBA at which both large primary and secondary follicles are depleted, and Brca1 and Rad51 mRNA were increased by 1 µM DMBA exposure after 2 days. Since these are components of the HR pathway that predominates in actively dividing cells, these data could suggest that the DMBA-induced DNA repair response occurs in granulosa cells. However, it has also been recently shown that genes involved in HR are expressed in the oocyte of mice and humans, and that their expression levels decline as the female ages (Titus et al., 2013).
In response to DNA damage, cells can also activate PARP1, an enzyme that catalyzes poly(ADP-ribosylation of a variety of proteins (D'Amours et al., 1999). Activation of PARP1 can also increase the accessibility of DNA repair enzymes and transcription factors to chromatin (Dantzer et al., 2006). In this study, Parp1 mRNA and protein were increased by both concentrations of DMBA. Also, Parp1 mRNA was observed to be increased prior to follicle loss in the ovaries exposed to 1 µM DMBA. There has been controversy concerning the role of PARP1 in the regulation of cell survival/death in response to DNA damage. Some studies have implicated PARP1 in the regulation of DNA repair and cell survival (De Vos et al., 2012; Wang et al., 1997; Ziegler and Oei, 2001) whereas others have implicated PARP1 in initiating cell death by either apoptosis (Muñoz-Gámez et al., 2009; Yu et al., 2002) or necrosis (Ha and Snyder, 1999). The data reported herein may indicate that PARP1 activation may stimulate cell death after DMBA exposure.
In summary, 8 days after DMBA exposure, caspase 3 and PARP1 protein levels are increased as measured in total ovarian homogenates by both 12.5 and 75 nM DMBA exposures. This data is matched by increased γH2AX and pATM in the oocyte of secondary follicles. The large primary follicles however do not have increased γH2AX or pATM at this same timepoint. This may indicate that either large primary follicles mounted their DNA repair response earlier than the secondary follicles, or that the large primary follicles have compromised DNA repair in response to DMBA exposure. The latter would fit with our follicle count data (Madden et al., under review) which demonstrated that large primary follicles were more sensitive to DMBA exposure, compared to secondary follicles. Taken together, the data presented herein indicates that exposure to DMBA causes ovotoxicity through DSB formation and that the ovary mounts a protective response in order to repair this damage. The response to DSBs however is not sufficient to completely prevent pre-ovulatory follicle loss at the DMBA levels investigated however it is possible that this is the case at lower exposure levels. Taken together these data raise concern about the fertility impacts of low level DMBA exposure to females.
Acknowledgments
Conflict of interest statement
The project described was supported by award number R00ES016818 to AFK and by a fellowship from the American Association of University Women to SG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Contributor Information
Shanthi Ganesan, Email: shanthig@iastate.edu.
Poulomi Bhattacharya, Email: poulomib@iastate.edu.
Aileen F. Keating, Email: akeating@iastate.edu.
References
- Barlow C, Liyanage M, Moens PB, Tarsounas M, Nagashima K, Brown K, Rottinghaus S, Jackson SP, Tagle D, Ried T, Wynshaw-Boris A. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development. 1998;125:4007–4017. doi: 10.1242/dev.125.20.4007. [DOI] [PubMed] [Google Scholar]
- Baskaran N, Rajasekaran D, Manoharan S. Coumarin protects 7,12-dimethylbenz(a) anthracene-induced genotoxicity in the bone marrow cells of golden Syrian hamsters. Int. J. Nutr. Pharmacol. Neurol. Dis. 2011;2:167–173. [Google Scholar]
- Bolognesi C, Parrini M, Aiello C, Rossi L. DNA damage induced by 7,12-dimethylbenz[a]anthracene in the liver and the mammary gland of rats exposed to polycyclic aromatic hydrocarbon enzyme inducers during perinatal life. Mutagenesis. 1991;6:113–116. doi: 10.1093/mutage/6.2.113. [DOI] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol. Appl. Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Knauff EAH, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol. Metab. 2007;18:58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Calsou P, Delteil C, Frit P, Drouet J, Salles B. Coordinated assembly of Ku and p460 subunits of the DNA-dependent protein kinase on DNA ends is necessary for XRCC4–ligase IV recruitment. J. Mol. Biol. 2003;326:93–103. doi: 10.1016/s0022-2836(02)01328-1. [DOI] [PubMed] [Google Scholar]
- Chiruvella KK, Sebastian R, Sharma S, Karande AA, Choudhary B, Raghavan SC. Time-dependent predominance of nonhomologous DNA end-joining pathways during embryonic development in mice. J. Mol. Biol. 2012;417:197–211. doi: 10.1016/j.jmb.2012.01.029. [DOI] [PubMed] [Google Scholar]
- D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- Dantzer F, Amé JC, Schreiber V, Nakamura J, Ménissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. In: Judith LC, Paul M, editors. Methods in Enzymology. Academic Press; 2006. pp. 493–510. [DOI] [PubMed] [Google Scholar]
- De Vos M, Schreiber V, Dantzer F. The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem. Pharmacol. 2012;84:137–146. doi: 10.1016/j.bcp.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol. Appl. Pharmacol. 2002;184:107–115. [PubMed] [Google Scholar]
- Dobbs TA, Tainer JA, Lees-Miller SP. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair (Amst.) 2010;9:1307–1314. doi: 10.1016/j.dnarep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Mitchell LA, Lauer FT, Burchiel SW. p53 and ATM/ATR regulate 7,12-Dimethylbenz[a]anthracene-induced immunosuppression. Mol. Pharmacol. 2008;73:137–146. doi: 10.1124/mol.107.039230. [DOI] [PubMed] [Google Scholar]
- Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J. Cell Biol. 2010;190:197–207. doi: 10.1083/jcb.200911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. PNAS. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35:3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Sipes IG. Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu. Rev. Pharmacol. Toxicol. 1996;36:307–331. doi: 10.1146/annurev.pa.36.040196.001515. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Davis JR, Bedrnicek JB, Marion SL, Christian PJ, Barton JK, Brewer MA. Ovarian neoplasm development by 7,12-dimethylbenz[a]anthracene (DMBA) in a chemically-induced rat model of ovarian failure. Gynecol. Oncol. 2009;112:610–615. doi: 10.1016/j.ygyno.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa Y, Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol. Appl. Pharmacol. 2009;234:361–369. doi: 10.1016/j.taap.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H. Cigarette smoking and early menopause. West. J. Med. 1979;130:235. [PMC free article] [PubMed] [Google Scholar]
- Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, Xu J, Matikainen T, Benito Hernandez A, Nunez G, Casper RF. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J. Clin. Invest. 2007;117:3971–3978. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madden JA, Hoyer PB, Devine PJ, Keating AF. Acute 7,12-dimethylbenz[a]anthracene exposure causes differential concentration-dependent follicle depletion and gene expression in neonatal rat ovaries. Toxicol. Appl. Pharmacol. 2013 doi: 10.1016/j.taap.2014.02.011. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison DR, Schulman JD. How xenobiotic chemicals can destroy oocytes. Contemp. Obstet. Gynecol. 1980;15:157. [Google Scholar]
- Mattison DR, Nightingale MS, Shiromizu K. Effects of toxic substances on female reproduction. Environ. Health Perspect. 1983;48:43–52. doi: 10.1289/ehp.834843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Muñoz-Gámez JA, Rodríguez-Vargas JM, Quiles-Pérez R, Aguilar-Quesada R, Martín-Oliva D, de Murcia G, de Murcia JM, Almendros A, de Almodóvar MR, Oliver FJ. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- Muqbil I, Azmi AS, Banu N. Prior exposure to restraint stress enhances 7,12-dimethylbenz(a)anthracene (DMBA) induced DNA damage in rats. FEBS Lett. 2006;580:3995–3999. doi: 10.1016/j.febslet.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Petrillo SK, Desmeules P, Truong T-Q, Devine PJ. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol. Appl. Pharmacol. 2011;253:94–102. doi: 10.1016/j.taap.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa KS, Sipes IG, Hoyer PB. Involvement of microsomal epoxide hydrolase enzyme in ovotoxicity caused by 7,12-dimethylbenz[a]anthracene. Toxicol. Sci. 2007;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Summers KC, Shen F, Sierra Potchanant EA, Phipps EA, Hickey RJ, Malkas LH. Phosphorylation: the molecular switch of double-strand break repair. Int. J. Proteomics. 2011;2011 doi: 10.1155/2011/373816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlova MP, Solovjeva LV, Tomilin NV. Mechanism of elimination of phosphorylated histone H2AX from chromatin after repair of DNA double-strand breaks. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010;685:54–60. doi: 10.1016/j.mrfmmm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol. Appl. Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013;5:172ra121. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai-Turton M, Nakamura BN, Luderer U. Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol. Reprod. 2007;77:442–451. doi: 10.1095/biolreprod.107.060368. [DOI] [PubMed] [Google Scholar]
- van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- Wang Z-Q, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K, Wagner EF. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ. ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis. 2003;24:1571–1580. doi: 10.1093/carcin/bgg137. [DOI] [PubMed] [Google Scholar]
- Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Ziegler M, Oei SL. A cellular survival switch: poly(ADP-ribosyl)ation stimulates DNA repair and silences transcription. Bio Essays. 2001;23:543–548. doi: 10.1002/bies.1074. [DOI] [PubMed] [Google Scholar]