Abstract
The Wnt pathway is a promising therapeutic and preventive target in various human cancers. The transcriptional complex of β-catenin/Tcf, a key mediator of canonical Wnt signaling, has been implicated in human colon cancer development. Current treatment of colon cancer depends on traditional cytotoxic agents with limited effects. Therefore, the identification of natural compounds that can disrupt the β–catenin/TcF complex to suppress cancer cell growth with fewer adverse side effects is needed. To identify compounds that inhibit the association between β-catenin and Tcf, we used computer docking to screen a natural compound library. Aesculetin, also known as 6,7-dihydroxycoumarin, is a derivative of coumarin and was identified as a potential small molecule inhibitor of the Wnt/β-catenin pathway. We then evaluated the effect of aesculetin on the growth of various human colon cancer cell lines and its effect on Wnt/β-catenin signaling in cells and in an embryonic model. Aesculetin disrupted the formation of the β-catenin/Tcf complex through direct binding with the Lys312, Gly307, Lys345 and Asn387 residues of β-catenin in colon cancer cells. Additionally, aesculetin effectively decreased viability and inhibited anchorage-independent growth of colon cancer cells. Aesculetin potently antagonized the cellular effects of β-catenin-dependent activity and in vivo treatment with aesculetin suppressed tumor growth in a colon cancer xenograft mouse model. Our data indicate that the interaction between aesculetin and β-catenin inhibits the formation of the β-catenin/Tcf complex, which could contribute to aesculetin’s positive therapeutic and preventive effects against colon carcinogenesis.
Keywords: aesculetin, human colon cancer, Wnt, β-catenin, Tcf
Introduction
The Wnt family of secreted glycoproteins is highly conserved and regulates many biological processes including development and disease (1–6). In the embryonic development process, appropriate activation of Wnt signaling regulates cell proliferation, differentiation and determination of cell fate (7–11). Inappropriate activation of the Wnt signaling pathway is implicated in human diseases, including various human cancers (1, 2, 6, 12–14). In particular, deregulation of the Wnt signaling pathway is a critical event in colon carcinoma tumorigenesis (15–18). Thus, the Wnt signaling pathway is considered a key therapeutic and preventive target for cancer (19, 20).
In the absence of active Wnt signaling, the β-catenin destruction complex, which is comprised of GSK-3β (glycogen synthase kinase-3β), APC (adenomatous polyposis coli) and Axin, catalyzes the phosphorylation of β-catenin leading to its proteosomal degradation. Activation of the canonical Wnt signaling pathway through the formation of a ligand-activated receptor complex inhibited the formation of the β-catenin destruction complex, which allows β-catenin to accumulate and subsequently translocate to the nucleus. Nuclear β-catenin directly binds with the T-cell factor (Tcf)/lymphoid enhancer factor (LEF) family and these interactions stimulate transcription of Wnt target genes, whose promoters contain binding sites of the Tcf-transcription factor (3, 21–23).
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second most frequent cancer reported in females (24). In the majority of colon cancers, the canonical Wnt/β-catenin pathway is constitutively active (15–18). Approximately 90% of colon cancers exhibit mutation of the APC or Axin genes, which leads to a disruption of the β–catenin destruction complex and accumulation of β–catenin. Multiple mutations lead to the nuclear accumulation of β–catenin and subsequent formation of a nuclear β–catenin/Tcf transcription complex (17, 25, 26). This leads to the inappropriate activation of its target genes, including c-myc (27) and cyclin D1 (28, 29), and also plays an essential role in proliferation of colon cancer cells. Therefore, disrupting the β–catenin/Tcf complex and inhibiting the nuclear function of β–catenin is considered to be therapeutically beneficial and could be useful for preventing colon cancer.
Here, we performed virtual structure-based screening of a natural product compound library using the crystal structure of the β–catenin/Tcf complex (PDB ID:1JPW). Aesculetin (6,7-dihydroxy-2-chromeone), a derivative of coumarin that is present in many medicinal plants (30–33), was identified as a potential inhibitor of β–catenin/Tcf-mediated transcription. Aesculetin exhibits many pharmacological effects, including inhibiting lipooxygenase (34, 35) and acting as an anticoagulant (36). Additionally, inhibition of cancer cell growth by aesculetin has been reported (37–39). Although aesculetin has shown anti-proliferative effects in cancer cells, the molecular mechanism by which this occurs has not been investigated carefully. In the present study, we demonstrated that aesculetin exhibits inhibitory effects against human colon cancer cells by directly targeting β–catenin, leading to the disruption of the β–catenin/Tcf complex. These results suggested that aesculetin has a significant therapeutic and preventive potential against human colon cancer.
Materials and Methods
Reagents
Aesculetin and anti-β-actin were purchased from Sigma-Aldrich (St. Louis, MO). McCoy’s 5A and RPMI1640 medium were obtained from Thermo Fisher Scientific (Barrington, IL). Basal Medium Eagle (BME), gentamicin, penicillin/streptomycin and L-glutamine were obtained from Invitrogen (Carlsbad, CA). [γ-32P]-ATP and the chemiluminescence detection kit were obtained from Amersham Pharmacia Biotech (Pittsburgh, PA). CNBr-Sepharose 4B beads were purchased from GE Healthcare (Piscataway, NJ). The MTS solution was purchased from Promega (Madison, WI). Antibodies against β-catenin and cyclin D1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against c-Myc and Tcf4 were purchased from Cell Signaling Biotechnology (Danvers, MA).
Plasmid constructs
The Myc-tagged β-catenin TM mutant, in which Lys312, Gly307, Lys345 and Asn387 was changed to Glu, Val, Glu, and Ala, respectively, was generated using the QuickChange II site directed mutagenesis kit (Stratagene, Cedar Creek, TX).
Cell culture
All cell lines were purchased from American Type Culture Collection and were cytogenetically tested and authenticated before the cells were frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 wk. HCT116 human colon cancer cells were cultured in McCoY’s 5A medium supplemented with 10% (v/v) fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and HCT15 and DLD-1 human colon cancer cells were cultured in RPMI 1640 medium supplemented with 10% (v/v) FBS.
Cell viability assay
Cells were seeded (2 × 103 cells per well) in 96-well plates and incubated for 12 h and then treated with different doses of aesculetin. After incubation for 72 h, 20 µl of Cell Titer96 Aqueous One Solution (Promega) were added and cells were incubated for 1 h at 37°C in a 5% CO2 incubator. Absorbance was measured at 492 nm.
Preparation of aesculetin-Sepharose 4B beads
To activate Sepharose 4B beads, aesculetin and Sepharose 4B powder (0.3 g) were suspended in 1 mM HCl. Then the coupled solution [0.1 M NaHCO3 (pH 8.3) and 0.5 M NaCl] was added and rotated overnight at 4°C. The mixture was washed with coupling buffer, and transferred to 0.1 M Tris-HCl buffer (pH 8.3). The excess of uncoupled aesculetin was removed by washing with 0.1 M acetate buffer (pH 4.0) and 0.1 M Tris-HCl buffer (pH 8.0) containing 0.5 M NaCl.
Cell-based pull-down assay
Proteins (500 µg) of HCT116, HCT15 and DLD-1 cells extracted with reaction buffer were mixed with Sepharose 4B beads (as a negative control) or aesculetin-Sepharose 4B beads (100 µl) in reaction buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 mg/ml BSA, 0.2 mM PMSF, and 1× protease inhibitor mixture]. After incubation with gentle rocking overnight at 4°C, the beads were washed 5 times with buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, and 0.02 mM PMSF] and binding was visualized by Western blotting.
Anchorage-independent cell growth
In brief, cells (8 × 103 per well) suspended in Basal Medium Eagle (BME) supplemented with 10% FBS were added to 0.3% agar with different doses of aesculetin in a top layer over a base layer of 0.6% agar. The cultures were maintained at 37°C in a 5% CO2 incubator for 2 wk and then colonies were counted under a microscope using the Image-Pro Plus Software (v.4) program (Media Cybernetics).
Western blot analysis
Cell lysates were prepared with lysis buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 10% glycerol and protease inhibitor cocktail tablet]. The protein concentration was measured using the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). A horseradish peroxidase-conjugated secondary antibody (Pierce Biotechnology) was used and the signal was detected with chemiluminescence reagent (Amersham).
Total RNA isolation and Reverse Transcription-PCR (RT-PCR)
Total RNA was extracted from cells using the TRIzol reagent (Tel-Test, Inc., Friendswood, TX) by following the manufacturer’s instructions. cDNA was synthesized using the Superscript pre-amplification system (Life Technologies, Inc., Carlsbad, CA). PCR reactions were performed using the following conditions: 94°C for 5 min and 25–28 amplification cycles at 94°C for 30 sec, the appropriate annealing temperature for 45 sec, and 72°C for 30 sec, and a final extension at 72°C for 10 min. The PCR primers were GAPDH (forward; 5’-CTCAGACACCATGGGG AAGGT-3’) and reverse; 5’-TGATCTTGAGGCTGTTGTCATA-3’); c-myc (forward; 5’-TGTCAAGAGGCGAACACACAACGTC-3’and reverse; 5’-ATCTTTCAGTCTCAAGACT CAGCCA-3’); and cyclin D1 (forward;CCTGTCCTACTACCGCCTCA, reverse; 5’-TCCTCCT CTTCCTCCTCCTC-3’).
Immunoprecipitation assay
HCT116 and HCT15 cells were treated with the indicated concentration of aesculetin for 24 h and then disrupted with lysis buffer [50 mM Tris (pH 8), 250 mM NaCl, 5 mM EDTA, 0.1% NP-40, 10% glycerol, and 1 × protease inhibitor cocktail]. Cell lysates were cleared by centrifugation and immunoprecipitations were performed by incubating overnight with anti-β-catenin. Protein A/G Plus Agarose (Santa Cruz) was added and the solution was incubated for 3 h at 4°C. Unbound proteins were removed by washing 4 times with lysis buffer. Bound proteins were harvested by boiling in sample buffer, and resolved by electrophoresis in 8% SDS-polyacrylamide gels. β-catenin and Tcf4 proteins were visualized using a chemiluminescence reagent (Amersham).
Transfection and luciferase reporter gene assay
Transient transfection was performed using jetPEI (VWR, Radnor, PA) and assays to detect firefly luciferase and Renilla activities were performed according to the manufacturer’s manual (Promega). Briefly, cells were seeded into 96-well plates and co-transfected with 50 ng of the Renilla luciferase internal control gene and 100 ng of the TOP-flash luciferase reporter construct containing three tandem Tcf consensus binding sites upstream of luciferase cDNA, or the FOP-flash luciferase reporter construct, a plasmid with mutated Tcf binding sites. After 12 h of transfection, cells were incubated with the indicated concentration of aesculetin for another 24 h. Luciferase and Renilla activities were measured using substrates included in the reporter assay system (Promega). The luciferase activity was normalized to Renilla activity.
Xenopus experiment
Xenopus laevis embryos were obtained by artificial fertilization. Vitelline membranes were removed by immersing embryos in a 2% cysteine solution (pH 8). Embryos were injected with 250 pg of β-catenin mRNA alone or together with the indicated concentration of aesculetin and then cultured to stage 40 in 67% Leibovitzs L-15 medium (Invitrogen) containing bovine serum albumin (BSA; 1 mg/ml), 7 mM Tris-HCl (pH 7.5), and gentamicin (50 µg/ml). Xenopus embryos at stage 40 do not have vertebrae, but have the notochord, which is a precursor of the backbone.
Xenograft studies in nude mice
Female athymic nude mice (6- to 7-wk old) were purchased from Central Lab Animal Inc (Seoul, Korea) and maintained under “specific pathogen-free” conditions on the basis of the guidelines established by the University of Seoul National University (Seoul, Korea) Institutional Animal care and Use Committee (SNU120106-4). Mice were divided into 4 groups: (i) untreated vehicle group (n = 10); (ii) 20 mg aesculetin/kg of body weight (n = 10); (iii) 100 mg aesculetin/kg of body weight (n = 10); (iv) no cells and 100 mg aesculetin/kg of body weight (n = 10). HCT116 cells (2.5 × 106 cells/100 µl) suspended in serum-free McCoy 5A medium were injected subcutaneously in the flank of each animal. Aesculetin or vehicle was administered intraperitoneally 3 times per wk for 11 days. DMSO (4 %) and polyethylene glycol (40 %) were diluted with PBS buffer and used as the vehicle. At the end of the experiment, mice were sacrificed, and tumors were extracted. Tumor volume was calculated from measurements of 2 diameters of the individual tumor base using the following formula: tumor volume (mm3) = (length × width × height × 0.52).
Immunohistochemical staining
For histopathological examination, paraffin sections (4 µm) were stained with hematoxylin and eosin. Immunohistochemical staining for Ki-67 (Lab Vision Corporation, Fremont, CA), cyclin D1 (Santa Cruz Biotechnology) or c-Myc (Novous Biologicals, Littleton, CO) was performed using the indirect avidin–biotin-enhanced horseradish peroxidase method (Vector Laboratories). For quantitation, each slide was scanned to obtain an overall impression of the staining patterns and 10 representative × 200 power (Ki-67 and cyclin D1) or × 400 (c-Myc) photomicrographs were taken with a digital camera, avoiding gross necrotic areas. The positively stained cancer epithelial cells within each photomicrograph were counted. Counting the total number of cancer cells was aided with the Image Pro+ image-processing program. The Ki-67 and cyclin D1 indices were based on the counting of ~7000 total cells per tumor slide.
Virtual Screening
To find potential inhibitors of β-catenin, a molecular docking method was developed using the Glide module from Schrödinger Suite 2011 (40, 41) and used to perform the virtual screening. A crystal structure of a human Tcf-4/β-catenin complex (PDB ID:1JPW) (42, 43) was downloaded from the PDB Bank (44) for virtual screening studies. This is an X-ray diffraction structure with a resolution of 2.5 Å. Waters, metals, and Tcf-4 were stripped from the structure and then hydrogens and atom charges are added to the structure using the Protein Preparation Wizard in Schrödinger suite 2011 with the standard procedure outlined. Two pockets were generated respectively within a 30-Å3 grid based on the binding site of Tcf-4 with β-catenin. One pocket was centered with Lys312 and the other with Lys435. The 2-D TCMD (Traditional Chinese Medicine Databse) structure database, which consists of about 9000 structures of natural products (45), was first converted to a 3-D structure database using the LigPrep module of the Schrödinger Suite of software and then used for virtual screening. High throughput virtual screening (HTVS) docking is usually first performed because it is intended for the rapid screening of large numbers of ligands followed by standard and extra precision (SP and XP) docking.
Statistical analysis
All data are presented as means ± S.D. of triplicate samples from at least 3 independent experiments. Differences between means were assessed by one-way analysis of variance (ANOVA) and the minimum level of significance was set at p < 0.05.
Results
Computer modeling of the β-catenin and aesculetin complex
To find potential inhibitors of β-catenin, we performed structure-based in silico screening using a molecular docking method as described in Materials and Methods. The crystal structure of β–catenin bound to the Tcf protein has been determined and two major pockets of β–catenin for binding with Tcf were identified (42, 43). Based on the crystal structure, we screened a small molecule library to identify compounds that could possibly bind to the two pockets of β–catenin, sites that are critical for the binding of Tcf to β–catenin. We used in silico screening to select 3 top-ranked compounds and tested their effect on human colon cancer cell growth. Of the three, aesculetin was the most effective (Supplementary Fig. 1). Aesculetin (Fig. 1A), also known as 6,7-dihydroxycoumarin, is a derivative of coumarin that is present in many medicinal plants (30–33). The computational prediction of the binding affinity between aesculetin and β–catenin was predicted to be very good with a score of −6.75 kcal/mol. In addition, computer modeling results predicted that aesculetin could directly bind to the Lys312, Gly307, Lys345 and Asn387 residues of β-catenin through hydrogen bonds (Fig. 1B). Therefore, we suggest that aesculetin might contribute to the inhibition of the β–catenin/Tcf complex formation by directing interacting with β–catenin.
Figure 1. Modeling of aesculetin binding with β-catenin.
(A) Chemical structure of aesculetin. (B) Aesculetin binds with β-catenin. Hydrogen bonds are formed between aesculetin and β-catenin at the Lys312, Gly307, Lys345 and Asn387 residues of β-catenin. Images were generated with the UCSF Chimera program.
Aesculetin directly binds with β-catenin
To confirm the results of the computer docking model, we determined whether aesculetin could directly bind to β-catenin. We performed pull down assays using aesculetin-conjugated Sepharose 4B beads with cell lysates of human colon cancer cells. These results showed that aesculetin directly bound to β-catenin precipitated from HCT116, HCT15 and DLD1 cell lysates (Fig. 2A). Additionally, molecular docking of the aesculetin and β-catenin complex indicated that aesculetin binds with β-catenin at Lys312, Gly307, Lys345 and Asn387. In order to confirm this prediction, we generated myc-tagged wildtype β-catenin and mutant (TM) β-catenin plasmids in which Lys312, Gly307, Lys345 and Asn387 were replaced with Glu, Val, Glu and Ala, respectively. Lysates from 293T cells were transfected with myc-tagged wildtype or TM β-catenin and were incubated with aesculetin-conjugated Sepharose 4B beads for a pull down assay. These results indicated that mutant (TM) β-catenin failed to bind aesculetin (Fig. 2B), which supports the computer docking model of the predicted binding sites between aesculetin and β-catenin.
Figure 2. Direct binding of aesculetin and β-catenin disrupts the β-catenin/Tcf complexes.
(A) Aesculetin directly binds to β-catenin. Colon cancer cell lysates were incubated with aesculetin-conjugated Sepharose 4B beads (Aes-sepharose) or Sepharose 4B beads alone, and then the pulled down proteins were analyzed by immunoblotting. (B) Lys312, Gly307, Lys345 and Asn387 of β-catenin are crucial for binding with aesculetin. Myc-tagged wildtype (WT) or mutant (TM) β-catenin was transfected into 293T cells. The pulled down proteins were analyzed by immunoblotting. (C) Aesculetin dose-dependently disrupts the β-catenin/Tcf complex. Nuclear extracts derived from aesculetin-treated colon cancer cells were used for an immunoprecipitation assay.
We then investigated whether the direct binding of aesculetin with β-catenin could interrupt the association of β–catenin with Tcf4. Tcf4 in nuclear extracts aesculetin-treated HCT116 and HCT15 cells was co-immunoprecipitated with anti-β–catenin. Indeed, aesculetin suppressed the association of β–catenin with Tcf4 in a dose-dependent manner (Fig. 2C). These results suggested that aesculetin might be able to attenuate the association of the β–catenin/Tcf4 transcriptional complex by directly interacting with β–catenin.
Aesculetin inhibits β–catenin/Tcf transactivation
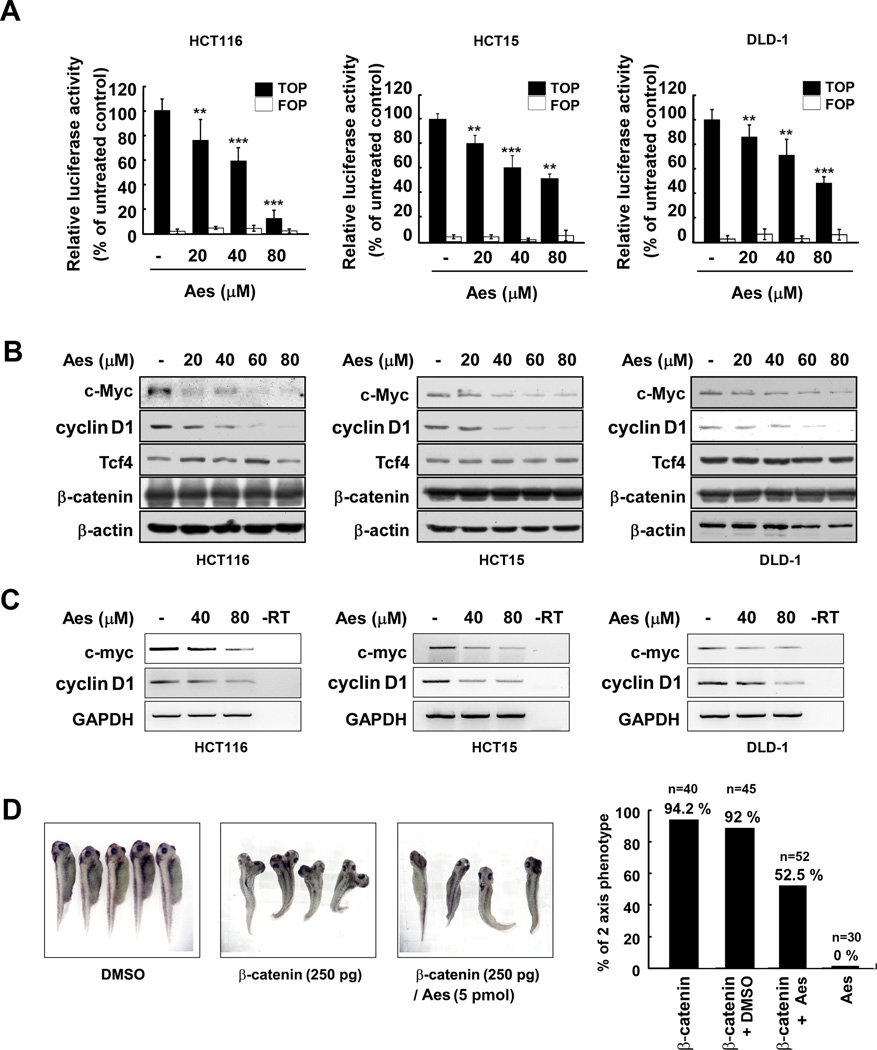
To confirm the effect of aesculetin on the β–catenin/Tcf transcriptional complex in cells, we measured TOP/FOP-luciferase activity in HCT116, HCT15 and DLD1 colon cancer cells. Colon cancer cells were co-transfected with a luciferase reporter gene containing 3 tandem Tcf consensus binding sites (TOP) or a mutated Tcf binding site (FOP) and the Renilla-luciferase reporter gene as a normalizing transfection control. At 12 h post-transfection, cells were treated for 24 h with the indicated concentration of aesculetin. TOP or FOP-luciferase activity was measured and normalized to Renilla-luciferase activity. Data are shown as relative values compared with untreated control. Aesculetin effectively suppressed the TOP-luciferase activity in a dose-dependent manner, without significantly affecting FOP-luciferase activity (Fig. 3A). These results indicated that aesculetin inhibited β–catenin/Tcf complex signaling, which is consistent with the finding that aesculetin disrupted the association of the β–catenin/Tcf transcriptional complex.
Figure 3. Effects of aesculetin on the β-catenin/Tcf pathway.
(A) Aesculetin inhibits the transcriptional activity of the β-catenin/Tcf complex in colon cancer cells. Colon cancer cells were co-transfected with reporter genes harboring the β-catenin/Tcf binding site (TOP-flash) or a mutant β-catenin/Tcf binding site (FOP-flash), respectively, and the Renilla gene. Cells were treated for 24 h with the indicated concentration of aesculetin. The luciferase activity was normalized to Renilla activity. (B–C) Colon cancer cells were incubated for 24 h with the indicated concentration of aesculetin. (B) Aesculetin inhibits the expression of target proteins, c-Myc and cyclin D1, of β-catenin/Tcf in colon cancer cells. c-Myc and cyclin D1 protein levels were determined by immunoblotting. (C) Aesculetin decreases the mRNA level of the cyclin D1 and c-myc genes. –RT indicates negative control of reverse transcription (RT). (D) Aesculetin inhibits β-catenin-induced axis duplication of Xenopus embryos.
Next, we examined the expression of c-myc and cyclin D1, which are direct target genes of the β–catenin/Tcf complex. We treated HCT116, HCT15 and DLD1 colon cancer cells with aesculetin and assessed the protein abundance of c-Myc and cyclin D1. Treatment with aesculetin for 24 h substantially repressed the protein levels of c-Myc and cyclin D1, but had no effect on the levels of β–catenin or Tcf (Fig. 3B). We also examined the mRNA levels of c-myc and cyclin D1 in aesculetin-treated human colon cancer cells. Consistent with the protein abundance of c-Myc and cyclin D1, the mRNA level of c-myc and cyclin D1 was attenuated by aesculetin (Fig. 3C). Taken together, these results indicated that aesculetin potently antagonizes the cellular effects of β–catenin/Tcf-dependent transactivation.
To further confirm the inhibitory effects of aesculetin on the Wnt/β–catenin signaling pathway, we determined the effect of aesculetin on Wnt/β–catenin signaling in vivo. During early Xenopus development, ectopic expression of β–catenin on the future ventral side leads to duplication of the embryonic body axis. This developmental model has been well established to investigate the regulation of Wnt/β–catenin signaling in vivo (46). Thus, this developmental model pathway provides rigorous methods for determining the biological effects of compounds on the β–catenin/Tcf pathway. About 90% of embryos injected with 250 pg of β–catenin mRNA showed duplication of ventral dorsal axis (Fig. 3D). In contrast, co-injection of β–catenin mRNA with 5 pmol of aesculetin inhibited the induction of axis duplication, with no contribution from the DMSO vehicle (Fig. 3D). These experiments further support the notion that aesculetin inhibits the β–catenin/Tcf-dependent signaling pathway.
Aesculetin decreases viability and inhibits anchorage-independent growth of colon cancer cells
To determine the biological effects of aesculetin in cells, HCT116, HCT15 and DLD1 colon cancer cells were treated for 72 h with various concentration of aesculetin and cell viability was assessed by the MTS assay. Aesculetin decreased viability of all 3 cell lines in a dose-dependent manner (Fig. 4A). Additionally, we evaluated the effect of aesculetin on anchorage-independent cell growth. HCT116, HCT15 and DLD1 colon cancer cells were seeded with aesculetin in 0.3% agar and incubated for 3 weeks. Data showed that aesculetin significantly suppressed anchorage-independent cell growth in dose-dependent manner (Fig. 4B). These results indicated that aesculetin had anti-proliferative and anti-tumorigenic effects against human colon cancer cells.
Figure 4. Anticancer activity of aesculetin.
(A) Aesculetin inhibits proliferation of colon cancer cells. Cells were treated with aesculetin for 72 h and proliferation was analyzed using the MTS assay. (B) Aesculetin inhibits anchorage independent cell growth. The asterisk(s) indicate a significant (*p < 0.05; **p < 0.01; ***p < 0.001) inhibitory effect of aseculetin on cancer cell growth and anchorage-independent cell growth.
Aesculetin inhibits tumor growth in a xenograft mouse model
To examine the antitumor activity of aesculetin in vivo, HCT116 cancer cells were injected into the right flank of individual athymic nude mice. Mice were injected intraperitoneally with vehicle or aesculetin at 20 or 100 mg/kg body weight 3 times a week for 2 weeks. Aesculetin treatment suppressed xenograft tumor development in mice. Treatment with 20 and 100 mg/kg aesculetin significantly inhibited HCT116 tumor size by 44 and 64%, respectively, relative to the vehicle-treated mice (Fig. 5A). All mice, including those treated with only aesculetin, were viable at the end of the experiment and body weight loss was not observed in any mice treated with aesculetin. This indicates that the doses used were not overtly toxic to the animals.
Figure 5. Aesculetin inhibits xenograft tumor growth.
(A) The average tumor volume of control and aesculetin-treated mice. Aesculetin suppresses colon tumor growth. (B) Aesculetin inhibits cyclin D1 and c-Myc expression in tumor tissues. The tumor tissues derived from groups treated with vehicle or 20 or 100 mg aesculetin/kg of body weight were immunostained with the indicated antibody. Positively stained cells were counted and the values were converted to a graphical representation. The asterisk(s) indicate a significant (*p < 0.05; **p < 0.01) inhibitory effect of aesculetin on expression of Ki-67, cyclin D1 and c-Myc.
Using the xenograft tumor tissues, we examined the effect of aesculetin on a tumor proliferation marker, Ki-67. The expression of Ki-67 was markedly inhibited by treatment with aesculetin. In addition, we investigated the effect of aesculetin on the direct targets of Wnt/β–catenin signaling, cyclin D1 and c-Myc by immunohistochemical analysis of HCT116 xenograft tumor tissues. Expression of cyclin D1 and c-Myc also was suppressed by treatment with aesculetin (Fig. 5B). These data suggested that aesculetin inhibited HCT116 colon tumor development by suppressing Wnt/β–catenin signaling.
Discussion
Colorectal cancer is the third most common cancer in men and women and is the third leading cause of cancer death (39, 40). Thus, the causes and methods of preventing and treating colon cancer are a high priority. Especially, abnormal activation of Wnt signaling has been tightly linked to colon cancer. Therefore identifying small molecules targeting Wnt signaling is a promising preventive or treatment strategy against cancer.
In the current study, we identified a natural product, aesculetin, as a potent inhibitor of Wnt signaling. Previously, the inhibitory effect of aesculetin on cell proliferation in many cell lines has been reported (32, 33, 43, 44). However, the molecular mechanism of the anti-proliferation effect of aesculetin against colon cancer cells is not clearly understood. In the present study, we demonstrated that aesculetin could disrupt the interaction between β–catenin and Tcf by targeting β–catenin, leading to suppressed proliferation of 3 different colon cancer cell lines without exhibiting any significant cytotoxic effects on the viability of normal human colon cells (Supplementary Fig. 2). We also demonstrated that aesculetin potently antagonized the cellular effects of β-catenin-dependent activity in human colon cancer cells and in an animal model (Fig. 3). Moreover, aesculetin effectively decreased viability and inhibited colony formation of human colon cancer cells (Figure 4) and tumorigenesis in vivo (Fig. 5). A high level of the β–catenin /Tcf transcriptional complex caused by the accumulation of β–catenin is associated with human colon cancer and colon carcinogenesis (9, 14, 16, 41, 42). Overall, our results suggest that aesculetin could be a potential chemopreventive agent against colon carcinogenesis. In particular, our results showing that aesculetin could effectively inhibit β–catenin-induced morphogenesis in the frog provide strong evidence supporting the potential preventive effect of aesculetin.
Recently, a number of existing drugs and natural compounds were reported to be antagonists of Wnt signaling. However, their molecular mechanisms of action and cellular targets are largely unknown, making their applications in cancer prevention and treatment and drug development very limited. In the present studies, we identified aesculetin as a potent natural inhibitor of the Wnt/β–catenin signaling pathway and elucidated its molecular mechanism of action. Thus we suggest that aesculetin could be a potent cancer therapeutic and preventive agent. However, several challenges remain to be addressed in the development of clinically useful Wnt pathway inhibitors. We screened many natural compounds that might affect the binding of Tcf to β–catenin. However, biochemical and structural studies showed that the region of β–catenin that binds to Tcf overlaps other binding partners, such as E-cadherin and APC (47, 48). In the future, we will address whether aesculetin can disrupt other interactions or whether it is selective for the β–catenin/Tcf protein-protein interaction. Additionally, whereas aesculetin, a nontoxic natural compound, appears to be potentially useful in treating or preventing cancer, its efficacy, safety, and toxicity need to be elucidated.
In conclusion, we identified aesculetin as a potent inhibitor of Wnt/β–catenin signaling, which acts by targeting β-catenin to effectively suppress proliferation of human colon cancer cells both in culture and in vivo. Overall, these results indicate that aesculetin could be a potential therapeutic or preventive and safe agent against human colon cancers.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by The Hormel Foundation and National Institutes of Health grants CA120388, CA1666011, CA172457 and R37 CA081064 and by the World Class University Grant, Ministry of Education, Science and Technology, Republic of Korea (Grant no. R31-2008-000-10103-0).
Footnotes
Disclosure of Conflicts of Interest: The authors have declared that no competing interests exist.
Author Contributions: The author(s) have made the following declarations regarding their contributions: Conceived and designed the experiments: SYL, TGL. Performed the experiments: SYL, TGL, HC, SKJ, HJL. Analyzed the data: SYL, YJS, KWL, AMB, ZD. Contributed reagents/materials/analysis tools: SYL, TGL, MHL, DJK, AS. Edited and wrote paper: SYL, TGL, AMB. Supervised experiments and provided overall direction: ZD
References
- 1.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nature reviews Genetics. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 4.Moon RT. Teaching resource. Canonical Wnt/beta-catenin signaling. Science's STKE : signal transduction knowledge environment. 2004;2004:tr5. doi: 10.1126/stke.2402004tr5. [DOI] [PubMed] [Google Scholar]
- 5.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. Wnt signaling and cancer. Genes & development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 7.Sokol SY. Wnt signaling and dorso-ventral axis specification in vertebrates. Current opinion in genetics & development. 1999;9:405–410. doi: 10.1016/S0959-437X(99)80061-6. [DOI] [PubMed] [Google Scholar]
- 8.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annual review of cell and developmental biology. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annual. review of cell and developmental biology. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty MP, Kamerzell TJ, Dawn B. Wnt signaling and cardiac differentiation. Progress in molecular biology and translational science. 2012;111:153–174. doi: 10.1016/B978-0-12-398459-3.00007-1. [DOI] [PubMed] [Google Scholar]
- 12.Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, Bouchal J. Wnt signaling in prostate development and carcinogenesis. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2011;155:11–18. doi: 10.5507/bp.2011.016. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E, et al. Activation of different Wnt/beta-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene. 2002;21:5548–5556. doi: 10.1038/sj.onc.1205686. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi K, Hennighausen L. Beta-catenin: a transforming actor on many stages. Breast cancer research : BCR. 2003;5:63–68. doi: 10.1186/bcr566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 16.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 17.Fearnhead NS, Wilding JL, Bodmer WF. Genetics of colorectal cancer: hereditary aspects and overview of colorectal tumorigenesis. British medical bulletin. 2002;64:27–43. doi: 10.1093/bmb/64.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Moravec M. Colorectal cancer and canonical Wnt signalling pathway. Casopis lekaru ceskych. 2012;151:335–342. [PubMed] [Google Scholar]
- 19.Herbst A, Kolligs FT. Wnt signaling as a therapeutic target for cancer. Methods Mol Biol. 2007;361:63–91. doi: 10.1385/1-59745-208-4:63. [DOI] [PubMed] [Google Scholar]
- 20.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Current cancer drug targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cellular signalling. 1999;11:777–788. doi: 10.1016/s0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 22.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Behrens J. The role of the Wnt signalling pathway in colorectal tumorigenesis. Biochemical Society transactions. 2005;33:672–675. doi: 10.1042/BST0330672. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 25.Buchert M, Athineos D, Abud HE, Burke ZD, Faux MC, Samuel MS, et al. Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo. PLoS genetics. 2010;6:e1000816. doi: 10.1371/journal.pgen.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nature reviews Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 27.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 28.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 30.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life sciences. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubols GB, Vianna DD, Medina-Remon A, von Poser G, Lamuela-Raventos RM, Eifler-Lima VL, et al. The Antioxidant Activity of Coumarins and Flavonoids. Mini reviews in medicinal chemistry. 2012 doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- 32.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life sciences. 2000;66:709–723. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 33.Adfa M, Yoshimura T, Komura K, Koketsu M. Antitermite activities of coumarin derivatives and scopoletin from Protium javanicum Burm. f. Journal of chemical ecology. 2010;36:720–726. doi: 10.1007/s10886-010-9807-1. [DOI] [PubMed] [Google Scholar]
- 34.Panossian AG. Inhibition of arachidonic acid 5-lipoxygenase of human polymorphonuclear leukocytes by esculetin. Biomedica biochimica acta. 1984;43:1351–1355. [PubMed] [Google Scholar]
- 35.Sekiya K, Okuda H, Arichi S. Selective inhibition of platelet lipoxygenase by esculetin. Biochimica et biophysica acta. 1982;713:68–72. [PubMed] [Google Scholar]
- 36.Awaad AS, Al-Jaber NA, Soliman GA, Al-Outhman MR, Zain ME, Moses JE, et al. New biological activities of Casimiroa edulis leaf extract and isolated compounds. Phytotherapy research : PTR. 2012;26:452–457. doi: 10.1002/ptr.3690. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko T, Tahara S, Takabayashi F. Inhibitory effect of natural coumarin compounds, esculetin and esculin, on oxidative DNA damage and formation of aberrant crypt foci and tumors induced by 1,2-dimethylhydrazine in rat colons. Biological & pharmaceutical bulletin. 2007;30:2052–2057. doi: 10.1248/bpb.30.2052. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Xiao YL, He XR, Qiu GF, Hu XM. Aesculetin-induced apoptosis through a ROS-mediated mitochondrial dysfunction pathway in human cervical cancer cells. Journal of Asian natural products research. 2010;12:185–193. doi: 10.1080/10286020903427336. [DOI] [PubMed] [Google Scholar]
- 39.Matsunaga K, Yoshimi N, Yamada Y, Shimizu M, Kawabata K, Ozawa Y, et al. Inhibitory effects of nabumetone, a cyclooxygenase-2 inhibitor, and esculetin, a lipoxygenase inhibitor, on N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Japanese journal of cancer research : Gann. 1998;89:496–501. doi: 10.1111/j.1349-7006.1998.tb03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrödinger Suite Schrödinger. LLC; 2011. [Google Scholar]
- 41.Miller JR, Mikol Y. Surveillance for diarrheal disease in New York City. Journal of urban health : bulletin of the New York Academy of Medicine. 1999;76:388–390. doi: 10.1007/BF02345678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta-catenin/Tcf complex. Cell. 2000;103:885–896. doi: 10.1016/s0092-8674(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 43.Poy F, Lepourcelet M, Shivdasani RA, Eck MJ. Structure of a human Tcf4-beta-catenin complex. Nature structural biology. 2001;8:1053–1057. doi: 10.1038/nsb720. [DOI] [PubMed] [Google Scholar]
- 44.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Research. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou JJ, Xie GR, Yan XJ. Traditional Chinese medicines: Molecular structures, natural sources and applications. China: Chemical Industry Press; 2004. [Google Scholar]
- 46.Moon RT, Kimelman D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. BioEssays : news and reviews in molecular, cellular and developmental biology. 1998;20:536–545. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 47.Sommer T, Hirsch C. San1p, checking up on nuclear proteins. Cell. 2005;120:734–736. doi: 10.1016/j.cell.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Xing Y, Hinds TR, Zheng J, Xu W. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. Journal of molecular biology. 2006;360:133–144. doi: 10.1016/j.jmb.2006.04.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.