Abstract
Introduction
Erectile dysfunction (ED) remains a major complication after radical prostatectomy. The use of adipose tissue-derived stem cells (ADSC) has shown promising results for the treatment of ED. However, the mechanisms of action for stem cell therapy remain controversial, with increasing evidence pointing to paracrine pathways.
Aim
To determine the effects and to identify the mechanism of action of ADSC and ADSC-derived lysate in a rat model of cavernous nerve (CN) crush injury.
Methods
Thirty-two male Sprague-Dawley rats were randomly divided into four equal groups: one group underwent sham operation, while three groups underwent bilateral CN crush. Crush-injury groups were treated at the time of injury with intracavernous injection of ADSC, lysate, or vehicle only (injured controls). Erectile function was assessed by cavernous nerve electrostimulation at 4 weeks. Penile tissue was collected for histology.
Main Outcome Measures
Intracavernous pressure increase upon CN stimulation; neuronal nitric oxide synthase (nNOS) content in the dorsal penile nerve; smooth muscle content, collagen content, and number of apoptotic cells in the corpus cavernosum.
Results
Both ADSC and lysate treatments resulted in significant recovery of erectile function, as compared to vehicle treatment. nNOS content was preserved in both the ADSC and lysate group, with significantly higher expression compared to vehicle-treated animals. There was significantly less fibrosis and a significant preservation of smooth muscle content in the ADSC and lysate groups compared to injured controls. The observed functional improvement after lysate injection supports the hypothesis that ADSC act through release of intracellular preformed substances or by active secretion of certain biomolecules. The underlying mechanism of recovery appears to involve neuron preservation and cytoprotection by inhibition of apoptosis.
Conclusions
Penile injection of both ADSC and ADSC-derived lysate can improve recovery of erectile function in a rat model of neurogenic erectile dysfunction.
Keywords: Mesenchymal stem cells, neurogenic erectile dysfunction, rat model of cavernous nerve injury, apoptosis, fibrosis, paracrine
Introduction
Prostate cancer is one of the most prevalent men’s health problems of the current time. Contemporary data show that the lifetime risk of prostate cancer is 1 in 6 men, and of these cancers, 94% are clinically localized, which are to date preferably treated by radical prostatectomy (RP) [1]. Although advancing anatomical knowledge and technology have decreased the rates of erectile dysfunction (ED) following RP, it remains challenging to fully preserve potency in RP patients. In the past decade, both clinical and preclinical research efforts have focused on stimulating nerve regeneration and prevention of corpus cavernosum fibrosis [2,3]. There is an expanding body of preclinical evidence demonstrating the efficacy of neuroimmunophilin ligands, neurotrophins and growth factors in improving neuroregeneration [2–7]. More recently, stem cell therapy has evolved as a potential therapy in the prevention of ED following cavernous nerve (CN) injury, thereby providing an autologous and curative therapeutic option [8–10].
Early projects in stem cell research for ED were conducted using embryonic stem cells which are complex to harvest and culture, let alone the ethical repercussions of the use of embryonic material and the reported tumerogenicity of this type of stem cell [8,11]. Therefore, adult stem cells, particularly those from bone marrow (BMSC), are increasingly investigated for their potential in tissue repair and regeneration. Recently, adipose-tissue derived stem cells (ADSC), another type of mesenchymal stromal cells found perivascularly in the adipose tissue, have become a valuable resource in stem cell therapy due to their abundance and ease of isolation [12–16]. ADSC closely resemble BMSC in differentiation and therapeutic potential, and are capable of expressing and secreting a broad spectrum of growth factors and cytokines [16–19]. However, although cell incorporation and differentiation in diseased tissues are possible mechanisms for ADSC’s therapeutic effects, recent studies suggested the involvement of paracrine pathways [20–24]. This study aimed to investigate whether ADSC are capable of restoring erectilefunction in a rat model of CN crush injury, to clarify the mechanisms by which ADSC exert their beneficial effects, and to examine whether injection of stem cell-lysate improves erectile function, based on the actions of ADSC-derived soluble molecules.
Methods
Study Design
Thirty-two male Sprague Dawley rats (12 weeks old) were obtained from Charles River Laboratories (Wilmington, MA, USA), and were randomly divided into four equal groups. The animals were maintained on a 12 hours light/dark cycle and had access to standard rat chow and water ad libitum. All rats underwent resection of the paratesticular pad of adipose tissue to procure ADSC. One week later, one group underwent sham surgery and injection of vehicle (sham group). The remaining 24 animals underwent bilateral CN crush injury and received either intracavernous injection of vehicle (vehicle group), ADSC derived lysate (lysate group), or ADSC (ADSC group). Four weeks after treatment, all rats underwent erectile function evaluation. The animals were then sacrificed and the penis was harvested for histology. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
ADSC Culture
ADSC were harvested and cultured according to a standardized protocol [25–28]. The ADSC cultured with this technique were extensively characterized in previous studies [12,29]. Briefly, under 2% isoflurane anesthesia all animals underwent surgery consisting of a lower abdominal midline incision and bilateral resection of the paratesticular adipose tissue. Adipose tissue was kept in phosphate buffered saline (PBS) on ice until processing of the samples. The adipose tissue was rinsed with PBS, minced into small pieces, and then incubated in a solution containing 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37°C with vigorous shake for 15 seconds in 20-minute intervals. The top lipid layer was removed and the remaining liquid portion was centrifuged at 1000g for 10 min at room temperature. The pellet was treated with 160 mM NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in 10 ml. Dulbecco’s Modified Eagle Medium (DMEM) supplemented with Streptomycin, Fungizone, Penicillin and 10% fetal bovine serum (FBS). The suspension was filtered through a 70-µm cell strainer, plated at a density of 1 × 106 cells in a 10-cm dish and cultured at 37 °C in 5% CO2. After 24 hours the cells were rinsed with PBS. On day 5 of cell culture, cells were labeled with the thymidine analog 5-ethynyl-2-deoxyuridine (EdU; Invitrogen, Carlsbad, CA, USA) for 48 hours, resulting in approximately 100% labeling of ADSC. This step was not applied in the culture dishes designated for the preparation of the lysate. When reaching confluence, approximately 1 million cells were harvested and suspended in 400 µL PBS and kept on ice until injection.
Preparation of ADSC Derived Lysate
For the processing of the cell lysate, spent culture-media was pipetted out from each dish, replaced with 10 ml of deionized H2O, and incubated at room temperature for a duration of 30 min. to allow for osmotic rupture of the cell membranes. Then cell-free lysate was prepared by subjecting the ADSC to three freeze–thaw cycles using an ethanol/dry ice bath to further dissociate the lysed cell sediments, followed by centrifugation at 1000g to remove insoluble material. Per animal, 400 µl of lysate was prepared and kept on ice until injection.
Cavernous Nerve Crush Injury
Surgery was performed under 2% isoflurane anesthesia with the animal placed on a heating pad. The prostate gland was exposed through a lower abdominal midline incision. The major pelvic ganglion (MPG) and CN were exposed on either side of the prostate. In sham animals, the abdomen was then closed. In the treatment and vehicle groups, standardized bilateral CN crush injury was performed by application of a designated needle driver 5 mm. distant to the origin of the cavernous nerve at the MPG for the duration of two minutes. The abdomen was then closed and the penis exposed through the lower abdominal midline incision. A tourniquet was applied to the base of the penis at the level of the symphysis to allow the ADSC and trophic factors in the lysate to adhere. Immediately after application of the tourniquet, 200 µL of PBS (sham and vehicle groups), lysate, or ADSC suspension was injected in both corpora cavernosum at the mid-penile level. Pressure was applied for one minute to the injection site after injection, to prevent backflow of treatment suspension. The tourniquet was released 3 minutes after injection of the treatment suspension.
Determination of Erectile Function
Four weeks after intracavernous injection, erectile function was assessed. Under Ketamine (100 mg/kg) and Midazolam (5mg/kg) anesthesia, the MPG and CN were exposed bilaterally via midline laparotomy. A 23G butterfly needle was inserted into the proximal left corpus cavernosum, filled with 250 U/mL heparin solution and connected to a pressure transducer (Utah Medical Products, Midvale, UT, USA) for intracavernous pressure (ICP) measurement. The ICP was recorded at a rate of 10 samples/second using a computer with LabView 6.0 software (National Instruments, Austin, TX, USA). A bipolar stainless-steel hook electrode was used to stimulate the CN directly (each pole 0.2 mm in diameter, separated by 1 mm) via a signal generator (National Instruments) and custom-built constant-current amplifier generating monophasic rectangular pulses with stimulus parameters of 1.5 mA, 20 Hz, pulse width 0.2 ms, and duration 50 seconds. Three stimulations were conducted per side and the erection with maximum increase in ICP was included for statistical analysis in each animal. Systemic blood pressure was recorded using a 23G butterfly needle inserted into the aorta at the level of the iliac bifurcation, for the calculation of the ICP increase/mean arterial pressure (MAP) ratio. After functional testing, animals were euthanized using Pentobarbital IP (200 mg/kg) followed by bilateral thoracotomy. The penis was then harvested for histology.
Histology
Immunofluorescence
Freshly dissected tissue was fixed for 4 hr with cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in buffer solution containing 30% sucrose. Tissues were frozen in optimum cutting temperature compound (Sakura Finetek, Torrance, CA, USA), and stored at −80 °C until use. Sections were cut at 6 µm, adhered to charged slides, air dried for 5 minutes, and rehydrated with 0.05 M PBS. After rinsing, sections were washed in PBS followed by 30 minutes room-temperature incubation with 3% goat serum/PBS/0.3% triton X-100. After draining solution from sections, tissues were incubated overnight at room temperature with primary antibodies, followed by 1 hr immersion in 1:500 dilution of secondary antibody conjugated with Alexa-488 fluor (Invitrogen) or Texas Red (Vector Laboratories, Burlingame, CA). The primary antibodies were: rabbit anti-nNOS (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), mouse anti-neuron-specific β-III Tubulin (TU-20, Abcam Inc., Cambridge, MA, USA), mouse anti-α-smooth muscle actin (α-SMA, Sigma-Aldrich, St. Louis, MO, USA).
For tracking of ADSC, slides were incubated with freshly made Click-iT reaction cocktail, which contained Alexa-594 fluor (Invitrogen) for 30 min at room temperature [30]. Nuclear staining was performed with 4’,6-diamidino-2-phenylindole (DAPI; D-3571, Invitrogen).
TUNEL Staining
Quantification of apoptotic cells was performed by detecting DNA damage in-situ using the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL; Roche diagnostics, Mannheim, Germany) staining and counterstaining by DAPI in frozen sections. The slides were evaluated using fluorescence microscopy.
Collagen Staining
For collagen subtypes I and III histochemistry, slides were rehydrated in PBS, immersed in picro-sirius red stain (American master tech scientific, Lodi, CA, USA) for one hour and rinsed with 0.5% acetic acid water twice. Slides were evaluated for total collagen content. Using normal light microscopy, Collagen I is located mainly in the trabeculae and the tunica albuginea and consists of thick, bright red, transversely oriented bundles. Collagen III is visible as less abundant, subendothelial, thinner reticular meshwork of pink fibrils which are interwoven among the more abundant collagen I fibers [31].
Digital Analysis of Sections
Three mid-penile tissue sections per animal were included for statistical analysis. Slides were photographed and recorded using a Retiga 1300 digital camera (QImaging, Surrey, Canada) attached to a Nikon E300 microscope (Nikon Instruments, Melville, NY, USA). Computerized histomorphometric analysis was performed using Image-Pro Plus 5.1 software (Media Cybernetics, Bethesda, MD, USA). For statistical analysis of neural nitric oxide synthase (nNOS) content, the ratio of the number of nNOS-positive fibers per nerve over the total area of the nerve in pixels was calculated at a magnification of ×400, and all the nerve branches of the dorsal nerve on each slide were included in the analysis. For analysis of smooth muscle and collagen content, the corpus cavernosum was photographed at a magnification of ×40, and the ratio between the area staining positive for α-smooth muscle actin (SMA) or collagen/total area within the tunica albuginea was used for statistical analysis. The apoptotic index was expressed as the number of TUNEL and DAPI-positive cells in 3 randomly chosen high-power fields (HPF, ×400) of the corpus cavernosum per animal, which were photographed and digitally analyzed.
Statistical Analysis
The results were analyzed using Medcalc version 11.0.0.0 (Medcalc software, Belgium). To test the difference between the means of multiple treatment groups, ANOVA and post-hoc analysis with the Tukey-Kramer test was used. Results were considered statistically significant if p<0.05. Data are shown as mean ± standard error of the mean (SEM).
Results
Erectile Function
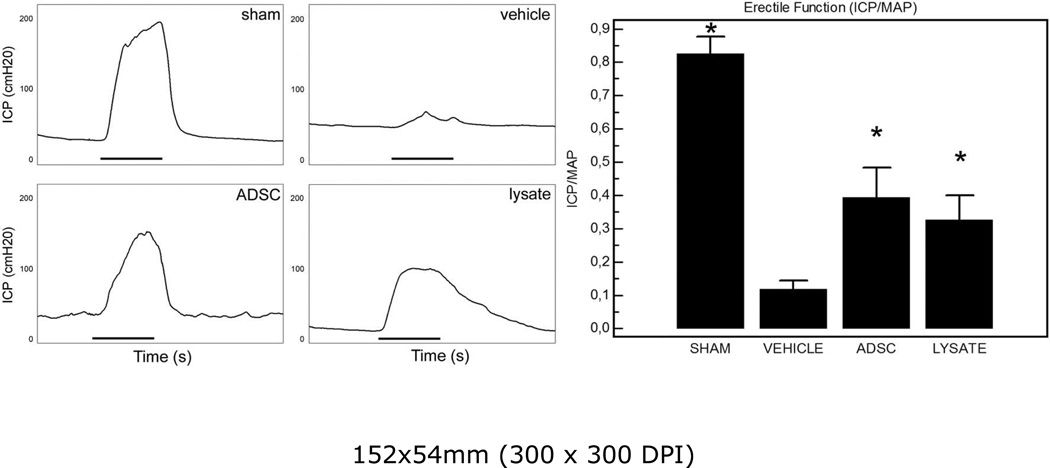
The effects of injection of ADSC or lysate after bilateral CN crush injury on erectile function are shown in figure 1. CN crush injury consistently resulted in erectile dysfunction. This finding is reflected by markedly decreased ICP/MAP responses to electrostimulation in the vehicle group compared to sham. Upon stimulation of the distal portion of the cavernous nerve, partial recovery of the erectile response was seen both in the ADSC and the lysate group. Both ADSC and lysate injections resulted in a significant increase of ICP/MAP ratio compared to injured controls treated with vehicle. The MAP did not differ significantly among groups. Furthermore, there was no significant difference in erectile function recovery between the ADSC and lysate group.
Figure 1.
Left panel: representative recordings of ICP-registration upon stimulation of the distal cavernous nerve. Black bar represents one electrical stimulus of 50 s. Right panel: results of ICP-measurement expressed as the ratio ICP/MAP. *: P<0.05 compared to vehicle-treated group.
nNOS Positive Nerve Fibers in the Dorsal Penile Nerve
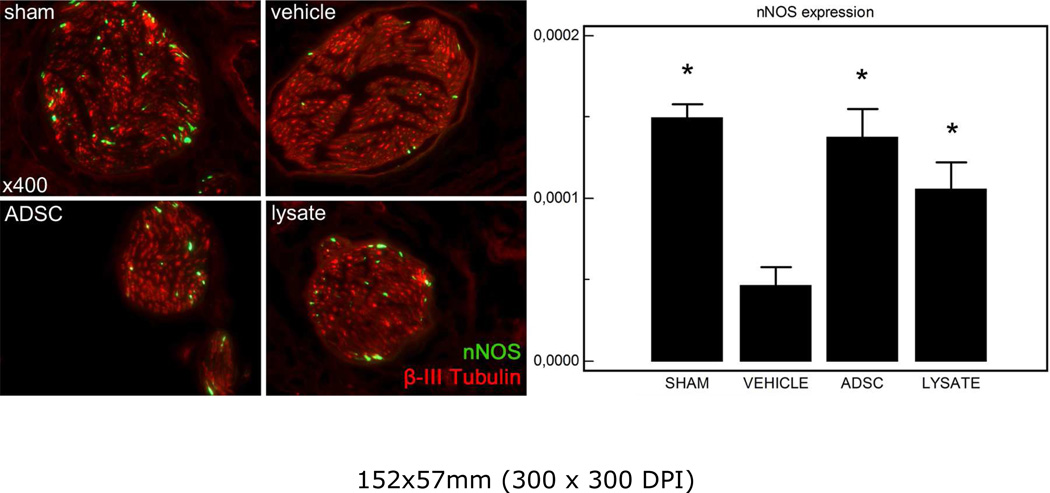
The dorsal penile nerves were immunostained for β-III-tubulin to identify nerve fibers and for nNOS to quantify nNOS content in the dorsal nerves. Representative images of each group are shown in figure 2. There was a significant decrease in nNOS content of the dorsal penile nerves after bilateral crush injury of the cavernous nerve. Following therapy with ADSC or lysate the number of nNOS containing fibers was significantly higher in both groups compared to vehicle treated injured controls.
Figure 2.
Left panel: representative images of the dorsal penile nerve of each group. Original magnification ×400. Right panel: result of nNOS quantification expressed as the number of nNOS-expressing fibers/area of the nerve (in pixel). *: P<0.05 compared to vehicle-treated group.
Histomorphometric Analysis of the Corpus Cavernosum
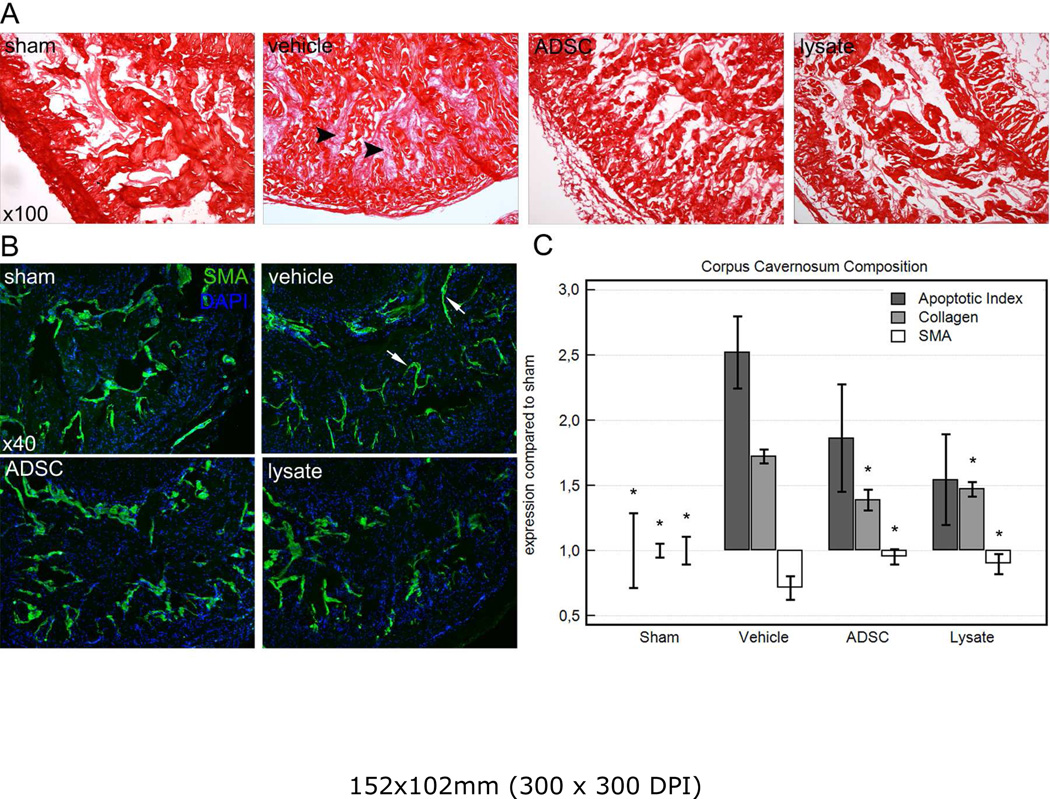
Computerized histomorphometric analysis showed a statistically significant decrease in smooth muscle content following CN crush injury. In the sham group, smooth muscle volumetric density of the corpus cavernosum was 5.57 ± 0.59% compared to 3.20 ± 0.50% after CN crush injury in the vehicle-treated group. Smooth muscle loss was mainly attributable to a thinning of the layer of smooth muscle cells located in the subendothelial space in the corpus cavernosum, as is shown in figure 3. After treatment with ADSC or lysate, smooth muscle content restored to 5.39 ± 0.34% and 5.07± 0.44% respectively. Total volumetric density of collagen was 84.12 ± 2.63% in vehicle treated rats, which was a significant increase compared to sham operated rats (48.79 ± 2.55%), which seemed to be attributed to an obliteration of the sinusoidal space with mainly collagen subtype III. After treatment with ADSC or lysate, there was a significant decline in corpus cavernosum fibrosis compared to injured controls, reflected by a collagen density of 67.74 ± 3.99% and 71.83 ± 2.78% respectively. Figure 3 shows the changes in the corpus cavernosum following CN crush injury and the effects of treatment on the composition of the corpus cavernosum. Fluorescence microscopy showed very few EdU-labeled cells in the cavernous tissue, which were located in the subendothelial space lining the sinusoids.
Figure 3.
Summary of the changes in the corpus cavernosum in each group. A: Changes in collagen content following CN crush injury and treatment. Note the obliteration of the sinusoidal spaces with reticular subtype III collagen (arrowheads) in the vehicle-treated group. Original magnification ×100. B: smooth muscle content in the corpus cavernosum in each group. Note the thinning of the subendothelial smooth muscle layer after CNI in vehicle treated rats (arrows), and how this was prevented by treatment with ADSC or lysate. Original magnification ×40. C: Graph summarizing the changes in the corpus cavernosum as relative expression as compared to sham-operated animals. *: P<0.05 compared to vehicle-treated group.
Quantification of Apoptosis
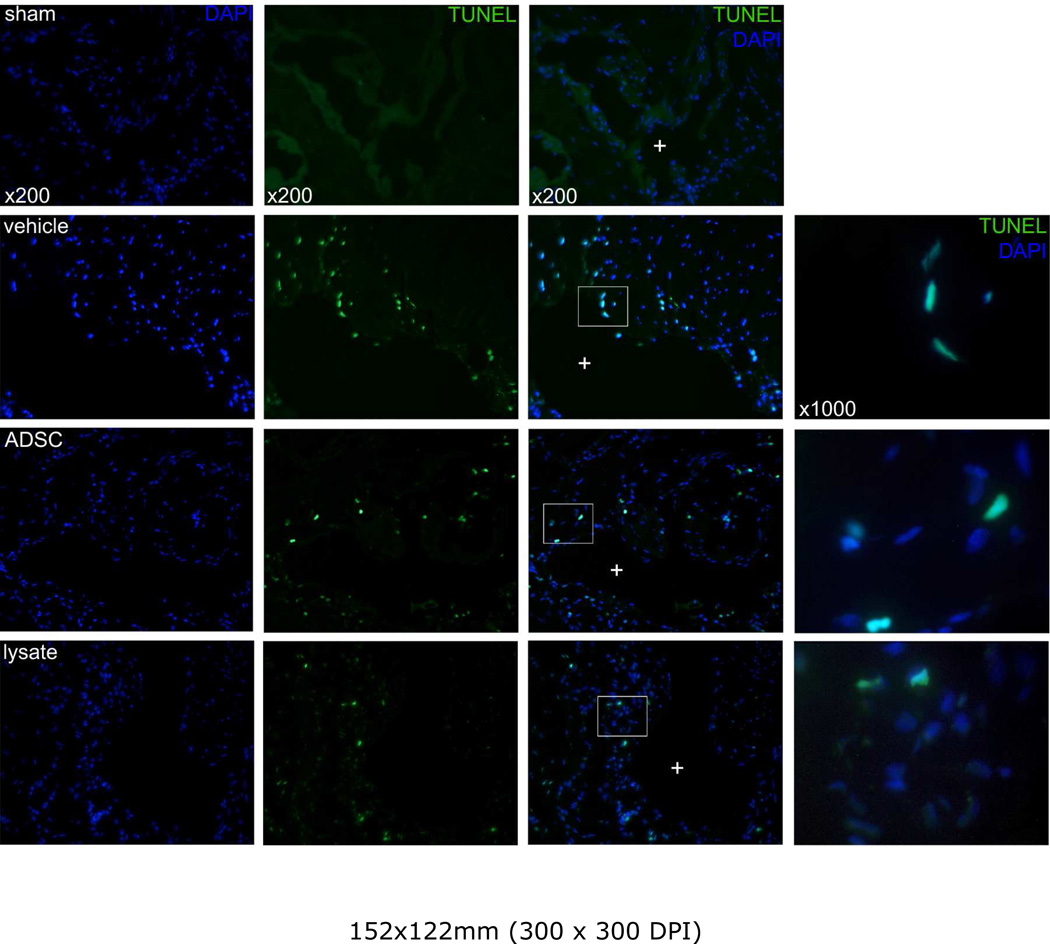
TUNEL staining showed nuclear colocalization with DAPI. Only cells positive for both TUNEL and DAPI were considered positive for apoptosis. Apoptotic cells were located predominantly in the close vicinity of the sinusoids in the endothelium and in the subendothelial tissue (figure 4), indicating apoptosis of predominantly endothelial and smooth muscle cells. Apoptosis was significantly more abundant in animals that underwent crush injury compared to sham. Treatment with ADSC and lysate were able to reduce the apoptotic index, although the differences with the injured controls treated with vehicle were nonsignificant (figure 3).
Figure 4.
Representative images of a lacunar sinusoid (+) in the corpus cavernosum. Notice the perisinusoidally located apoptosis at the endothelial and subendothelial tissue, and high-power magnification of nuclear colocalization of DAPI and TUNEL-signals. Original magnification: ×200, ×1000.
Discussion
Previously, our group obtained promising results with the use of ADSC for erectile dysfunction caused by diabetes or dyslipidemia [21,23]. In the present study we report beneficial functional effects of ADSC therapy on erectile function after CN crush injury. The beneficial functional effects were accompanied by increased nNOS content in the dorsal penile nerve and reduced fibrosis in the corpus cavernosum when compared to injured controls treated with vehicle.
This study was preceded by a small number of studies investigating other types of stem cells in the treatment of ED following cavernous nerve injury. Kendirci and colleagues have shown functional benefit of injection of a subpopulation of BMSC after CN injury [10], and a recent study by Fall et al. showed functional improvement of erectile function and reduction of penile apoptosis after bilateral cavernous nerve ablation in rats treated with mononucleated bone-marrow cells [9]. In the study by Fall et al. the labeling dye was traced in the erectile tissue; however, it was not clear whether the dye remained in the penis after cell death and lysis, or whether the cells were incorporated, differentiated and alive [9]. In the present study, we used EdU as a labeling azide, which is incorporated into the nuclear DNA and therefore makes it easy to colocalize with DAPI [30]. By this tracking method, we observed a very limited number of EdU-positive cells in the corpus cavernosum at 28 days following injection. The paucity of EdU-expressing cells at 28 days after injection, argues against sustained incorporation of ADSC into the host tissue. While we only studied one time-point in this project, in a previous study, we observed a time-dependent decrease in the number of ADSC in the corpus cavernosum following injection, suggesting that the beneficial effects of ADSC therapy are established early after injection [21].
In recent years, there has been an increasing body of literature describing the trophic effects of ADSC on cytoprotection, cell survival, immunomodulation and modulation of the extracellular matrix of endogenous tissues [18]. These effects are closely related to a variety of soluble factors secreted by ADSC. Molecules such as various growth factors, hematopoietic factors, a number of interleukins, tumor necrosis factor-α (TNF-α), vascular endothelial growth factor, and a variety of neurotrophins such as brain derived neurotrophic factor (BDNF), neurturin, neurotrophic factor 3 (NT3) and nerve growth factor (NGF) have been identified in the secretome of these stem cells [18,32]. The beneficial effect on erectile function we observed in the ADSC group might be, in part, attributable to the presence of neurotrophins. Various neurotrophins, such as NT3, BDNF, and NGF, have been shown to enhance neuroregeneration of nNOS-expressing neurons in vivo and in vitro [2–4,33–35]. The neuroregenerative effects after intracavernous injection of these factors have been attributed to retrograde axonal transport towards the MPG, where these molecules modulate neuronal growth and survival [36]. The hypothesis that paracrine pathways are involved in these effects is supported by our data showing a similar nNOS-content in the dorsal penile nerve fibers in the rats treated with the lysate as in those treated with ADSC. Lysate treatment exposes the tissues to soluble factors contained in ADSC, without allowing live cells to directly act on the host tissue [37]. Furthermore, we observed a comparable preservation of corpus cavernosum architecture and reduction of the apoptotic index between the two treated groups, suggesting a complimentary role for antifibrotic and anti-apoptotic properties of ADSC-derived biomolecules, rather than replacement of apoptotic endothelial, smooth muscle and mesenchymal cells. Perhaps the strongest argument supporting the hypothesis that ADSC work in a paracrine fashion is provided by the fact that the cell-free lysate therapy resulted in a comparable functional recovery as the ADSC therapy did.
A multitude of studies demonstrate that ADSC engraftment into the host tissue and in-situ survival is too limited to be therapeutically beneficial and enhance organ function [11,20–23,37–39]. Furthermore, mesenchymal stem cells have been shown to induce acute improvement in target organ function less than 72 h after injury, precluding differentiation as a cause due to time required [11]. The third, and most convincing argument for the paracrine pathway is that in-vitro and in-vivo research has revealed that the functional improvement of injury seen in stem cell therapy can be replicated by utilizing either cell free lysate, or conditioned stem cell culture media [37,40,41]. In our experience, we have been able to show significantly improved neurite sprouting in MPGs that were co-cultured with ADSC as compared to co-culture with smooth muscle cells [32]. Both lysate and conditioned media contain a broad variety of cytokines, growth factors, and putatively also microvesicles containing (micro)RNA, which are held responsible for the beneficial effects seen with lysate and media therapy [42,43]. These data combined, suggest that stem cells may improve injured organ performance and limit injury not via differentiation but rather via complex paracrine actions.
Our study does have some limitations. First, although the injection of cell-free lysate resulted in similar improvement as the ADSC-therapy did, we realize that these data only provide us with indirect evidence supporting the paracrine pathway. Furthermore, we do not know what the exact contents of the lysate are, and how these are responsible for the observed functional improvement and structural preservation. Therefore, further investigations aimed to identify the proteomic characteristics of the cell-free lysate should allow us to gain further insight into the possible mechanisms of erectile functional improvement. Different time points will also be studied to examine changes in the effects of ADSC in time. Identification of the components of the lysate might have important implications on the future development of novel stem cell based therapies including enhancement of the secretory profile of stem cells by the overexpression of certain paracrine signaling molecules. These investigations will be conducted in the near future to further clarify the role of paracrine signaling in stem cell therapy for ED. Second, we observed a paucity of ADSC in the corpus cavernosum. Although this finding also provides indirect clues concerning the mechanisms of action of ADSC, further studies are indicated to investigate the fate and the behavior of the ADSC after intracavernous injection. The question remains whether these cells exert their effects by their demise, or whether they home to other tissues to improve function indirectly instead of having direct effects on the effector tissue, the corpus cavernosum. Studies involving the tracking of ADSC after injection have been initiated to address these issues.
Conclusions
ADSC are easily accessible and abundant in adipose tissue and may therefore hold great promise towards a cure for erectile dysfunction following radical prostatectomy and other radical pelvic surgeries. The functional and histomorphometric benefits of both ADSC and lysate injection over vehicle-treatment, support the hypothesis that ADSC act through release of intracellular preformed substances or by active secretion of certain biomolecules. The underlying mechanism of recovery appears to involve neuron preservation and cytoprotection by inhibition of apoptosis.
References
- 1.Shao YH, Demissie K, Shih W, Mehta AR, Stein MN, Roberts CB, et al. Contemporary risk profile of prostate cancer in the united states. J Natl Cancer Inst. 2009;101:1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albersen M, Joniau S, Claes H, Van Poppel H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv Urol. 2008:594868. doi: 10.1155/2008/594868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bella AJ, Lin G, Lin CS, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6(Suppl 3):347–352. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bella AJ, Lin G, Cagiannos I, Lue TF. Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian J Androl. 2008;10:54–59. doi: 10.1111/j.1745-7262.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- 5.Sezen SF, Lagoda G, Burnett AL. Role of immunophilins in recovery of erectile function after cavernous nerve injury. J Sex Med. 2009;6(Suppl 3):340–346. doi: 10.1111/j.1743-6109.2008.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagoda G, Sezen SF, Burnett AL. FK506 and rapamycin neuroprotect erection and involve different immunophilins in a rat model of cavernous nerve injury. J Sex Med. 2009;6:1914–1923. doi: 10.1111/j.1743-6109.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 7.Fandel TM, Bella AJ, Lin G, Tantiwongse K, Lin CS, Pohl J, et al. Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2008;5:1866–1875. doi: 10.1111/j.1743-6109.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 8.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;t;94:904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 9.Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, et al. Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2009;56:716–725. doi: 10.1016/j.eururo.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 10.Kendirci M, Spees JL, Trost L, Whitney MJ, Prockop DJ, Hellstrom WJ. Adult bone marrow stem cells isolated by the p75 nerve growth factor receptor into the corpora cavernosa promoted recovery of erectile function in cavernous nerve injury. J sex med. 2006;3(suppl 5):384. (abstract OR-008). [Google Scholar]
- 11.Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143:577–581. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 14.Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 15.Kuhbier JW, Weyand B, Radtke C, Vogt PM, Kasper C, Reimers K. Isolation, characterization, differentiation, and application of adipose-derived stem cells. Adv Biochem Eng Biotechnol. 2010 doi: 10.1007/10_2009_24. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, et al. Perivascular multi-lineage progenitor cells in human organs: Regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20:429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Recent advances in andrology-related stem cell research. Asian J Androl. 2008;10:171–175. doi: 10.1111/j.1745-7262.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 18.Salgado AJ, Reis RL, Sousa N, Gimble JM. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2009 doi: 10.2174/157488810791268564. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Tholpady SS, Katz AJ, Ogle RC. Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:398–402. doi: 10.1002/ar.a.10039. [DOI] [PubMed] [Google Scholar]
- 20.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J sex med. 2010 doi: 10.1111/j.1743-6109.2009.01697.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YC, Shindel AW, Ning G, Lin G, Harraz AM, Wang G, et al. Adipose derived stem cells ameliorate hyperlipidemia-associated detrusor-overactivity in a rat model system. J. urol. 2010 Mar;183(3):1232–1240. doi: 10.1016/j.juro.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, Lin CS, et al. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, Suzuki E, Oba S, Nishimatsu H, Kimura K, Nagano T, et al. Adipose tissue-derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral artery. Am J Physiol Heart Circ Physiol. 2010;298:H415–H423. doi: 10.1152/ajpheart.00391.2009. [DOI] [PubMed] [Google Scholar]
- 25.Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008;449:69–79. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- 26.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 29.Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003;3:705–713. doi: 10.1517/14712598.3.5.705. [DOI] [PubMed] [Google Scholar]
- 30.Lin G, Huang YC, Shindel AW, Banie L, Wang G, Lue TF, et al. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11:864–873. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro AC, Costa WS, Cardoso LE, Sampaio FJ. Organization and relative content of smooth muscle cells, collagen and elastic fibers in the corpus cavernosum of rat penis. J Urol. 2000;164:1802–1806. [PubMed] [Google Scholar]
- 32.Bella AJ, Garcia M, Lin G, Fandel TM, Brant WO, Lue TF. Adult adipose tissue derived stem cells enhance neurite outgrowth from the major pelvic ganglion of the rat. CUAJ. 2007;1:200. (abstract 2.01). [Google Scholar]
- 33.Bella AJ, Lin G, Tantiwongse K, Garcia M, Lin CS, Brant W, et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: Part I. J Sex Med. 2006;3:815–820. doi: 10.1111/j.1743-6109.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: Part 2. J Sex Med. 2006;3:821. doi: 10.1111/j.1743-6109.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 35.Bakircioglu ME, Lin CS, Fan P, Sievert KD, Kan YW, Lue TF. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol. 2001;165:2103–2109. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- 36.Hiltunen JO, Laurikainen A, Klinge E, Saarma M. Neurotrophin-3 is a target-derived neurotrophic factor for penile erection-inducing neurons. Neuroscience. 2005;133:51–58. doi: 10.1016/j.neuroscience.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Burst VR, Gillis M, Putsch F, Herzog R, Fischer JH, Heid P, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2009;114:e107–e116. doi: 10.1159/000262318. [DOI] [PubMed] [Google Scholar]
- 40.Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone BH, March KL, Farlow MR, Du Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci Lett. 2009;462:76–79. doi: 10.1016/j.neulet.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Wei X, Ma Z, Feng D, Tu P, Johnstone BH, et al. Adipose stromal cells-conditional medium protected glutamate-induced CGNs neuronal death by BDNF. Neurosci Lett. 2009;452:238–240. doi: 10.1016/j.neulet.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: Role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 43.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]