Abstract
Children with ASD demonstrate significant heterogeneity in their profiles of social interaction and stress responsivity. We evaluated behavior and stress response in 52 male children ages 8 to 12 in a naturalistic playground interaction paradigm involving a child with autism spectrum disorder, a typically developing peer, and a same-age confederate. Younger children in the ASD group engaged in 5.8 times more approach behavior and showed a lower cortisol response than their older peers. Those that verbally initiated with their peers also showed a higher cortisol response. Older children with ASD exhibited the highest stress responsivity, while younger children with ASD showed more willingness to approach others without apparent stress. Intervening early and often may contribute to improvements in social engagement in youth with ASD.
Keywords: autism, cortisol, play, social, stress, responder
Introduction
Autism spectrum disorder (ASD) refers to a range of pervasive developmental disorders that vary in symptoms and intensity of impairment across three domains: reciprocal social interaction, verbal and nonverbal communication, as well as restricted and repetitive interests and behaviors. According to the current DSM-IV-TR classification autistic disorder (AD) is defined by symptom presentation prior to the age of three across all three domains (APA 2000). Pervasive developmental disorder-not otherwise specified (PDD-NOS) is diagnosed when a child demonstrates impairment across all three domains, but full criteria for autistic disorder are not met. Asperger syndrome (AS) is characterized by deficits in social behavior and a repertoire of inflexible thoughts and behaviors while exhibiting typical cognitive and language development (APA 2000).
It is well known that play facilitates cognitive, motor, and social development skills (Boucher et al. 1998; Ginsburg 2007; Lieberman and Yoder 2013; Pellegrini and Smith 1998). However, as a result of a variety of challenges involving poor reciprocal social skills, restricted interests, and limited imagination, children with ASD seldom engage in spontaneous and age-appropriate play with peers (Honey et al. 2007; Yuill et al. 2007). Their need for sameness and repetition can interfere with social interaction as the demands of adapting to the dynamics of unfamiliar social situations require behavioral flexibility (Geurts et al. 2009). Subsequently, children with ASD may prefer to engage in self-play at a distance or engage in repetitive behavior and movements (BLINDED; Humphrey and Symes 2011) instead of engaging with peers (Loftin et al. 2008).
When engaged in play with their peers many children with ASD display signs of stress or anxiety, especially as they enter adolescence (Bellini 2006). Furthermore, they have difficulty understanding the perspective of others and sharing affective states (Baron-Cohen et al. 2009; Gutstein and Whitney 2002); . Because children with ASD exhibit deficits in age-appropriate social interaction skills, they frequently fail to establish interpersonal relationships, especially with peers (Krasny et al. 2003). In addition to facing challenges establishing and maintaining friendships, children with ASD are often the target of ridicule and rejection by peers (Church et al. 2000). Although these children may demonstrate improvement in some areas of social functioning in response to intervention and development, they also appear to gain insight into their lack of social competence (Knott et al. 2006). It has been proposed that this greater awareness may subsequently result in increased anxiety (Bellini 2006) and enhanced stress in social interactions with peers (BLINDED).
The hypothalamic-pituitary-adrenal (HPA) axis regulates many bodily processes and interactions, including reactions to psychological stress (Herman and Cullinan 1997). The primary stress hormone in humans is cortisol, which maintains a diurnal rhythm with a peak in the morning and lowest levels in the evening, and is secreted from the adrenal cortices in response to stress (Hennessey and Levine 1979; Herman and Cullinan 1997). Higher functioning children with ASD do not tend to exhibit temporal displacement of cortisol secretion (Marinovic-Curin et al. 2008; Richdale and Prior 1992); however, they show a more variable diurnal rhythm compared to typically developing children (BLINDED). Additionally, evening cortisol levels tend to be more elevated than typically developing peers, which have been associated with greater behavioral stress throughout the day (BLINDED).
In regards to stress responsivity, higher cortisol levels have been reported in ASD populations in response to a variety of novel stimuli and situations, including MRI procedures (BLINDED), phlebotomy (Spratt et al. 2012), and school integration (Richdale and Prior 1992). It has been suggested that individuals with ASD demonstrate increased responsiveness to acute stress, as opposed to a pattern of persistent hyperarousal (Tordjman et al. 1997). Context, as well as insight into the nature of the psychosocial stress, appears to be important mediating factors in determining HPA axis responsiveness. For example, a well-established psychosocial stressor, the Trier Social Stress Test (Buske-Kirschbaum et al. 1997), does not reliably activate the HPA axis in individuals with ASD (Jansen et al. 2003; Jansen et al. 2006; Lanni et al. 2012). Conversely, relatively benign social situations with peers result in enhanced cortisol response in many children with ASD (BLINDED).
The study of play via careful observation can provide important insight into developmental, psychological, and physiological factors that impact social engagement in ASD. Specifically, playground observation in a naturalistic setting is a useful and valid approach to understanding biobehavioral social profiles in children with ASD (BLINDED). It has been demonstrated that older children with ASD who attempted to engage in solicited play with same-age peers show increased stress responsivity as measured by salivary cortisol when compared to younger children with ASD and typically developing peers. Children with ASD were also differentiated on a number of behavioral variables including less time spent socially interacting with others, engagement in more peripheral play, and extended self-play on the playground equipment. However, more careful analyses were needed in a larger sample of children across different states of play to establish broader behavioral profiles based on age and stress response.
The objective of the current study was to extend previous findings in several important ways. First, we investigated the behavioral and biological response profiles of children with and without ASD during periods of free play rather than focusing solely on solicited cooperative play. Secondly, we aimed to stratify children into cortisol responders and non-responders and compare their cortisol profile to their behavioral characteristics. These aims were accomplished in a larger sample of children with high functioning ASD. Based on previous research, we hypothesized the following. In regards to stress responsivity we predicted that children with ASD would demonstrate: 1) heightened cortisol responsivity compared to typically developing children, 2) cortisol responsivity in the children with ASD would be modified by age, and 3) cortisol responsivity would be modified by factors of social engagement. Behaviorally, we predicted that children with ASD would demonstrate: 1) significant differences in their pattern of play with peers especially in regards to equipment use, approach behavior, avoidance rate and the amount of time socially interacting with peers, 2) age would significantly moderate social engagement and 3) differences in behaviors based on the independent and solicited group play would exist.
Methods
Participants
The study sample consisted of 52 unmedicated, pre-pubertal, healthy, male children between 8 and 12 years old, 26 with ASD (21 with autistic disorder, 5 with PDD-NOS, no children with Asperger syndrome) and 26 typically developing children. This narrow age range was selected to enhance the likelihood that children would engage together on the playground. Diagnosis was based on the Diagnostic and Statistical Manual (DSM-IV-TR) criteria(APA 2000) and established by all of the following: (1) a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with ASD expertise; (2) clinical judgment and (3) corroborated by a total score on the social-communication scale of the ADOS (Lord et al. 2000), administered by research-reliable personnel, with a total score at or above the ASD threshold for Module 3. There were no significant age differences across the diagnostic groups for children with AD (10.0 years) or PDD-NOS (11.1 years). Approximately half of the sample participated in a previous report that focused on other research questions related to the peer interaction paradigm (BLINDED).
Informed written consent was obtained from parents and verbal assent was obtained from research participants prior to inclusion in the study. Parents were informed that they would help collect salivary samples of their child from home and complete questionnaires. Participants were told they would be playing on a playground with peers and providing saliva samples. The University Institutional Review Board approved the study which was conducted in compliance with the Code of the Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki) http://www.wma.net/e/policy/b3.htm.
Cortisol Sampling Protocol
Well-established salivary collection protocols were carefully followed, which included using consistent collection materials and methods, controlling the intake and time of drinks, food, and prescription medications, as well as using standardized procedures (BLINDED; Hanrahan et al. 2006). Basal levels of salivary cortisol were collected for six diurnal cycles consisting of three samples per day (morning, afternoon, and evening), over three consecutive days, over two consecutive weeks, for a total of 18 samples. Participants were instructed to avoid eating for a minimum of 1 hour prior to sampling, which was confirmed by parental report. The afternoon sample was collected between 13:00 and 16:00.
The basic procedure involved giving the participant Trident® Original Sugarless chewing gum to act as a salivary stimulant. The child then emitted saliva into a collection tube via passive drool. Following training, baseline samples were collected by parents and then labeled and stored temporarily in the home refrigerator. For the social stress protocol, 4 salivary samples were obtained, including (S1) a sample taken after arrival, just prior to the playground peer interaction within a relaxed and private waiting room, (S2) immediately post-play, (S3) 20-minutes post-play, and (S4) 40-minutes post-play.
Cortisol Storage and Assays
All of the samples were transported to the Endocrinology Laboratory by research personnel, and then stored in a −20 C freezer. Prior to assay, samples were thawed and centrifuged at 6000 rpm for 10 minutes to separate the aqueous component from mucins and other suspended particles. Assays were performed using coated-tube radioimmunoassay kits (Siemens Medical Solutions Diagnostics, Los Angeles). Assay procedures were modified to accommodate overall lower levels of cortisol in human saliva relative to plasma as follows: 1) standards were diluted to concentrations ranging from 2.76 to 345 nmol/L, 2) sample volume was increased to 200µl, and 3) incubation times were extended to 3 hours. Serial dilution of samples indicates that the modified assay displays a linearity of 0.98 and a least detectable dose of 1.38 nmol/L. Intra-assay and inter-assay coefficients of variation were 3.91% and 5.26%, respectively.
Peer Interaction Playground Paradigm
Previously, we designed a peer interaction that blends prescribed sequences of play while allowing flexibility in the protocol to allow natural play behavior to occur. The peer interaction occurred on a fenced 60’ by 60’ playground containing large equipment and open space for cooperative games. Research personnel remained in the building so as not to impinge on the natural environment. The entire transaction was recorded via four cameras and sound equipment. Two fixed video cameras were housed on the building exterior in glass cases that rotated to observe the entire play area. Adjacent to the playground was a concealed video-control room where the cameras were monitored and controlled. In addition, two portable cameras were used to record the play through windows. The different camera viewpoints were recorded and synced with voice recordings that were later used for behavioral coding.
The interaction paradigm consisted of a child with ASD, a typically developing child, and a confederate of the same age and gender. The confederate provided behavioral structure to the free play by permitting key interactive sequences to occur within an otherwise natural interaction and social setting. Importantly, the confederate also helped to maintain an even level of interactive play in part to prevent increased aerobic activity which could affect cortisol levels. Each child wore a microphone clipped to their shirt and the confederate also wore earphones that enabled direct communication with research personnel who provided cues to ensure appropriate responses at key points in time. The two participants were simultaneously escorted to the playground. Each play session had a total duration of 20-minutes, which was divided into four 5-minute periods, which were rigorously timed to maintain paradigm consistency.
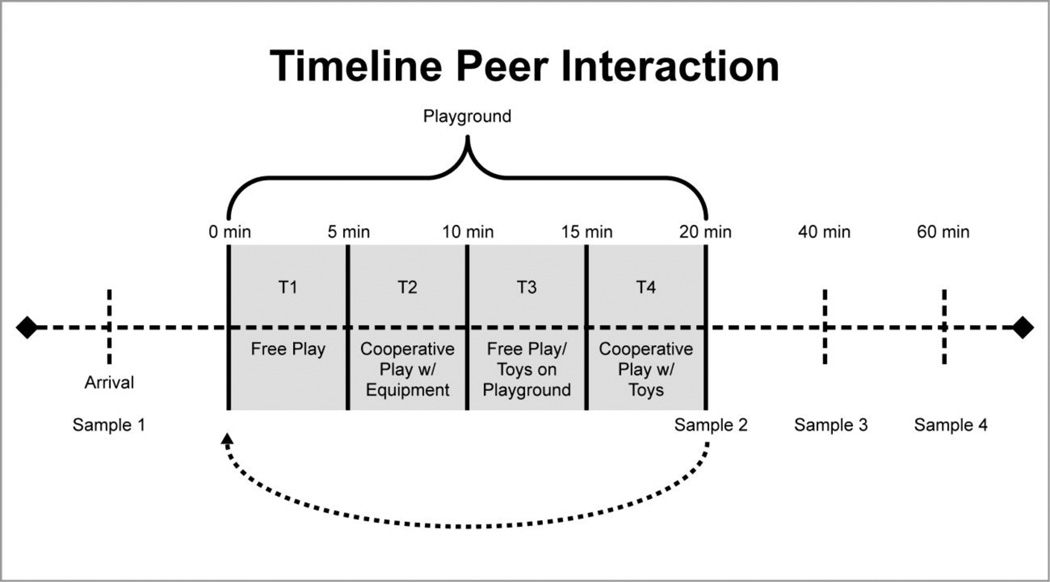
As shown in Figure 1, the first period involves free play in which the three children engage in independent gross motor play (T1). During the second period the confederate solicits interaction on the play equipment for cooperative play (T2). The third period is also free play and includes the introduction of a box of toys (T3). Finally, during the fourth period, the confederate again solicits the two participants to engage with him in a cooperative game involving the previously introduced toys (T4).
Figure 1.
Peer interaction paradigm timeline of events. The 20 min interaction consists of four 5 min periods: The first period involves free play in which the three children engage in independent gross motor play (T1). During the second period the confederate solicits interaction on the play equipment for cooperative play (T2). The third period is also free play and includes the introduction of a box of toys (T3). During the fourth period, the confederate again solicits the two participants to engage in a cooperative game involving toys.
Behavior Coding
The Observer XT Version 8.0 software was used for the collection and analysis of the interaction observational data (Noldus 2008). Data were analyzed using both standard ethological approach by examining frequency, duration, and direction of the target behaviors and using a transactional approach (i.e., a who does what to whom format) based on a predefined list of operationalized behaviors (Lyons et al. 1990; Lyons et al. 1992; Mason et al. 1993; Mendoza and Mason 1989). The transactional approach evaluates “bouts” of engagement that begin with an overture by one participant initiating a sequence of behaviors occurring between two or more children. The transactional bout identifies a participant’s attempt to alter their immediate state of association with a target by a cooperative or competitive response. Within each bout, the immediate responses of the participants and that of the other children were coded. Shifts in orientation and proximity are also coded and assist in determining the end of a bout. Coding was accomplished by observing and coding each participant’s behavior separately while simultaneously viewing all camera viewpoints. Reliability was calculated for a random sample of 25% of observations using the Noldus Observer software. Cohen’s Kappa is considered a robust measure of inter-rater reliability as it controls for the possibility of raters agreeing by chance; Inter-rater reliability was calculated at K = 0.80 while test-retest reliability was K = 0.89. Gesturing, equipment use and group equipment use were used to code basic playground activities. Approach and avoid were coded when subjects deliberately increased or decreased distance to another subject. Proximity captured when subjects deliberately moved to within 10 feet of another subject but did not interact, while initiation and rejection captured subjects verbally starting or terminating interactions.
Diagnostic Variables
Autism Diagnostic Observation Schedule (ADOS)
The ADOS (Lord et al. 2000) is a semi-structured interview designed to assess behaviors characteristic of ASD across domains of functioning. A score of 8 or greater on the social-communication domain of the ADOS Module 3 was required for inclusion. The test authors report interrater reliability for domains as follows: Communication .73 to .84, Social .78 to .84, and Communication-Social Interaction Total .82 to .92, (Lord et al. 1999). Internal consistency using Cronbach’s alpha are reported as follows across the modules: Social Interaction (.86 to .91) and Communication (.74 to .84).
Wechsler Abbreviated Scale of Intelligence (WASI)
The WASI (Wechsler 1999) is a measure of cognitive ability used to obtain an estimate of intellectual functioning. Inclusion in the study required an estimated IQ of 75 or higher. According to the manual, test-retest reliability for children is .85, interrater reliability is >.9 and construct validity ranges from .87 when compared to the full Wechler tests.
Pubertal Development Scale (PDS)
The PDS is a parent report measure allowing an estimate of the participant’s level of pubertal development (Petersen et al. 1988), and has a reported correlation to physician rating of 0.61 – 0.67. Enrollment was limited to those who had not formally entered puberty defined as a score of 3 based on ratings of “1” (change not yet begun) in each of three categories: voice, pubic hair, and facial hair. The PDS was used as a proxy measure for inclusion/exclusion since pubertal development was not assessed through physical exam or used as a dependent measure.
Social Communication Questionnaire (SCQ)
The SCQ (Rutter et al. 2003) is a screening tool for ASD and according to the manual, the discriminative validity of the SCQ to differentiate ASD from non-ASD diagnoses ranges from .74 to .94. Furthermore, the SCQ demonstrates strong discrimination between ASD and non-ASD cases (sensitivity 0.88, specificity 0.72) and between autism and nonautism cases (sensitivity 0.90, specificity 0.86) (Chandler et al. 2007). . Scores of ≥15 are suggestive of ASD while scores of ≥22 are suggestive of autism. Any typical child with a score of 10 or higher was excluded.
Dependent Variables
Salivary cortisol
The primary outcome variable of interest for characterizing the stress response was salivary cortisol. Because salivary cortisol measurements are positive and skewed toward large values a log transformation was performed to achieve approximate normality; thus log-transformed values were used in all analysis. As noted, the peer interaction included four salivary cortisol samples taken 20 minutes apart for each subject: S1 – (baseline), S2 – (immediately post-play), S3 – (20 minutes post-play), and S4 – (40 minutes post play). Importantly, there is an approximate 20 minute lag from the time an event occurs until the change in cortisol can be detected in saliva. For example, the S2 measurement taken immediately after the peer interaction is measuring the subject’s cortisol levels at the beginning of the peer interaction while the S3 measurement is measuring the cortisol levels at the end of the peer interaction. All peer interactions were held in the afternoon between 13:00 and 16:00 for comparison to afternoon values from the diurnal study.
Independent Variables
Diagnosis
Diagnosis was a primary independent variable included in all of the models. It was treated as a categorical variable with 2 levels (ASD, Typical).
Sampling time
Sampling time was a primary independent variable included in all of the cortisol response models. It was treated both categorically and continuously in different models.
Additional Variables
Average afternoon cortisol
Average afternoon cortisol was calculated based on the 6 afternoon values for each participant taken during the diurnal study to represent the expected (“normal”) afternoon cortisol level. The arrival values might not represent an accurate baseline; therefore this measurement was included as a covariate in the adjusted stress response models.
Cortisol responder status
Due to the stressor, cortisol responder status was calculated using the following steps: 1) the standard deviation for afternoon cortisol was found for each child, 2) the maximum change in cortisol from arrival (S1) to either the post-play (S2) or 20 minute post play (S3) was calculated, 3) if the maximum change represented an increase in cortisol levels greater than 1.645 standard deviations the subject was labeled a responder. Otherwise the subject was assumed to have normal afternoon variability, to not be experiencing a stress response, and labeled a nonresponder. A response of this magnitude or greater should only occur by chance 5% of the time and therefore represents a significant change. An increase from baseline to the post-play measurement indicated a stress response to the beginning of the peer interaction, while an increase to the 20 minute post play measurement indicated a stress response to the end of the peer interaction.
Statistical Analysis
Between-group analyses were performed across all demographic, diagnostic, and inclusion variables using independent two-sample t-tests if the assumption of normality held true; otherwise the equivalent nonparametric test was used. Variances were compared using Levene’s test of homogeneity to determine if the equal variance assumption between the groups was valid. If the equal variance assumption was not met, the Welch-Satterthwaite degree of freedom approximation was used.
Cortisol stress response
The stress response profile consisting of the four cortisol measurements was characterized using repeated measures linear mixed-effects models, with diagnosis and time of sample measurement (S1, S2, S3, S4) as the primary independent variables, and included interaction terms between diagnosis and time. This model treated sample time categorically where the variables S2, S3, and S4 are indicator variables (relative to S1) and imposes little restriction on the mean response over time or on the covariance structure among the repeated measures (Fitzmaurice et al. 2004) (pg. 103). This base model was then expanded and by including average afternoon cortisol, continuous age, and IQ as potential covariates or effect modifiers (i.e. two-way interactions with either diagnosis or time). Model coefficients for the mean response were based on maximum likelihood estimation and fixed effects were tested using a Wald test. A random intercept was included for each subject and the error terms between subjects were assumed to be independent, normally distributed, and to have a common variance.
Playground behaviors
The percentage of time spent interacting for each time period was calculated, transformed using the arcsine function treating it as a continuous variable, and analyzed using ordinary least squares linear regression. The other behavior variables collected during the four time periods of the peer interaction were analyzed separately using generalized linear models for count data with diagnosis as the primary predictor variable. After examining the unadjusted relationships between diagnosis and behavior the playground behavior variables were then adjusted for continuous age, IQ, and responder status.
Poisson regression models are widely employed in the analysis of count data and were the primary regression method used. These models allow direct comparison of average behavior rates between children with ASD and typical children. It is known, however, that counts often display substantial extra Poisson-variation, or overdispersion, relative to a Poisson model (Lawless 1987). Therefore negative binomial regression models were also fit to account for the extra variation. A likelihood ratio test was used to compare the negative binomial model to the Poisson model for final model selection. All statistical analysis was performed using the R statistical program (R 2009), and the repeated measures analysis utilized the nonlinear and linear mixed effects models package (Pinheiro et al. 2009).
Results
Baseline analyses were conducted between 26 children with ASD and 26 typically developing children to assess differences between the groups based on age and clinical variables. This demographic information for the sample participants can be found in Table 1.
Table 1.
Between-group Comparisons of Demographic, Diagnostic, and Inclusion Variables
| ASD | Typically Developing |
||||||
|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t | df | p |
| Age | 10.2 | 1.2 | 10.0 | 1.5 | 0.49 | 50.0 | .6245 |
| IQ | 94.9 | 17.2 | 121.6 | 11.5 | −6.57 | 50.0 | < .001 |
| SCQ | 24.7 | 6.3 | 2.2 | 2.0 | 16.58 | 27.3 | < .001 |
Note. ASD = Autism Spectrum Disorder; SCQ = Social Communication Questionnaire
Characterizing Cortisol Stress Response
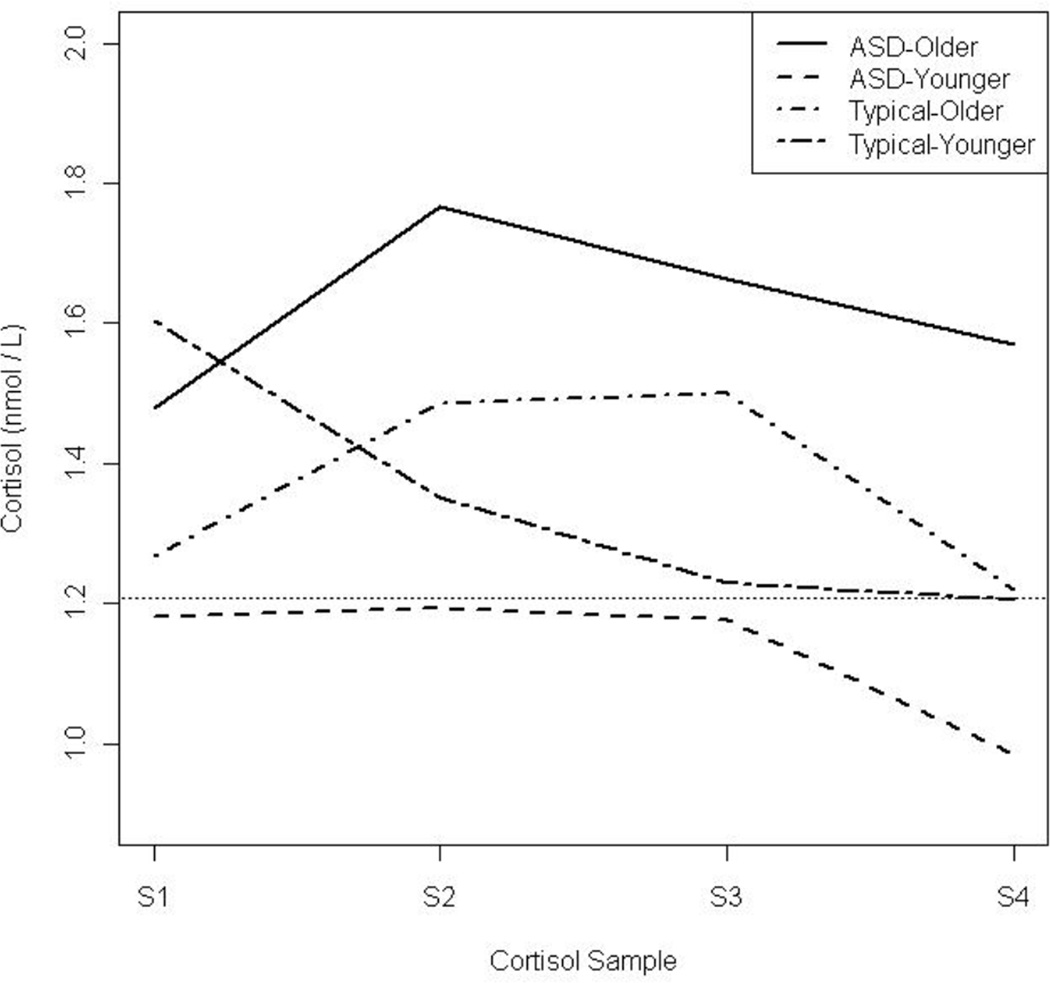
Preliminary unadjusted analysis between diagnosis, time, and cortisol level revealed a significant relationship when compared to a trivial model with constant cortisol level. This model, which treated sampling time as a factor, was a significant improvement over the trivial model (p = 0.048). Next, after the model was adjusted for age and average afternoon cortisol level (IQ was not found to be significant), the repeated-measures analysis of the cortisol values revealed a similar relationship between diagnosis and measurement time (Table 2), regardless of whether time was treated categorically or continuously with a quadratic term. In both models there was a strong interaction between diagnosis and age such that children with ASD had higher average cortisol levels compared to typical children of the same age. In addition there were strong time and age interactions, and the cortisol response profile appeared to change significantly with age regardless of diagnosis. The second model, selected based on minimizing the AIC, was a highly significant improvement compared to the model containing only diagnosis and time (p < 0.001) and is visualized in Figure 2, with age dichotomized into a “younger” and an “older” group based on a median split within each diagnostic group for the purpose of illustrating interacting relationships.
Table 2.
Estimated Coefficients and Standard Errors for Repeated Measures Regression of Log-Cortisol on Diagnosis and Measurement Time Adjusted for Age and Average Afternoon Cortisol
| Variable | Estimate | SE | df | t | p |
|---|---|---|---|---|---|
| Intercept | 2.01 | 0.50 | 149 | 4.05 | < .001 |
| Diagnosis | −2.19 | 0.65 | 47 | −3.35 | .002 |
| S2 | −1.44 | 0.35 | 149 | −4.09 | < .001 |
| S3 | −1.55 | 0.45 | 149 | −3.46 | < .001 |
| S4 | −1.27 | 0.50 | 149 | −2.52 | .013 |
| Age | −0.11 | 0.05 | 47 | −2.27 | .280 |
| Diagnosis*Age | 0.22 | 0.06 | 47 | 3.38 | .002 |
| S2*Age | 0.15 | 0.03 | 149 | 4.32 | < .001 |
| S3*Age | 0.15 | 0.04 | 149 | 3.51 | < .001 |
| S4*Age | 0.11 | 0.05 | 149 | 2.29 | .024 |
| Average Afternoon Cortisol | 0.37 | 0.14 | 47 | 2.66 | .011 |
Note. S2 = Indicator Function for Time Period 2; S3 = Indicator Function for Time Period 3; S4 =Indicator Function for Time Period 4
Figure 2.
Observed average response profiles by age and diagnosis. The horizontal line represents the overall average afternoon cortisol level from the diurnal study used for comparison to the response profiles. Age dichotomized based on a median split within each diagnostic group for the purpose of illustrating the interacting relationships.
The horizontal line represents the overall average afternoon cortisol level from the diurnal study used for comparison to the response profiles. The proportion of cortisol responders was 38.5% of the ASD group and 19.2% of the typical group.
Explaining Playground Behaviors
The observed behavioral data (see Table 3) was analyzed using both Poisson and negative binomial regression models for count data with diagnosis as the main predictor variable. An unadjusted diagnosis effect was not present for any of the variables during either the independent gross motor free play period (T1) or independent cooperative free play period (T3). During the solicited gross motor play time period (T2) there was a significant difference in the rate of rejections such that children with ASD rejected 3.72 times more often than typical children. In addition, the percentage time interacting was highly significant (p = 0.002) during that time period, with typical children interacting on average 78.5% of the time compared to 50.5% for children with ASD. During the solicited cooperative play time period (T4), there was a significant difference in the rate of equipment use, proximity, and rejections (all p <0.05). Children with ASD used equipment by themselves 3.92 times more often, approached without initiating 6.73 times more often, and rejected 5.11 times more often than their typical peers. Percentage of time interacting was also significantly different between the groups (p < 0.001) with typical children interacting 91.1% of the time on average compared to 61.5% for children with ASD.
Table 3.
Observed Playground Behaviors for the Four Peer Interaction Time Periods by Diagnosis
| Period 1 | Period 2 | Period 3 | Period 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD | Typical | ASD | Typical | ASD | Typical | ASD | Typical | |||||||||
| Variable | M | V | M | V | M | V | M | V | M | V | M | V | M | V | M | V |
| Approach | 0.62 | 0.13 | 0.76 | 0.86 | 1.00 | 1.58 | 0.52 | 0.84 | 0.42 | 0.33 | 0.63 | 1.38 | 0.54 | 0.50 | 0.60 | 0.83 |
| Avoid | 0.46 | 0.58 | 0.32 | 0.39 | 1.68 | 0.89 | 0.40 | 1.26 | 0.38 | 0.89 | 0.25 | 0.28 | 0.46 | 0.82 | 0.16 | 0.22 |
| Gesture | 0.31 | 0.70 | 0.32 | 0.48 | 0.60 | 1.25 | 0.50 | 1.43 | 0.38 | 0.49 | 0.54 | 0.69 | 0.38 | 0.41 | 0.72 | 2.30 |
| Equipment | 4.27 | 4.52 | 4.72 | 8.63 | 2.56 | 6.34 | 1.60 | 3.92 | 2.35 | 3.28 | 1.58 | 5.81 | 1.88 | 5.15 | 0.48 | 0.43 |
| Group Eq. | 0.73 | 2.76 | 0.72 | 1.21 | 2.12 | 6.11 | 3.72 | 10.46 | 1.96 | 7.56 | 3.08 | 8.69 | 2.08 | 4.31 | 2.68 | 2.48 |
| Proximity | 0.77 | 1.30 | 92.00 | 1.49 | 0.68 | 2.31 | 0.36 | 0.41 | 0.54 | 0.90 | 0.38 | 0.51 | 0.54 | 1.38 | 0.08 | 0.08 |
| Initiate | 0.87 | 2.12 | 0.75 | 0.98 | 0.71 | 1.01 | 0.43 | 0.62 | 0.91 | 1.13 | 0.73 | 1.83 | 0.09 | 0.09 | 0.39 | 0.43 |
| Reject | 0.04 | 0.04 | 0.08 | 0.17 | 0.81 | 0.96 | 0.22 | 0.18 | 0.23 | 0.47 | 0.14 | 0.12 | 2.00 | 30.00 | 0.39 | 0.89 |
Note. V = variance; Eq. = equipment
Independent gross motor play (T1)
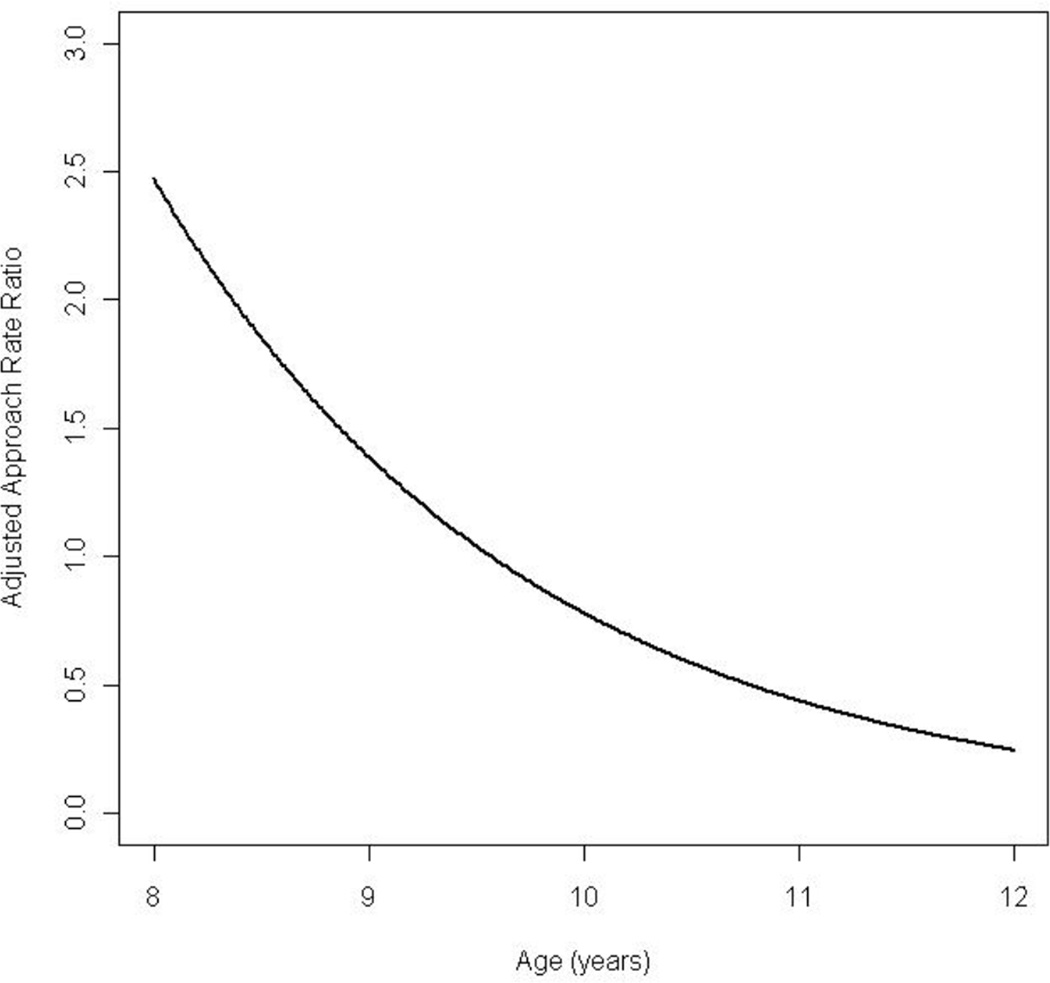
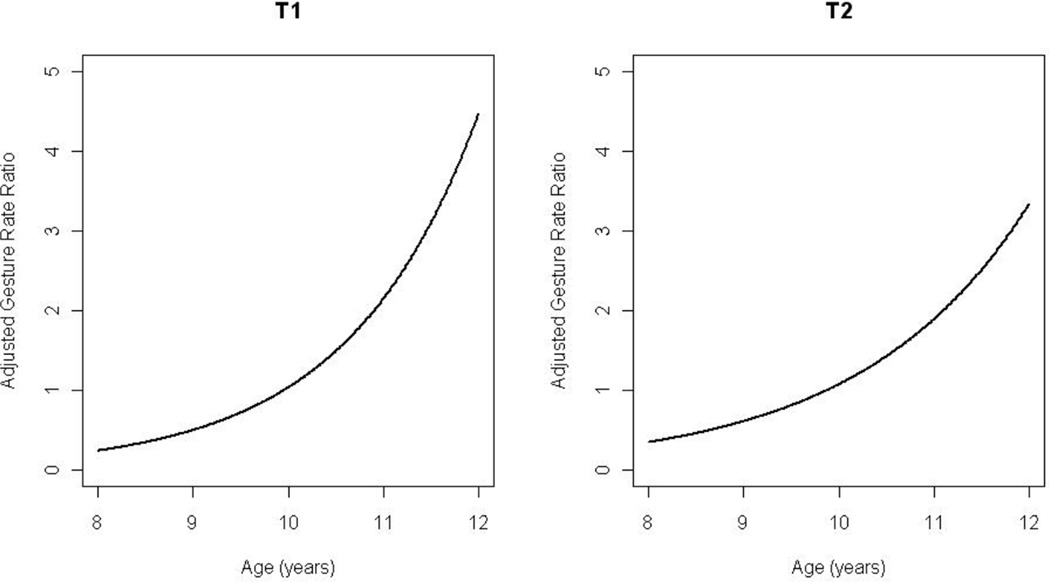
There was significant modification of the diagnosis effect based on subject age for rate of approach. It appears that for younger children with ASD, the approach rate was greater than younger typical children, while the reverse held true for older children (Figure 3). In addition, within the ASD group, younger children approached at a rate 5.8 times the approach rate of older children. Under the Poisson assumption the opposite relationship held for the rate of gesturing, where the rate ratio was greater than 1 for older children and less than 1 for younger children (Figure 4). Within the ASD group, older children gestured at a rate 2.83 times the rate of younger children.
Figure 3.
Rate ratio function of approach adjusted by age for time period T1 comparing children with autism spectrum disorders to typical children.
Figure 4.
Rate ratio function of gestures adjusted by age for time period T1 (left) and time period T2 (right) comparing children with autism spectrum disorders to typical children.
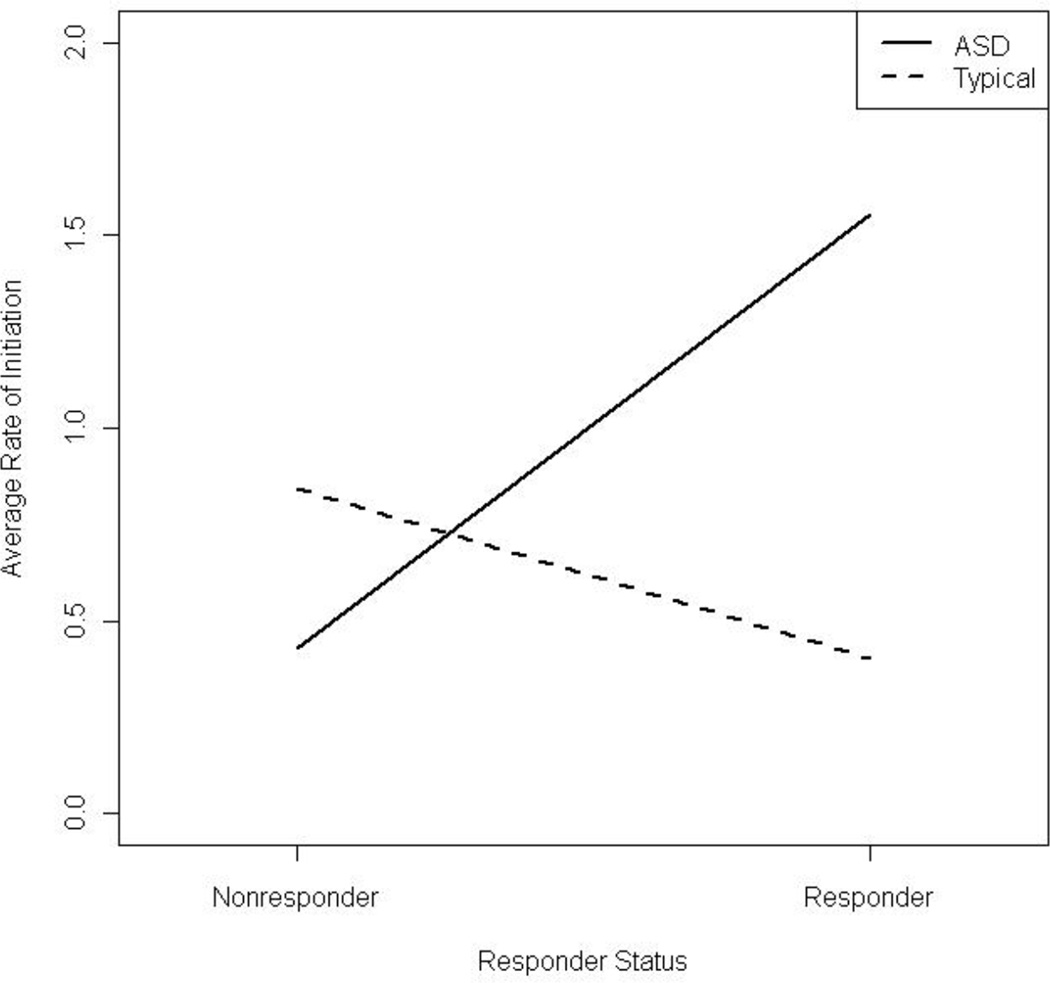
Among cortisol responders, the rate of verbal initiations for children with ASD was 3.86 time the rate for typical children (Figure 5), while for cortisol nonresponders the rate of verbal initiations for typical children was 1.96 times the rate for children with ASD. Additionally, within the ASD group, comparing initiation rates between responders and nonresponders revealed that responders initiated at a rate 3.63 times greater than nonresponders. There was no significant adjusted relationship between diagnosis and the rate of avoidance, equipment use individually or in a group, proximity, or rejections.
Figure 5.
Average rates of verbal initiation adjusted by diagnosis and responder status.
Solicited gross motor play (T2)
Among older children, the rate of gesturing was greater for children with ASD compared to typical peers, while among younger children was less (Figure 4). Within the ASD group, older children gestured at a rate 3.19 times the rate for younger children. After adjusting for IQ, the expected rate of approach for children with ASD was 4.14 times the expected rate of typical children. There appeared to be significant effect modification by age for the relationship between rate of avoidance and diagnosis such that older children with ASD avoided at a much higher rate than older typical children. After adjusting for cortisol responder status, children with ASD used equipment individually at a rate 1.82 time that of typical children. There was no significant adjusted relationship between diagnosis and the rate of group equipment use, proximity, or rejections.
Independent cooperative play (T3)
After adjusting for IQ, the avoidance rate among older children with ASD was greater than older typical children, while among younger ages the typical children avoid at a higher rate than children with ASD. There was no significant adjusted relationship between diagnosis and any of the other behavior variables.
Solicited cooperative play (T4)
The rejection rate between the groups was modified significantly by age, such that younger children with ASD rejected at a rate greater than younger typical children and the effect declined as the ages of the children increased. There was no significant adjusted relationship between diagnosis and any of the other behavior variables, including percentage of time spent interacting.
Discussion
The primary objectives of the current study were to evaluate the biological response profiles of children with and without ASD in response to a benign social interaction on a playground environment. Our aims were to extend previous findings evaluating biobehavioral profiles during solicited cooperative play (BLINDED) by 1) using a larger sample of children with ASD, 2) analyzing periods of free play, and 3) stratifying children into cortisol responders and non-responders.
During solicited cooperative play periods, the cortisol stress response profile revealed significant age modification of both diagnosis and time of measurement; thus supporting the previous report (BLINDED). These relationships are best described in Figure 2, where it appears that older children tend to experience a stress response (an increase in cortisol levels) due to the peer interaction paradigm. In addition, the levels continued to increase from sample S2 to S3 indicating an increasing response over the course of the 20 minute peer interaction. Older children with ASD had higher average cortisol levels at all sample points. On the other hand, younger children’s levels declined gradually over time and children with ASD had lower levels than typical children. Thus, once the playground interaction began, the older children with ASD mounted additional physiological arousal due to interaction. Meanwhile, the younger ASD children ostensibly found the playground interaction stress reducing as reflected by their diminishing cortisol levels.
The modification of diagnosis effect by age is apparent in the markedly different patterns and levels of cortisol across time between younger and older children with ASD, while the typical children’s average levels are relatively similar at all sample points. The peer interaction paradigm was designed to emulate a “real life” social interaction. It would not be considered an inherently stress-inducing paradigm. Rather it would generally be hypothesized to be a benign stressor and less provoking than an actual playground environment. The playground interaction involved only three children and included solicited interaction considered sources of sensory stimulation. The fact that the older portion of the ASD group exhibited increased stress under such conditions provides evidence that social stress would presumably be even more acute in a natural play environment such as during recess in a school setting.
Given that the younger children with ASD showed such a diminished stress response suggests that they may not have the awareness or history of negative social exchanges that can contribute to enhanced stress responsivity in social situations (Bellini 2006). Teaching coping techniques early in development might ward off the additional stress social situations create as the child grows older and gains greater insight into the social world and roles within it. For example, it may be beneficial to normalize the experience of increased arousal in the presence of peers by teaching the child to be more aware of their own psychological and emotional states. In the process, they can learn to reframe negative thinking about socializing, as well as become familiar with strategies on how to initiate and respond in ways that lead to positive interaction outcomes. Interventions, such as cognitive behavior therapy, may be valuable in addressing underlying thoughts processes and behavior that interfere with positive social exchanges (White et al.,2010 Wood et al., 2009).
In addition, it appears that the older children with ASD and the younger typical children arrived to the peer interaction with similar, but relatively high levels of cortisol. The initial higher cortisol levels could be attributed to anticipatory stress related to the upcoming “social experiment” since the children were informed as part of the study they would be engaging in play with others. On the other hand, the younger children with ASD and the older typical children arrived with expected afternoon cortisol levels, indicating that the younger children with ASD were presumably unaware of any social threat in the anticipated situation. Older typical children were either naturally neutral or better at coping with the novel, social situation.
Analysis of the playground behaviors during the peer interaction revealed, as anticipated, that the children with ASD spent significantly less time interacting than typical children during the time periods in which interaction was solicited by the confederate (T2 and T4) as previously shown (BLINDED). However, during independent play when children were left to their own devices, no significant differences between the groups were observed. This suggests that when allowed to make their own choices without bids from the confederate, children with ASD were choosing to engage in self-play.
Importantly, when age was included in the model intriguing patterns emerged based on developmental level and social behaviors. For example, younger children with ASD approached more during free play than younger typical children while the reverse held true for older children (Figure 3). In addition to approaching less, the older ASD children tended to more frequently avoid their peers. .Again, this supports the notion that as children with ASD age they become more reticent about initiating with peers, whereas younger children with less insight and fewer past negative experiences inhibiting their behavior (Knott et al. 2006) make more social overtures. While social approach may be reduced, the opposite pattern was revealed for gesturing, as older children with ASD demonstrated a much higher rate of gesture use, implying better developed social communication skills. Taken together these observations suggest that while young children with ASD engage in a higher rate of approach behavior, they also use fewer and seemingly less developed nonverbal social communication modalities .
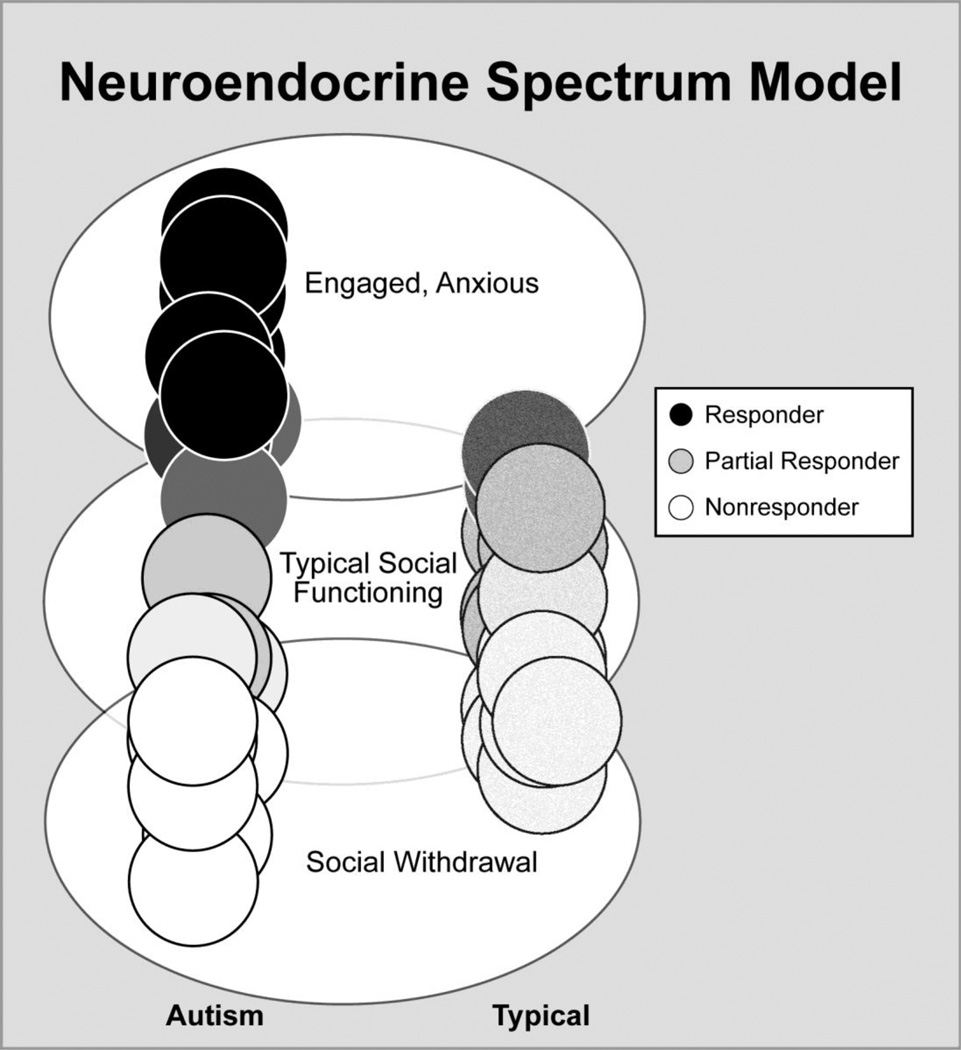
In regards to periods of unsolicited free play and cortisol responder status, children with ASD characterized as cortisol responders engaged in significantly more verbal initiations than typical peers. The opposite pattern emerged for typically developing children; it was the cortisol nonresponder group that showed more verbal initiations during free play. In other words, cortisol level appears to be driven by both factors of age and social engagement such that older children with ASD that show heightened cortisol make more verbal social overtures despite enhanced arousal. In contrast, the cortisol nonresponders in the typically developing group show more verbal social engagement. Taken a step further, the findings imply that typically developing children readily engage other children and find an absence of stress when they interact whereas children with ASD that choose to willfully initiate play with others do so despite experiencing significant physiological arousal. These findings build on our hypothesized neuroendocrine spectrum model (BLINDED), in which social engagement intersects with stress responsivity in children with ASD to reveal biobehavioral profiles. As shown in Figure 6, at one end of the spectrum some children with ASD readily engage with peers despite an increase in their level of stress whereas at the other end are children who interact less with peers and show diminished stress response. It is unclear if physiological arousal a) influences the initiation of engagement with peers, b) is a response to the engagement or c) if both causal and response mechanisms are at work. It is also apparent that developmental factors to include age and experience modulate the interplay of social behavior and stress responsivity in ASD.
Figure 6.
Neuroendocrine spectrum model. The model shows how social engagement intersects with stress responsivity in children with ASD to reveal biobehavioral profiles. At the top end of the spectrum some children with ASD readily engage with peers despite increase in their level of stress shown by higher cortisol level, whereas at the other end are children who interact less with CORTISOL RESPONSIVITY DURING PLAY 30 peers and show diminished cortisol release. In contrast to the notable variability in ASD children, typically developing children show a much more restricted range of social behavior and physiological response.
It is important to note that there are changes in the regulation and responsivity of the HPA axis in typical development characterized by increases in basal activity as well as stress responsiveness (Gunnar et al. 2009; Stroud et al. 2009). The higher cortisol levels in the children with and without ASD provide support for the growing literature showing associated changes in HPA activity with developmental maturation e.g., (Adam 2006); Walker et al. 2001). In our study we also show differences in the set point as well as stress responsivity at the prepuberty stage (Petersen et al. 1988). Therefore, the finding of profoundly increased cortisol levels in older children with ASD may be considered within a developmental framework in which stress responsivity is already expected to rise to some degree, but is even more pronounced than in their typically developing peers.
In summary, many of the same patterns observed through analysis of the physiological response were also found during analysis of the behavioral profiles during free play. Age was a prominent modifier of the diagnosis effect such that younger children with ASD approached at a much higher rate during the beginning of the peer interaction than younger typical children and showed lower levels of stress across all samples. Older children with ASD tended to avoid interactions during free play, but when they did interact they demonstrated greater use of coordinated gestures. The younger children also used fewer non-verbal social cues. Notably, the cortisol responders with ASD tended to be older and engaged in more verbal initiation during free play, meaning that they were more socially active but also more physiologically stressed.
Although the sample in the study is well characterized, the 8 to 12 year age range is rather narrow. Further, the sample is limited to male children with ASD characterized by average IQ, verbal language ability, and rather low rates of repetitive and stereotyped behaviors. Thus, the sample limits the generalizability of the findings across dimensions of functioning. Current efforts are underway to extend this protocol to female participants, a broader age range, a more diverse cognitive sample of ASD participants, and implemented on a larger school-based playground.
Conclusion
The current investigation revealed distinct patterns of stress responsivity, developmental changes and social engagement in ASD supporting the hypothesis that as children with ASD age they may develop enhanced physiological arousal from socially interacting with peers. For children who choose to initiate activity with novel peers they must override a learned and physiological response that otherwise seeks to avoid such social exchanges. Meanwhile, younger children with ASD show an enhanced willingness to approach others and seem to do so without apparent stress. These results suggest that if we intervene early and often with positive social exchanges while providing instruction in self-awareness, initiation, and coping strategies that children with ASD may benefit from a decreased stress response and be more willing to approach same age peers for play and social interaction.
Acknowledgements
This work was supported in part by National Institute of Health R01 MH085717 awarded to (Blinded). Portions of these data were completed as part of (Blinded) dissertation. We are grateful to the children and families who continue to support our research.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Baron-Cohen S, Golan O, Ashwin E. Can emotion recognition be taught to children with autism spectrum conditions? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2009;364(1535):3567–3574. doi: 10.1098/rstb.2009.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini S. The development of social anxiety in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2006;21(3):138–145. [Google Scholar]
- Boucher J, Lewis V, Collis G. Familiar face and voice matching and recognition in children with autism. J Child Psychol Psychiatry. 1998;39(2):171–181. [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1324–1332. doi: 10.1097/chi.0b013e31812f7d8d. [DOI] [PubMed] [Google Scholar]
- Church C, Alisanski S, Amanullah S. The social, behavioral, and academic experiences of children with Asperger syndrome. Focus on Autism and Other Developmental Disabilties. 2000;15(1):12–20. [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, Levine S. Variable cortisol circadian rhythms in children with autism and anticipatory stress. J Psychiatry Neurosci. 2008a;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, Levine S. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry and Neuroscience. 2008b;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress and sensory sensitivity in children with autism. Autism Research. 2009;2:32–39. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, Ryan N, Mendoza S. Elevated cortisol during play is associated with age and social engagement in children with autism. Mol Autism. 2010;1(1):13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. John Wiley & Sons; 2004. [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg KR. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics. 2007;119(1):182–191. doi: 10.1542/peds.2006-2697. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein SE, Whitney T. Asperger syndrome and the development of social competence. Focus on Autism and Other Developmental Disabilties. 2002;17(3):161–171. [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl Nurs Res. 2006;19(2):95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hennessey JW, Levine S. Stress, arousal, and the pituitary-adrenal system: a psychoendocrine hypothesis. Vol. 8. Progress in Psychobiology and Physiological Psychology; 1979. [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Honey E, Leekam S, Turner M, McConachie H. Repetitive behaviour and play in typically developing children and children with autism spectrum disorders. J Autism Dev Disord. 2007;37(6):1107–1115. doi: 10.1007/s10803-006-0253-4. [DOI] [PubMed] [Google Scholar]
- Humphrey N, Symes W. Peer interaction patterns among adolescents with autistic spectrum disorders (ASDs) in mainstream school settings. Autism. 2011;15(4):397–419. doi: 10.1177/1362361310387804. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, van der Gaag RJ, van Engeland H. Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology. 2003;28(3):582–590. doi: 10.1038/sj.npp.1300046. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Wiegant VM, Westenberg HG, Lahuis BE, van Engeland H. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord. 2006;36(7):891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- Knott F, Dunlop AW, Mackay T. Living with ASD: how do children and their parents assess their difficulties with social interaction and understanding? Autism. 2006;10(6):609–617. doi: 10.1177/1362361306068510. [DOI] [PubMed] [Google Scholar]
- Krasny L, Williams BJ, Provencal S, Ozonoff S. Social skills interventions for the autism spectrum: essential ingredients and a model curriculum. Child Adolesc Psychiatr Clin N Am. 2003;12(1):107–122. doi: 10.1016/s1056-4993(02)00051-2. [DOI] [PubMed] [Google Scholar]
- Lanni KE, Schupp CW, Simon D, Corbett BA. Verbal ability, social stress, and anxiety in children with Autistic Disorder. Autism. 2012;16(2):123–138. doi: 10.1177/1362361311425916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless JF. Negative binomial and mixed poisson regression. The Canadian Journal of Statistics. 1987;15(3):209–225. [Google Scholar]
- Lieberman R, Yoder P. Play and Communication in Children with Autism Spectrum Disorder: A Framework for Early Intervention. Journal of Early Intervention. 2013 [Google Scholar]
- Loftin RL, Odom SL, Lantz JF. Social interaction and repetitive motor behaviors. J Autism Dev Disord. 2008;38(6):1124–1135. doi: 10.1007/s10803-007-0499-5. [DOI] [PubMed] [Google Scholar]
- Lopata C, Volker MA, Putnam SK, Thomeer ML, Nida RE. Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. J Autism Dev Disord. 2008;38(10):1866–1877. doi: 10.1007/s10803-008-0575-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule-WPS. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lyons D, Mason WA, Mendoza SP. Beyond the ethogram: Transactional analysis of behavior in primate social interchanges. American Journal of Primatology. 1990;20:209. [Google Scholar]
- Lyons D, Mendoza S, Mason W. Sexual segregation in squirrel monkeys (Saimiri sciureus): A transactional analysis of adult social dynamics. Journal of Comparative Psychology. 1992;106(4):323–330. doi: 10.1037/0735-7036.106.4.323. [DOI] [PubMed] [Google Scholar]
- Marinovic-Curin J, Marinovic-Terzic I, Bujas-Petkovic Z, Zekan L, Skrabic V, Dogas Z, et al. Slower cortisol response during ACTH stimulation test in autistic children. Eur Child Adolesc Psychiatry. 2008;17(1):39–43. doi: 10.1007/s00787-007-0632-1. [DOI] [PubMed] [Google Scholar]
- Mason W, Long D, Mendoza S. Temperment and Mother-Infant Conflict in Macaques: A Transactional Analysis. In: Mendoza WMS, editor. Primate Social Conflict. Albany, NY: SUNY Press; 1993. pp. 205–227. [Google Scholar]
- Mendoza SP, Mason W. Behavioral and endocrine consequences of heterosexual pair formation in squirrel monkeys. Physiol Behav. 1989;46(4):597–603. doi: 10.1016/0031-9384(89)90338-7. [DOI] [PubMed] [Google Scholar]
- Noldus . The Observer XT. Vol. 10.5. Wageningen, The Netherlands: Noldus Information Technology; 2008. [Google Scholar]
- Pellegrini AD, Smith PK. Physical activity play: the nature and function of a neglected aspect of playing. Child Dev. 1998;69(3):577–598. [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–131. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. Linear and nonlinear mixed effects models. 2009;Vol. 3 [Google Scholar]
- R. R Foundation for Statistical Computing. 2009. (Vol. Version 2.10.1) [Google Scholar]
- Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. J Autism Dev Disord. 1992;22(3):433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Spratt EG, Nicholas JS, Brady KT, Carpenter LA, Hatcher CR, Meekins KA, et al. Enhanced cortisol response to stress in children in autism. J Autism Dev Disord. 2012;42(1):75–81. doi: 10.1007/s10803-011-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, et al. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, Hall LM, et al. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry. 1997;38(6):705–715. doi: 10.1111/j.1469-7610.1997.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13(3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Yuill N, Strieth S, Roake C, Aspden R, Todd B. Brief report: designing a playground for children with autistic spectrum disorders--effects on playful peer interactions. J Autism Dev Disord. 2007;37(6):1192–1196. doi: 10.1007/s10803-006-0241-8. [DOI] [PubMed] [Google Scholar]