Abstract
Background
It has been suggested that synovitis causes joint pain. On non-contrast-enhanced MRIs synovial thickening cannot be assessed and on these images synovitis has been inconsistently associated with pain.
Objective
To assess synovial thickening in relation to knee pain severity among subjects in the Multicenter Osteoarthritis Study (MOST) using contrast-enhanced (CE) MRI.
Methods
MOST is a cohort study of people who have, or are at high risk of, knee osteoarthritis (OA). An unselected subset of 535 participants who volunteered underwent CE 1.5 T MRI of one knee. Synovitis was scored in six compartments and a summary score was created. Knee pain severity was assessed using the maximum item score on the Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain scale. The association between synovitis and pain severity was examined using a logistic regression model adjusting for age, sex, body mass index (BMI), MRI bone marrow lesions and effusions in the whole sample and in a subgroup without radiographic OA.
Results
454 of the 535 subjects undergoing CE MRI had complete data on synovitis and WOMAC pain. Mean age was 59 years, mean BMI 30 and 48% were women. In knees with moderate pain, 80% had synovitis. For knee pain, synovitis conferred a 9.2-fold increased odds compared with those without synovitis. In knees without radiographic OA (n=329), there was also an association of synovitis with an increased prevalence of pain.
Conclusion
Synovitis has a strong relation with knee pain severity, an association detected more clearly with CE MRI than suggested by previous studies using non-CE MRI measures of synovitis.
INTRODUCTION
In the past few years, reports on the relation of knee pain with structural features of osteoarthritis (OA) have emerged, triggered by the availability of MRI, which for the first time has permitted pathology in certain features of the knee to be detected—for example, the relationship between bone marrow lesions and knee pain.1
One feature thought to be associated with the existence and severity of pain in patients with knee OA is synovitis. The synovium is optimally imaged using gadolinium-enhanced MRI. The contrast takes advantage of increased vascular perfusion and capillary permeability of the synovium to highlight the synovial tissue, distinguishing it from synovial fluid and other adjacent articular structures. Only a handful of studies have examined the relation of synovitis and pain in knees and, until now, all have used non-contrast-enhanced images, making it difficult to obtain an accurate estimate of the extent of synovitis present, particularly if there is joint effusion.2–4 In recent work by Loeuille et al, synovial membrane inflammation assessed by contrast-enhanced (CE) MRI correlated well with synovial membrane inflammation confirmed by biopsy while synovitis on non-CE MRI did not.5 We are unaware of any study that has evaluated the relationship of knee pain with synovitis assessed using CE scans. Because these scans accurately image the extent of synovitis throughout the knee, we hypothesise that such a study would find a strong relationship of synovitis with knee pain and the amount of pain would correlate with the degree of synovitis.
We tested this hypotheses by obtaining CE MRIs in Multicenter Osteoarthritis Study (MOST) study subjects as part of a larger-scale study evaluating the correlates of knee pain. In addition, because many pathological features of OA coexist in patients with knee pain and OA, making it difficult to identify a single structural source of pain, we performed a substudy focusing on patients who had no radiographic knee OA. In such knees, multiple pathological features do not usually coexist and therefore studies of such knees can provide a clearer signal of the potential sources of pain.
PATIENTS AND METHODS
Study population
MOST is the parent study for this investigation of knee pain and synovitis. It is a prospective epidemiological study of 3026 people aged 50–79 years. Its overall goals are to identify risk factors for incident or progressive knee OA in a sample of people either with OA or at high risk of developing disease. Those considered at high risk included people who were overweight or obese, had knee pain, aching or stiffness on most of the past 30 days, had a history of knee injury that made it difficult to walk for at least 1 week, or had previous knee surgery. High risk from obesity was defined based on people weighing more than the Framingham Heart Study median weight for their age- and gender-specific group.6 All subjects were recruited from two US communities, Birmingham, Alabama and Iowa City, Iowa, through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. Each centre also recruited ethnic minorities according to their representation in the recruitment population. Subjects were excluded if they screened positive for rheumatoid arthritis, had ankylosing spondylitis, psoriatic arthritis, chronic reactive arthritis, had renal problems resulting in a need for haemodialysis or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), bilateral knee replacement surgery, inability to walk without the help of another person or walker or were planning to move out of the area in the next 3 years. People who had no contraindications to MRI and who were willing obtained extremity-based MRIs (see below) repeatedly throughout the study. The protocol for MOST was approved by institutional review boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University Medical Center.
For this investigation on synovitis and pain, a subset of MOST subjects with the following inclusion criterion were investigated: volunteered to undergo a 1.5 T CE MRI of one knee at the 30-month follow-up clinic visit. Radiographs had already been obtained and read and we selected the knee with the lower Kellgren and Lawrence (KL) grade for CE MRI. If the grade was the same for both knees, the dominant leg was chosen. Subjects with a KL grade of 4 in both knees were ineligible.
For those subjects with kidney disease, diabetes or over the age of 65, serum creatinine was determined and the glomerular filtration rate calculated before gadolinium injections. Those subjects with renal insufficiency were excluded from the study.
MRI measurements
The MOST parent study MRIs were performed on a 1.0 extremity-based OrthOne magnet (Oni Inc, Wilmington, Massachusetts, USA) for which CE scans were not advisable.
CE MRIs were obtained with 1.5 T system (Siemens Symphony) with a circumferential extremity coil. Axial and sagittal T1-weighted CE sequences were acquired (TR=600 ms, TE=13 ms, 3.0 mm slice thickness, 0.3 mm interslice gap, FOV 16×16 cm, matrix 512×512, ETL 1). Intravenous gadolinium (Magnevist (gadopentetate dimeglumine; Bayer HealthCare Pharmaceuticals, Bayer Shering PharmaAG, Berlin Germany) or Omniscan (gadodiamide GE Healthcare, New Jersey, USA)) was administered at a dose of 0.2 ml (0.1 mmol) kg body weight. Two minutes after completing the injection of the gadolinium, sagittal sequences were obtained immediately followed by the axial sequences.
The non-CE MRIs acquired in the parent study were obtained with a 1.0 T dedicated MR system (OrthOne) with a circumferential extremity coil using fat-suppressed, fast-spin, echo proton density-weighted sequence in two planes, sagittal (TR=4800 TE=35 slice thickness 3 mm, 0 mm interslice gap, FOV 14 cm and 288×192 matrix, NEX 2); and axial (TR 4700 TE 13.2 slice thickness 3 mm, 0 mm interslice gap, FOV 14 cm and 288×192 matrix, NEX 2) and a short time inversion recovery sequence in the coronal plane (TR=7820 TE=14, TI=100, slice thickness 3 mm, 0 mm interslice gap, FOV 14 cm and 256×256 matrix, NEX 2).
MRI reading
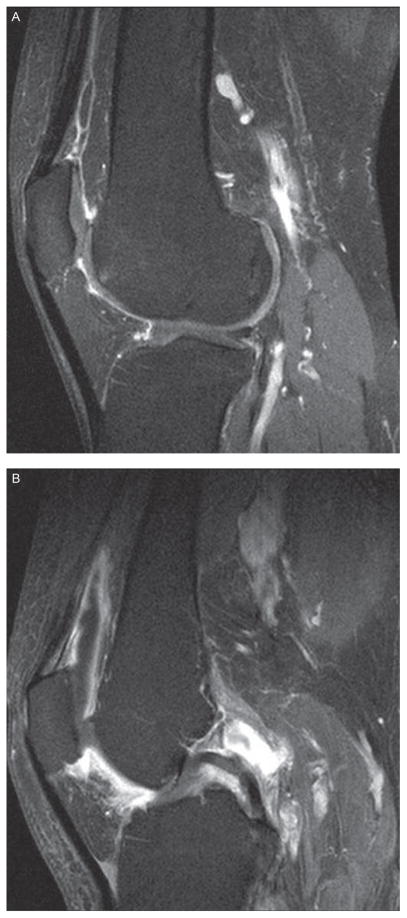
For the MRI readings we equated synovial thickening with synovitis. Synovitis on CE MRI using axial and sagittal T1-weighted sequences was scored separately at six sites (medial and lateral parapatellar recesses, suprapatellar pouch, infrapatellar fat pad and medial and lateral posterior condyles). The degree of synovitis was scored semiquantitatively (0–3) in the medial and lateral parapatellar recesses, suprapatellar pouch and the infrapatellar fat pad. Figures 1 and 2 illustrate synovitis scored semiquantitatively in saggital and axial sequences, respectively. For the posterior medial and lateral femoral condyles, we scored synovitis as either present (1) or absent (0). This semiquantitative scoring method has been validated in CE MRI.7 One reader (KB), trained by a musculoskeletal radiologist (AGr) and blinded to pain, scored the synovitis on CE magnetic resonance images.
Figure 1.
Sagittal sequence: (A) Score of 1 in suprapatellar pouch. Score of 1 in infrapatellar fat pad. (B) Score of 2 in suprapatellar pouch. Score of 3 in infrapatellar fat pad.
Figure 2.
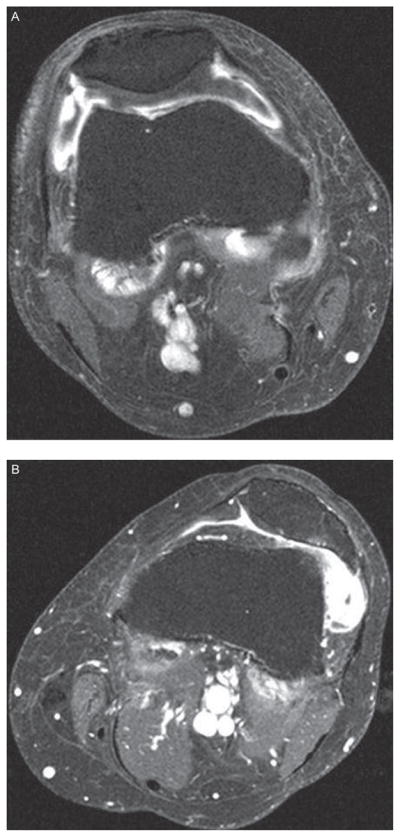
Axial sequence: (A) Score of 2 in lateral patellar recess. Score of 1 in medial patellar recess. (B) Score of 3 in lateral patellar recess. Score of 1 in medial patellar recess.
To obtain information about other MRI features related to pain, we used the non-CE MRI. Two musculoskeletal radiologists (AGu and FR), blinded to pain data, read non-CE MRIs for bone marrow lesions and effusions according to the Whole-Organ MRI Score (WORMS) method.8 Bone marrow lesions were scored from 0 to 3 based on size or volume of the lesion in each of five subregions in the medial and lateral compartments and four in the patellofemoral compartment as reported before.1 Effusion volume was scored 0–3 based on volume.
Assessment of knee pain
The 3.0 Likert version of the Western Ontario and McMaster Osteoarthritis Index (WOMAC) was administered at the 30-month clinic visit. For each of five pain questions subjects rated their pain from 0 (no pain) to 4 (extreme pain). For each subject knee, we used their worst pain score on any of the five WOMAC questions as their pain severity. For example, those rating their pain in all five questions as mild or one were categorised as mild. We also examined pain using the summary score of WOMAC pain scale for each subject knee.
Also at the 30-month clinic visit participants were asked a knee-specific question about frequent knee pain, as follows: “During the past 30 days, have you had pain, aching or stiffness in your knee on most days?” Positive responses to the frequent knee pain question for the knee that had had CE MRI were considered to indicate presence of frequent knee pain.
Only participants who completed the CE MRI within 30 days of the 30-month clinic visit were included in the analysis.
Analysis
Our primary analysis focused on the relation of pain with synovitis assessed using CE MRI. To obtain a summary score for the six regions in which synovitis was scored on CE MRI, we created the following categories for the six sites read: (1) normal or questionable, <4 sites scored as 1 and all other sites scored as 0; (2) some, ≥4 sites scored as 1 and/or ≤1 site scored as 2; (3) a lot, ≥2 sites scored as 2 and no score of 3; and (4) extensive, ≥1 site scored as 3. Owing to small numbers of MRIs classified as ‘a lot’ and ‘extensive’ synovitis, these categories were collapsed in the analysis. Interobserver (AGr, KB) and intra-observer (KB) agreements (weighted κ) for the summary score were 0.80 (95% CI 0.68 to 0.94) and 0.58 (95% CI 0.40 to 0.76), respectively.
We evaluated the association between synovitis and WOMAC pain using logistic regression. We controlled for the effects of age, sex, body mass index (BMI), MRI bone marrow lesions, MRI effusions and radiographic OA status on pain and synovitis. Because of the high co-occurrence of MRI features related to pain such as bone marrow lesions and effusions in OA knees,9 we also carried out subanalyses limited to knees with and without OA, and owing to the potential confounding influence of drugs used for pain, we did separate analyses excluding people taking non-steroidal anti-inflammatory drugs (NSAIDs) or drugs for pain. All analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina, USA).
RESULTS
A total of 2713 subjects participated in the 30-month follow-up visit of the MOST study, of which 535 had CE MRI in one knee. We excluded 15 knees with some synovitis variables missing usually because the superior extent of synovium was not visualised and 66 subjects in whom the interval between the WOMAC questionnaire and enhanced MRI was longer than 30 days. Therefore, we assessed synovitis and pain in one knee of 454 subjects. The subjects in the analysis who received CE MRI were more likely to be male, have a lower BMI and KL grade than the MOST subjects who did not receive contrast MRI at the 30-month follow-up visit. For the 454 subjects included in the analysis, the mean age was 59 years, BMI 29.7 and WOMAC pain score 2.6 (0–20) (see table 1). The mean, median and SD for number of days between WOMAC pain assessment and CE MRI was 2 days before CE MRI, same day as CE MRI and 5.5 days, respectively. Of the knees analysed 77.6% had a KL grade <2. The presence of synovitis was significantly correlated with KL radiological grade (Spearman correlation = 0.4723 (p<0.0001)).
Table 1.
Baseline characteristics
| Characteristics | All (N=454) | ROA (N=125) | No ROA (N=329) |
|---|---|---|---|
| Age (mean±SD (years)) | 59.2±7.2 | 61.0±8.1 | 58.6±6.7 |
| Women (%) | 48 | 53 | 45 |
| BMI (mean±SD (kg/m2)) | 29.7±5.0 | 31.2±5.1 | 29.1±4.9 |
| WOMAC pain (mean±SD) | 2.6±3.1 | 3.5±3.2 | 1.7±2.6 |
| Synovitis | |||
| None/questionable (%) | 33.3 | 4.8 | 44.4 |
| Some (%) | 51.4 | 56.0 | 49.5 |
| A lot (%) | 12.6 | 32.0 | 5.2 |
| Extensive (%) | 2.7 | 7.2 | 0.9 |
| Kellgren and Lawrence grade | |||
| 0 (%) | 60.1 | 11.2 | 78.7 |
| 1 (%) | 17.6 | 8.0 | 21.3 |
| 2 (%) | 10.4 | 37.6 | 0 |
| 3 (%) | 10.1 | 36.8 | 0 |
| 4 (%) | 1.8 | 6.4 | 0 |
| Analgesic/NSAID use (%) | 32.2 | 32.8 | 31.9 |
BMI, body mass index; NSAID, non-steroidal anti-inflammatory drug; ROA, radiographic osteoarthritis; WOMAC, Western Ontario and McMaster Osteoarthritis Index.
The odds of mild versus no WOMAC pain was 1.6 for some synovitis and 4.8 for either a lot/extensive synovitis (p=0.0017) (table 2). When we examined more severe pain, the relation of synovitis with pain was stronger (table 2). The association of pain with synovitis was similar when analysed by the summed WOMAC pain score. Whereas roughly 40% of our sample had no WOMAC knee pain, only 9/69 (13%) knees with either a lot or extensive synovitis had no WOMAC knee pain. Frequent knee pain was also associated with synovitis (OR of 1.5 (95% CI 0.9 to 2.7) and 2.8 (95% CI 1.3 to 6.2)) for some and a lot/extensive synovitis respectively (p for trend = 0.012).
Table 2.
Maximal pain on WOMAC item and synovitis on CE MRI
| All Subjects
|
Synovitis
|
p For trend | ||
|---|---|---|---|---|
| Worst pain on any WOMAC pain question | None/questionable (n=152) | Some (n=233) | A lot/extensive (n=69) | |
| None (n=188) | 84 | 95 | 9 | |
| Mild (n=145) | 40 | 77 | 28 | |
| Moderate/severe/extreme (n=121) | 28 | 61 | 32 | |
| Adj OR for mild pain vs no pain (95% CI)* | 1.0 | 1.6 (0.9 to 2.6) | 4.8 (1.8 to 12.6) | 0.0017 |
| Adj OR for moderate/severe/extreme pain vs no pain (95% CI)* | 1.0 | 2.0 (1.1 to 3.6) | 9.2 (3.2 to 26.3) | <0.0001 |
Adjusted for age, sex, BMI and MRI bone marrow lesions and effusions.
BMI, body mass index; CE, contrast-enhanced; WOMAC, Western Ontario and McMaster Osteoarthritis Index.
In a subset analysis of 329 subjects with KL ≤2 and no radiographic patellofemoral OA (table 3), the association of synovitis with WOMAC pain persisted with increased odds of pain for some and a lot/extensive synovitis (OR=1.4 and 5.1, respectively) (p for trend = 0.04). In subjects without radiographic OA, those with pain had a much greater prevalence of a lot/extensive synovitis than those without pain.
Table 3.
WOMAC knee pain (yes/no) and synovitis in CE MRI in a subset of subjects
| Synovitis
|
p For trend | |||
|---|---|---|---|---|
| None/questionable (n=146) | Some (n=163) | A lot/extensive (n=20) | ||
| No radiographic OA (n=329) | ||||
| None (n (%)) | 82 (56) | 74 (45) | 5 (25) | |
| Any knee pain (n (%)) | 64 (44) | 89 (55) | 15 (75) | |
| Adj OR for pain (95% CI)*, excluding effusion missing | 1.0 | 1.4 (0.8 to 2.3) | 5.1 (1.2 to 22.0) | 0.04 |
|
| ||||
| None/questionable (n=6) | Some (n=70) | A lot/extensive (n=49) | ||
|
| ||||
| Radiographic OA (n=125) | ||||
| None (n (%)) | 2 (33) | 21 (30) | 4 (8) | |
| Any knee pain (n (%)) | 4 (67) | 49 (70) | 45 (91) | |
| Adj OR for pain (95% CI)*, excluding effusion missing | 1.0 | 2.4 (0.2 to 22.7) | 6.6 (0.6 to 80.2) | 0.02 |
Adjusted for age, sex, BMI and MRI bone marrow lesions and effusions.
BMI, body mass index; CE, contrast-enhanced; OA, osteoarthritis; WOMAC, Western Ontario and McMaster Osteoarthritis Index.
A similar subset analysis of knees with radiographic OA also showed a relation of synovitis with pain (table 3). Additional analyses excluding people taking drugs or NSAIDs for pain showed similar findings with a strong and significant relation of synovitis with knee pain.
DISCUSSION
Our study demonstrates that synovitis is strongly related to pain severity, which is shown especially when analysed with CE MRI. This association with pain persisted after controlling for other MRI features that have been linked with pain, bone marrow lesions and effusions. It also persisted whether we analysed the summed WOMAC pain score or used a single question about the presence or absence of frequent knee pain. A subset analysis on those without radiographic OA also showed a significant relationship of synovitis with any pain on WOMAC.
Whereas others have studied the response of synovitis to intra-articular treatment using CE MRI,10 this is the first report of the relationship between synovitis and knee pain using CE MRI. Hill et al examined the relationship of synovitis on non-CE MRI with knee pain and attempted to distinguish between effusion and synovitis by oversampling knees with no or small effusions. Of the 150 knees selected, those with knee pain had a higher prevalence of synovial thickening than those without knee pain (73% vs 21%, respectively).2 However, in this same study examining all knees, the authors found no correlation between baseline knee pain and synovitis score, but did find a significant but modest correlation (r=0.21) of change in pain with change in synovitis.3 Non-CE MRI synovitis scores were validated against CE MRI synovitis scores in 20 of 270 subjects and of the 20, 13 had the same scores on both images. The most common error was for the non-CE image to underestimate the amount of synovitis (6 of 20). One other study combined a score for synovitis and effusion on non-CE images and found a relation with pain, but it is unclear whether that relation is due to effusion, which has been linked with pain.1,2,4
Differences across the populations studied may also explain the discrepancy between our finding of a strong association of synovitis with knee pain and the results of others who found a more modest association or none at all. In the studies of Hill et al only 17% of subjects were without knee pain, whereas in our study 41% reported no knee pain on the WOMAC, allowing for more variability in the knee pain outcome.2,3 Seventy-seven per cent of our participants had a KL score of <2 compared with only a few of the patients studied by Hill et al. Because mild or absent OA was common in our sample and multiple MRI lesions were therefore uncommon, we could examine synovitis separately from other structural changes occurring with knee OA.
There is evidence from other studies suggesting synovitis or inflammation in the knee as a source of pain. Intra-articular steroids decrease both inflammation in the synovium and lessen pain.10 NSAIDs and paracetamol may decrease pain in conjunction with a decrease in synovial tissue volume.11
Although we found a strong significant relationship of pain with synovitis even after controlling for other features of OA that cause pain, we cannot completely rule out their contribution to pain owing to the co-occurrence of features in the disease. We tried to overcome this by examining knees without OA, but a large proportion of them still exhibited effusions and, to a lesser extent, bone marrow lesions. We had too few knees (<100) to look at pain versus no pain in knees with both no radiographic OA and no bone marrow lesions or effusions.
A large number of our subjects without radiographic OA and knee pain had synovitis. The MOST study excluded subjects with rheumatoid and other forms of inflammatory arthritis. In examining those with a WORMS MRI reading in this subset without radiographic OA but with synovitis, we found that 140/146 of these subjects had some evidence of cartilage loss (WORMS scores at least 2 in at least one cartilage plate), suggesting they had mild OA not yet radiographically evident.
One limitation of our study is the lack of reliability data on CE MRI. We know of no data on the reproducibility of synovitis on repeat scans. In addition, although this study focused on the relationship between pain and a structural change in the knee (synovitis), the dissonance between the structural disease and the clinical symptoms must be mentioned and the importance of psychosocial variables in the reporting of knee pain.12 Finally, the results are only generalisable to populations with similar characteristics to those of the 454 participants in this study.
In summary, in the first study of CE MRIs to image synovitis, we found that synovitis was strongly related to the presence and severity of knee pain. We suggest that optimal characterisation of synovitis in OA using MRI will require contrast enhancement.
Acknowledgments
Funding NIH grants for MOST from NIA: DTF (U01 AG18820), Torner (U01 AG18832), Lewis (U01 AG18947), MN (U01 AG19069). NIH grants AR053161 and K23AR053855.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the institutional review board at Boston Medical School.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 2.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–7. [PubMed] [Google Scholar]
- 3.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres L, Dunlop DD, Peterfy C, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthr Cartil. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Loeuille D, Rat AC, Goebel JC, et al. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2009;17:1186–92. doi: 10.1016/j.joca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–97. vii. doi: 10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes LA, Grainger AJ, Keenan AM, et al. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology (Oxford) 2005;44:1569–73. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 8.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Molina G, Neogi T, Hunter DJ, et al. The association of bone attrition with knee pain and other MRI freatures of osteoarthritis. Ann Rheum Dis. 2008;67:43–7. doi: 10.1136/ard.2007.070565. [DOI] [PubMed] [Google Scholar]
- 10.Ostergaard M, Stoltenberg M, Gideon P, et al. Changes in synovial membrane and joint effusion volumes after intraarticular methylprednisolone. Quantitative assessment of inflammatory and destructive changes in arthritis by MRI. J Rheumatol. 1996;23:1151–61. [PubMed] [Google Scholar]
- 11.Brandt KD, Mazzuca SA, Buckwalter KA. Acetaminophen, like conventional NSAIDs, may reduce synovitis in osteoarthritic knees. Rheumatology (Oxford) 2006;45:1389–94. doi: 10.1093/rheumatology/kel100. [DOI] [PubMed] [Google Scholar]
- 12.Hadler NM. Knee pain is the malady – not osteoarthritis. Ann Intern Med. 1992;116:598–9. doi: 10.7326/0003-4819-116-7-598. [DOI] [PubMed] [Google Scholar]