Abstract
Background
Evidence suggests that periodontitis is associated with prevalent and incident type 2 diabetes mellitus (T2DM), raising the question of whether periodontitis treatment may improve glycemic control in patients with T2DM. Meta-analyses of mostly small clinical trials suggest that periodontitis treatment results in a modest reduction in glycosylated hemoglobin (Hb) A1c.
Purpose
The purpose of the Diabetes and Periodontal Therapy Trial (DPTT) was to determine if periodontal treatment reduces HbA1c in patients with T2DM and periodontitis.
Methods
DPTT was a phase-III, single-masked, multi-center, randomized trial with a planned enrollment of 600 participants. Participants were randomly assigned to receive periodontal treatment immediately (Treatment Group) or after 6 months (Control Group). HbA1c values and clinical periodontal measures were determined at baseline and 3 and 6 months following randomization. Medication usage and dosing were assessed at each visit. Periodontal treatment consisted of scaling and root planing for a minimum of two 90-minute sessions, plus the use of an antibacterial mouth rinse for at least 32 days afterwards. The primary outcome was change in HbA1c from baseline to 6 months and the trial was powered to detect a between-group difference of 0.6%. Secondary outcomes included changes in periodontal clinical measures, fasting plasma glucose, the Homeostasis Model Assessment (HOMA2) and the need for rescue diabetes or periodontal therapy.
Conclusion
Dental and medical researchers collaborated to recruit, treat and monitor participants with two chronic diseases to determine if treatment of one condition affects the status of the other.
Keywords: Diabetes, Diabetes Mellitus, Type 2, Periodontal Disease, Periodontitis, Glycosylated Hemoglobin, HbA1c
1. Introduction
Type 2 diabetes mellitus (T2DM) is a growing public health concern worldwide. Since 2008, diabetes has ranked as the seventh leading cause of death in the United States[1], with estimated annual direct costs of $245 billion [2]. Today, approximately 20.4 million U.S. adults have diabetes, with a third of all cases undiagnosed [3].
Diabetes mellitus is characterized by frequent episodes of hyperglycemia [4], which initiates chemical and molecular pathways associated with diabetes complications [5]. Accordingly, diabetes-related health care expenditures increase with decreasing glycemic control [6]. It is estimated that for each successive 1% increase in HbA1c above 6%, medical care costs increase by, respectively, 4%, 10%, 20% and 30% [7]. Thus, establishing and maintaining adequate glycemic control is an important means of reducing the morbidity, mortality and medical care costs associated with diabetes.
Periodontitis, a common bacteria-induced, oral inflammatory condition that destroys the supporting structures of the teeth [8], is associated with diabetes. Individuals with diabetes are about two and a half times more likely to have periodontitis than medically healthy controls [9]. Periodontitis also is more severe in those with than without diabetes [10, 11] and the disease tends to be most severe in patients with the poorest glycemic control [12]. Because of its consistent and severity-dependent association with diabetes, some have argued that periodontitis should be considered more formally as a diabetes complication [13]. Infections are known to adversely affect glycemic control [14]. Emerging evidence suggests that periodontal disease too may affect glycemia and risk for incident T2DM [15]. The mechanisms by which periodontitis may affect diabetes risk are not well established. A common hypothesis poses that periodontal inflammation and pathogenic bacteria and their byproducts trigger the production of cytokines, acute phase proteins and oxidative stress molecules that over time impair insulin sensitivity or action [16].
Because of the proposed bi-directional relationship between T2DM and periodontitis, researchers have studied whether periodontal treatment improves metabolic control in patients with T2DM. At the time the Diabetes and Periodontal Therapy Trial (DPTT) was being planned (early 2007), all published trials of T2DM were small, some lacked controls, and none was multi-centered. A meta-analysis of five published trials reported a decrease in HbA1c of 0.66% following periodontal therapy [17]. Only one trial, which was published after the meta-analysis, enrolled more than 100 participants, and that trial showed no significant effect of periodontal treatment on HbA1c [18]. Then and now, the research community has recognized the need for data from large, high-quality, multi-centered RCTs as the basis for clinical practice recommendations [17].
Given sufficient biological rationale, initial evidence from observational studies and small RCTs, and a compelling public health need to justify a Phase III RCT to evaluate the effects of periodontal treatment on glycemic control in patients with T2DM and periodontitis, the Diabetes and Periodontal Therapy Trial (DPTT) was developed.
2. Research Design and Methods
2.1 Aim and Design
DPTT’s aim was to determine the effect of non-surgical periodontal therapy on HbA1c in individuals with T2DM and chronic periodontitis when compared to no therapy. We hypothesized that participants receiving therapy would experience a 0.6% greater reduction in HbA1c when compared to untreated controls.
The DPTT was a single-masked, multicenter, randomized, controlled, Phase III clinical trial. Participants with moderately-controlled diabetes and moderate to advanced periodontitis were randomly assigned to receive immediate or delayed periodontal treatment. Participants were evaluated at baseline and at 3 and 6 months following randomization for multiple measures of diabetes control and periodontitis.
The trial was designed as a single-masked trial, with only the periodontal examiner masked to treatment assignment. Double masking would have required us to provide some type of “sham” periodontal therapy to control participants, which, to the best of our knowledge, had not been done in any previous trial in periodontology. An endpoint of treatment is the complete removal of hard and soft deposits from the tooth and root surfaces. Thus it is not possible to mask therapists. Periodontal therapy also frequently results in gingival (gum) recession and tooth sensitivity, especially to hot and cold temperatures. Treatment also removes the discolored calcified deposits that form at and just beneath the gum line. These signs and symptoms, which can be readily noticed by patients, would not be expected following some type of “sham” treatment. Thus, it is unlikely that the provision of a sham treatment would adequately mask control participants either.
The trial was led by a study chair (SE), who headed the trial’s Executive and Steering Committees. An independent Coordinating Center (CC) trained and certified site study personnel, developed the Manual of Operations and data collection forms, monitored trial activities, and processed and will analyze trial data. A central Core Laboratory performed the blood and biochemical tests and transmitted the results directly to the CC. A Data and Safety Monitoring Board reviewed study conduct and participant safety.
Clinical site selection was based on: 1) the clinical research experiences and collaborative history of site investigators; 2) access to a large numbers of patients with T2DM; 3) some geographic diversity within the United States; and 4) the presence of local facilities and personnel to conduct detailed periodontal examinations and collect, process and store blood samples. The number of clinical sites was based on the projected sample size, the timeline for recruitment, and the sites’ expected cumulative enrollment rate. Four clinical sites were proposed as part of the initial grant application; three were funded by the sponsor. Clinical sites were established first in Alabama (University of Alabama at Birmingham), Minnesota (University of Minnesota and Hennepin County Medical Center, both in Minneapolis) and Texas (University of Texas Health Science Center at San Antonio). Since recruitment was more challenging than expected and was lagging behind projections, two additional clinical sites (at Stony Brook University in New York and the University of Texas Health Science Center at Houston) were added later. These additional sites were selected based on similar criteria used for the initial sites as well as their potential to become certified and begin enrolling participants relatively quickly.
2.2 Study Outcomes
The trial’s primary outcome was change in HbA1c from baseline to the 6-month post-randomization visit. We selected this outcome because it is widely used in trials to monitor diabetes status [19], is associated with risk for diabetes-related complications [20], and was the primary outcome used in many previous trials in periodontology [21].
Secondary outcomes included:
HBA1c change from baseline to the 3-month visit
-
Change in clinical measures of chronic periodontitis (gingival index, bleeding on probing, probing depth, clinical attachment loss) at 3 and 6 months
Probing depth, clinical attachment loss and bleeding on probing are standard clinical measurements used to assess individual’s periodontal condition. The gingival index estimates gingivitis severity.
-
Change in fasting glucose, the Homeostatsis Model Assessment (HOMA2), and diabetes medications from baseline
The Homeostasis Model Assessment 2 (HOMA2) estimates steady-state beta cell function and insulin sensitivity from insulin and glucose measurements. HOMA2 values correlate well with results from euglycemic-hyperinsulinemic clamp studies [22].
Need for diabetes and periodontal rescue therapy
2.3 Eligibility
The trial’s inclusion and exclusion criteria are listed in the Text Box. These criteria were selected to maximize the chance that a participant would complete the 6-month trial and minimize the effects of co-morbidities, such as dialysis for kidney failure and immunosuppressive therapy, on a participant’s periodontal treatment response or medical management during the trial. We targeted individuals whose glycemia was not well controlled despite being under the regular care of a physician for T2DM. To minimize the number of diabetes medication changes during the trial, we set the upper limit for screening HbA1c values at <9% and excluded individuals with physician-directed medication changes within 3 months prior to randomization. The lower limit for HbA1c (≥7.0%) was chosen to increase the probability that a participant’s HbA1c could be reduced with periodontal treatment by 0.6%. Participants taking non-steroidal anti-inflammatory drugs for more than 7 consecutive days within the previous 2 months were excluded because this class of drugs may lower HbA1c [23].
We required participants to have moderate to advanced periodontitis at two or more tooth sites in each of two or more quadrants in the mouth (See Text Box). The deepened (>= 5mm) probing depth and clinical attachment loss measures had to be present at the same location (site) on a tooth. The qualifying sites in a quadrant, however, could be located on the same tooth. Notably, periodontal disease eligibility criteria in previous clinical trials have varied considerably, which may explain the heterogeneity in these trial results [24]. DPTT’s periodontal eligibility criteria were similar to those of at least two previous trials [25, 26] and were generally based on the joint Center for Disease Control and Prevention (CDC) and American Academy of Periodontology (AAP)’ s definition of “moderate” periodontitis [27]. Compared to the CDC/AAP definition, we used a higher threshold for clinical attachment loss (>= 5mm versus >= 4mm, indicating more severe disease), required that the clinical attachment loss and probing findings be on the same tooth site, and required there to be affected teeth in at least two (versus one) dental quadrants.
2.4 Recruitment
Because eligibility was based on features of two diseases, recruitment required broad and novel efforts. Participants were recruited from medical and dental clinics, through referrals from community medical practices, using posted flyers, brochures and educational materials, through radio and print advertisements, and through investigator appearances on local television or radio stations. We also established relationships with off-site dental and diabetes clinics, community groups and places of worship that serve high-risk patient populations, including African-Americans, Hispanics and Native Americans, and senior communities. Some affiliated medical clinics identified patients with recent HbA1c values near the study range and mailed Institutional Review Board (IRB)-approved recruitment materials to potentially eligible patients.
2.5 Prescreening and Screening
Dentists often are unaware of the details of their patient’s diabetes status and management [28], and physicians typically do not assess their patients’ periodontal condition. Thus, we prescreened participants referred from medical clinics for dental eligibility, and vice versa. In addition, patients with untreated periodontal disease frequently have untreated caries (decay or cavities)[29] or abscessed teeth. Because we sought to isolate the effects of periodontal treatment alone on HbA1c, we also screened for and excluded individuals with non-periodontal essential dental care needs (e.g., untreated gross caries or abscessed teeth).
Recruiters or Clinic Coordinators initially assessed an individual’s eligibility via phone or in person using an IRB-approved questionnaire. Individuals were asked about their recent medical and dental history, use of prescription and over-the-counter medications, and number of remaining natural teeth. Potentially eligible and interested individuals were scheduled for an in-person screening visit.
Participants provided written consent at the start of the screening visit. IRB-approved Spanish translations of the study forms were available for use. Certified interpreters also were available for non-English speakers at the time of consent and throughout the trial. Participants completed a medical/dental history questionnaire, received a dental and abbreviated periodontal examination, and provided a blood sample for HbA1c testing. Eligibility was based on the screening HbA1c value. (A participant’s HbA1c measurement at baseline (see below) was used as the baseline study value, but not to reconfirm eligibility.)
Dental radiographs were obtained to verify alveolar bone loss and detect non-periodontal oral infections (e.g., dental caries and abscessed teeth). Individuals who appeared to meet the eligibility criteria at screening were scheduled for a baseline visit. Otherwise eligible participants who had non-periodontal essential dental care needs were referred for treatment and offered to be rescreened once these needs were addressed.
2.6 Data collection
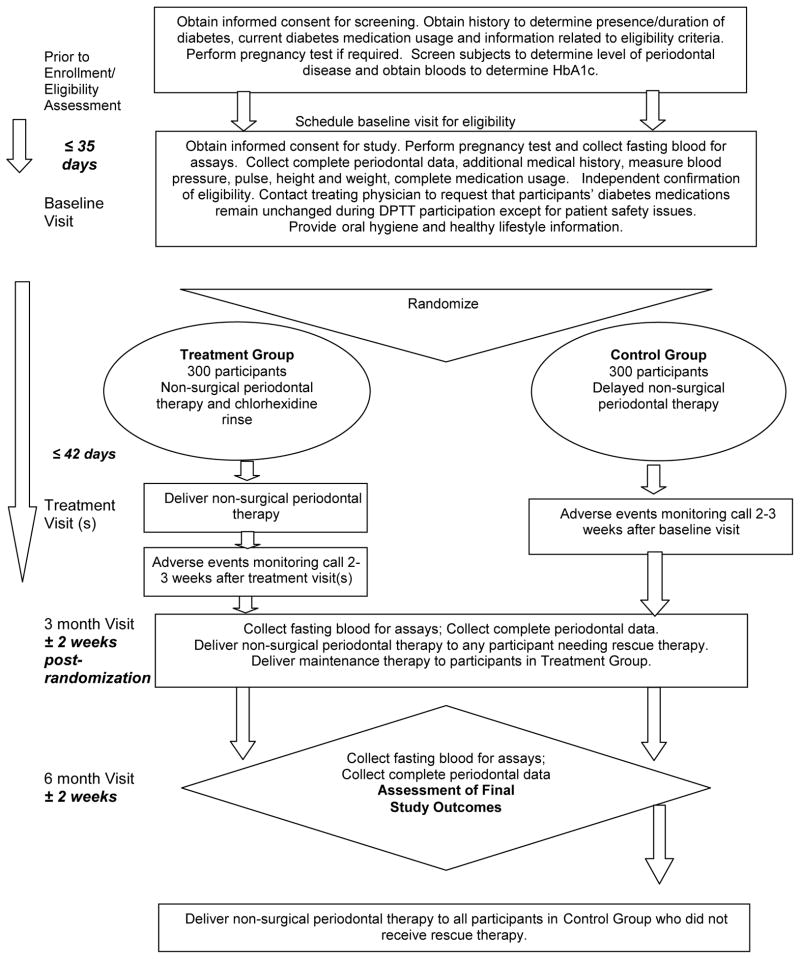
Trained and certified study personnel collected data and blood samples using standard procedures to assess a participant’s baseline periodontal and diabetes statuses and to track changes in each condition during the trial. The Table summarizes the data and blood collection by study visit. A participant’s flow through the trial is depicted in the Figure.
TABLE.
SCHEDULE OF STUDY ACTIVITIES BY VISIT
| Study Visits/Trial Phases | ||||||||
|---|---|---|---|---|---|---|---|---|
| Screening | Baseline Exam | Treatment & Follow-up Contacts | Final Visit | Post- final visit | ||||
| Activity | Recruitment/Prescreening | Screening −35 days to Day 0 |
Baseline/Randomization Day 0 |
Treatment visits 2–4 visits 0–42 days |
Follow-up Phone Call 2–3 weeks Post Treatment/Baseline |
3 month follow-up 3 mo± 2 wk |
6 month follow-up 6 mo± 2 wk |
Delayed Periodontal Therapy (Control Group) 0–28 days post 6 month visit |
| Consent | ||||||||
| Verbal Informed Consent | X | |||||||
| Written Informed Consent | X | X | ||||||
| Data Collection | ||||||||
| Recruitment Interview | X | |||||||
| Collect and enter contact information at recruitment and screening | X | X | ||||||
| Screening visit | X | |||||||
| Collect health care provider information | X | |||||||
| Pregnancy test | X | X | X | X | X | |||
| Oral/Periodontal Examination | X1 | X | X | X | ||||
| HbA1c assessment | X | X | X | X | ||||
| Essential Dental Care referral | X | X | ||||||
| Collect demographic/medical data and study measurements | X | X | X | |||||
| Document current medication use | X | X | X | |||||
| Draw fasting blood sample, isolate and store serum and plasma | X | X | X | |||||
| Confirm of eligibility/randomize | X | |||||||
| Periodontal therapy | X | X | ||||||
| Supportive maintenance therapy | Treatment Group | Treatment Group | ||||||
| Periodontal Rescue Therapy | as needed | referral | ||||||
| Diabetes Rescue Therapy | as needed | |||||||
| Post Treatment/Baseline Telephone Follow-up | X | |||||||
Partial exam at screening
FIGURE 1.
FIGURE SCHEMATIC OF DPTT STUDY DESIGN
Trained personnel interviewed participants at each study visit (Baseline, 3- and 6-months). Participants self-reported race and ethnicity, smoking/tobacco and alcohol use, daily exercise, dietary and dental history, education, employment, health insurance coverage, oral hygiene habits, and self-perceived overall and oral health, medical history and medication use. To enhance accuracy of medication reporting, participants were asked to bring their medications to each study visit. Study personnel reviewed the medications with the participant to record accurate dosing information. Study personnel measured blood pressure (in mm Hg), height (in m) and weight (in kg) in duplicate using calibrated equipment. The CC provided the same measurement devices to all sites to standardize the data collection process.
Data were recorded on standardized paper case report forms. Completed forms were scanned and transmitted to the CC through a secure electronic portal (SharePoint, Microsoft, Redmond, WA). The CC reviewed the forms, prepared edit statements as needed, and entered the data into Velos eResearch (Velos, Inc., Fairmount, CA) using double data entry with an adjudication process. Data were stored in an Oracle database (Oracle Corp., Redwood Shores, CA) then downloaded to SAS (Cary, NC) for analysis.
2.7 Blood collection and processing
Venous blood samples were obtained to assess a variety of diabetes-related variables, as described below, at each study visit. Samples collected at screening were non-fasting; thereafter samples were obtained following 8–12 hours of fasting. HbA1c was assessed at each visit from fresh whole venous blood collected in tubes containing EDTA. The baseline visit was completed within 35 days of the qualifying screening visit. Because eligibility was based on the screening HbA1c values, a relatively short time interval was chosen to minimize the likelihood of changes in HbA1c between the screening and baseline visits. All blood samples were either shipped within 4 days to the Core Laboratory (for HbA1c assessment) or processed and frozen at −70°Celsius for subsequent assessments of glucose, insulin, lipids and creatinine (Section 2.8). Approximately once a month, the frozen serum and plasma samples were shipped overnight on dry ice to the Core Laboratory for storage and analysis.
2.8 Outcome Assessments
2.8.1. Primary outcome: HbA1c
HbA1c was measured by the Core Laboratory using an automated high performance liquid chromatography method (Tosoh HPLC G7 Glycohemoglobin Analyzer, Tosoh Medics, Inc., San Francisco CA). The method was calibrated against National Glycohemoglobin Standardization Program (NGSP) standards, with a reference range of 4.3 – 6.0%. The laboratory’s coefficient of variation (CV) ranged from 1.4 – 1.9%. All laboratory personnel were masked to a participant’s group assignment.
2.8.2. Periodontal assessments
Calibrated and masked examiners assessed participants at the baseline and 3- and 6-month visits. Measures of dental plaque and gingivitis [30] were assessed at six locations (mesiobuccal, mid-buccal, distobuccal, distolingual, mid-lingual and mesiolingual sites) on each of six “index” teeth [31]. Missing index teeth were substituted with adjacent teeth in a pre-specified manner. Probing depth (PD, in mm), the distance from gingival margin to the cementoenamel junction (GM-CEJ, in mm), and bleeding following probing (BOP, scored as present or absent) were assessed at the same locations on all teeth, excluding third molars, using a manual periodontal probe (UNC–15, Hu-Friedy Mfg. Co., Chicago IL). Clinical attachment loss (CAL) was computed chair-side from the PD and CEJ-GM for immediate clinical interpretation. The CC also calculated CAL by computer for use in the analyses and to aid the clinical sites in identifying progressing sites. Whenever possible, the same examiner completed all examinations for a participant. Alveolar bone height was assessed from orthopantomographic radiographs obtained at baseline but was not used to determine eligibility.
A single “gold standard” examiner (SE) trained and calibrated the clinical examiners using established standards [32] with some modifications. The trial standards required exact or close agreement between examiners (i.e., compared to the gold standard examiner) and within examiners. For gingivitis assessments, examiners were required to achieve ≥75% and 70% exact agreement within and between examiners, respectively, and ≥95% agreement within 1 index unit, both within and between examiners. For PD and CAL measurements, the calibration standards were as follows: ≥80% intra-examiner agreement for PD ± 1 mm, ≥75% inter-examiner agreement for PD ± 1 mm, ≥95% intra-examiner reproducibility ± 2 mm for both parameters, ≥65% intra-examiner agreement for CAL ± 1 mm, and ≥60% inter-examiner agreement for CAL ± 1 mm. Examiners were calibrated before examining trial participants and annually thereafter.
Although examiners were calibrated using percentage agreement, we also computed Cohen’s kappa statistics to provide the reader with another measure of inter- and intra-examiner agreement. For examiners meeting the pre-specified standards, kappa values for inter-examiner agreement (comparing each examiner to the single gold standard) ranged from 0.75 to 1.00 for PD ± 1 mm, from 0.95 to 1.00 for PD ± 2 mm, from 0.63 to 1.00 for CAL ± 1mm, and from 0.89 to 1.00 for CAL ± 2mm. Kappa values for intra-examiner agreement ranged from 0.87 to 1.00 for PD ± 1 mm, were all 1.00 for PD ± 2 mm, ranged from 0.84 to 1.00 for CAL ± 1 mm and were all 1.00 for CAL ± 2 mm.
Kappa statistics were not computed for GI (the gingivitis index) because the kappa statistic is sensitive to skewed distributions, which is common for this index. Determining bleeding on probing (BOP) is an invasive procedure and sites are more likely to bleed at subsequent passes in a calibration trial. Thus, valid calibration protocols for BOP do not exist. Examiners were trained but not calibrated for the Plaque Index because plaque is removed when this particular index is scored [30].
2.8.3. Secondary Outcomes: Insulin, Glucose, and HOMA2
Insulin was measured in serum on a Roche Elecsys 2010 Analyzer (Roche Diagnostics Corp., Indianapolis, IN) using a sandwich immunoassay method with a chemiluminescent endpoint (Roche Diagnostics Corp.). The method is calibrated against the 1st IRP WHO Reference Standard 66/304 (NIBSC). The laboratory CV is 4.2%.
Glucose was measured in serum by the Roche hexokinase method on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corp.). The method is calibrated against Standard Reference Material 965a from the National Institute of Standards and Technology (NIST). The laboratory CV is 1.6%
HOMA2 values were determined from the insulin and glucose measurements using the HOMA2 Calculator, version 2.2 (http://www.dtu.ox.ac.uk/index.php?maindoc=/homa/). HOMA2 values were calculated for non-insulin users with fasting plasma glucose levels between 3.5 and 25.0 mmol/l and fasting plasma insulin levels between 20 and 400 pmol/l.
2.8.4. Lipid Panel and Creatinine
Plasma total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides and serum creatinine were measured or computed to characterize the study population. Lipids and creatinine were assayed by enzymatic methods using a Roche Modular P Chemistry Analyzer. The Core Laboratory’s cholesterol, HDL-cholesterol and triglyceride calibration methods and results are monitored by the CDC/NHLBI Lipid Standardization Program. The laboratory’s CV for the three lipid measures are 1.6%, 2.9% and 4.0%, respectively. The creatinine method is calibrated against NIST standard reference material SRM 967. The laboratory CV for creatinine is 2.3%. LDL-cholesterol was calculated using the formula of Friedewald et al. [33] if triglycerides were <400 mg/dL.
2.8.5. Medication use and changes
Because diabetes medication changes were a secondary trial outcome, Study Coordinators needed to accurately record and monitor participants’ medication use. To address the challenges of tracking the sheer and growing number of individual and combinatory hypoglycemic medications, we instituted several protocols to assist the Coordinators to record medications and dosages. First, physician investigators at each site reviewed medications reported at baseline for accuracy and completeness, and at subsequent study visits if questions arose about changes in drugs or dosing. The Medical Monitor, the CC and a trial diabetologist (MG) also developed a detailed glossary that included all FDA-approved oral and injectable hypoglycemic agents, by generic and brand name, and by formulation and dosing. Investigators also used the National Library of Medicine’s RxNorm (http://www.nlm.nih.gov/research/umls/rxnorm/overview.html) to provide standard coding for entering medications into Velos eResearch.
2.9 Masking
Laboratory personnel and periodontal examiners were masked to a participant’s group assignment. Masking was maintained using standard approaches. First, randomization assignments by individual participant were accessible in Velos eResearch only to the necessary CC personnel and the Clinical Site Coordinators. Participant IDs did not contain treatment assignment codes. Identical protocols and study forms were used to collect data from both Treatment and Control Group participants. Lastly, brief questionnaires administered to examiners at the 6-month visit evaluated whether the efforts to achieve masking of the periodontal examiners were successful.
2.10 Randomization Process
The CC confirmed a participant’s eligibility after reviewing the baseline and screening data. Randomization was conducted centrally by the CC using a site-specific randomization assignment sequence generated prior to the start of the study. Assignments to the Treatment and Control Groups were created through a custom computer program using a permuted block randomization scheme stratified by Clinical Site using block sizes of 2, 4 or 6. Once eligibility for an individual was confirmed, the CC Study Coordinator generated the randomization assignment electronically and notified the Clinic Coordinator by email or fax. The Clinic Coordinator then contacted the participant with the treatment group assignment. No other Clinical Site personnel other than the Study Therapist were informed of the assignments.
For randomized participants under the regular care of a dentist, a letter was sent informing the provider of their patient’s participation in the trial. Providers were asked not to provide periodontal care or tooth cleanings until their patient completed the trial. Participants could receive minor non-periodontal dental care as needed. Participants without a dentist of record were referred to one or more community clinics to address any treatment needs that arose during the trial.
2.11 Intervention
All participants received structured oral hygiene and healthy lifestyle instructions at the baseline, 3- and 6-month study visits.
2.11.1 Treatment Group
Participants randomized to the Treatment Group received full-mouth supra- and subgingival scaling and root planing (i.e., non-surgical treatment), which was completed using hand and sonic or ultrasonic instruments. Certified study therapists (licensed dentists or hygienists) completed the treatment over two or more sessions, each lasting approximately 90 minutes, and within 42 days of randomization. Local (injected) or topical anesthetics (e.g., lidocaine, benzocaine) were used as needed. Treatment was continued until the therapist and an independent examiner determined that the teeth and roots were free of dental plaque and calculus (tartar). Following each treatment visit, participants received a 16-ounce bottle of an antimicrobial mouth rinse (Chlorhexidine Gluconate 0.12%, 0.5 ounce rinse for 30 seconds twice daily). The rinse is approved by the Food and Drug Administration for reducing dental plaque and gingivitis and provides an adjunctive benefit to scaling and root planing [34, 35].
The intervention did not include systemic (peroral) antibiotics. Although antibiotics are useful adjuncts to mechanical therapy in the treatment of aggressive periodontitis [36], concerns about overall efficacy and gastrointestinal side effects, and growing concerns about antibiotic resistance contraindicate their routine use in chronic periodontitis patients [37]. Systemic antibiotics, however, were allowed as part of rescue treatment for Treatment Group participants (Section 2.12.2).
We defined the intervention based on the length of treatment and the achievement of complete plaque and calculus removal. We did not define treatment endpoints, i.e., minimal required improvements in selected clinical measures following treatment. Although some have advocated for the use of such endpoints [38], the minimal response needed to improve glycemic control in patients with T2DM has not been determined. The periodontology community also lacks a consensus regarding the definition of “successful” treatment in terms of PD and BOP reductions [39]. Thus, we standardized treatment by requiring therapists to be trained and certified, by specifying a minimum length of treatment, and by utilizing an independent examiner to verify complete plaque and calculus removal.
As is convention in clinical practice, Treatment Group participants also received full mouth scaling and polishing (i.e., a cleaning), and localized root planing as needed, 3 and 6 months following randomization.
2.11.2 Control group (Delayed treatment)
Control Group participants did not receive periodontal treatment during the trial unless their condition deteriorated (Section 2.12.2). Delaying treatment was not considered unethical because of the low risk for disease progression over 6 months [40] and because these individuals were monitored frequently and offered immediate (rescue) treatment if their disease progressed. After completing their 6-month visits, Control Group participants were offered the same treatment provided to the Treatment Group.
2.12 Trial monitoring
2.12.1 Adverse event reporting
Trial monitoring and reporting of adverse events were conducted in accordance with the Office for Human Research Protections guidelines and under the auspices of the trial’s Medical Monitor. DPTT was a low risk study and serious adverse events due to study participation were not expected. Events that occurred within two weeks of completion of therapy for the Treatment Group or within two weeks of the baseline visit for the Control Group were considered as study related and reported as adverse events. Serious or unanticipated adverse events within this window were reported to the local IRB as needed, to the Medical Monitor, and to the Contract Research Organization (Rho, Inc.), acting on behalf of the funding agency (NIDCR). Adverse events, expected or otherwise, that occurred outside of the two-week window were reported to the local IRBs as needed but were not considered to be study-related.
Clinic Coordinators interviewed participants by phone two weeks following the completion of treatment (for Treatment Group participants) or the baseline examination (for Control Group participants). Participants were given a diary card at the baseline visit to aid in recognizing and recording adverse events during this period. Participants were asked to record and report oral bleeding, pain or swelling, temperature sensitivity, difficulty chewing or eating, and signs and symptoms related to the use of the chlorhexidine mouth rinse (e.g., tooth discoloration and changes in taste). At each follow-up visit, study personnel questioned participants regarding oral symptoms, new medical conditions and other possible study-related adverse events.
2.12.2 Monitoring for Progressive Periodontal Disease
Examiners evaluated participants at 3 and 6 months for periodontitis progression, defined as an increase in CAL of >2 mm at any tooth site. Participants with five or fewer progressing sites received immediate treatment (scaling and root planing) by study therapists on the affected teeth. Participants with more than five progressing sites were considered to have generalized progressive disease, treatment for these individuals differed according to group assignment. Control Group participants received full-mouth scaling and root planning. Treatment Group participants were referred to a consulting periodontist and retreated with scaling and root planing. The periodontist could supplement re-treatment with systemic (peroral) antimicrobials (e.g. Augmentin, metronidazole, or the combination of both). The choice, and even use, of antibiotics was left up to the discretion of the consulting periodontist. Rescue therapy was administered as soon as possible after disease progression was detected. The CC analyzed the periodontal data and provided an independent assessment of the need for rescue therapy.
2.12.3 Diabetes Safety Monitoring
Although participants were required to be under the care of a physician, the trial took additional steps to monitor their medical safety. HbA1c values ≥ 9.5% at any study visit were communicated in writing to the participant and to his or her physician. Participants with evidence of symptomatic hypertension or hyper- or hypoglycemia were referred for immediate medical management. Participants also were informed about their current blood pressure and weight at each visit.
2.12.4. Data and Safety Monitoring
An independent Data and Safety Monitoring Board (DSMB) reviewed and approved the study protocol before implementation. The DSMB, which was appointed by the NIDCR and included experts in periodontology, diabetology, biostatistics and clinical trials methodology, provided advice to the NIDCR concerning participant safety, data quality, and trial performance. The Board reviewed monthly reports monitoring routine study progress and annual reports detailing recruitment, participant retention, trial safety, protocol adherence and data quality. The Board also reviewed results from the planned interim futility analysis (Section 2.14.2).
2.13. Quality Assurance
Study activities were monitored internally by the Steering Committee and externally by the DSMB. In collaboration with the Study Chair, the CC developed and implemented standards for training and certifying staff and examiners, by study role. In addition, the CC reviewed incoming data and generated data queries for the clinical sites, and communicated regularly with study investigators and personnel to identify and resolve procedural errors. The CC also prepared reports detailing study progress and quality, which were reviewed during monthly Steering Committee conference calls. The study also implemented periodic site monitoring by an independent quality assurance team acting on behalf of NIDCR (Rho, Inc.)
2.14. Sample size and data analyses
2.14.1. Sample Size
The study was powered to detect at least a 0.6% reduction in HbA1c associated with periodontal treatment. We assumed a 0.6% reduction in HbA1c in the Treatment Group, no reduction in the Control Group, and a standard deviation (SD) of 2.0% for the distribution of 6-month changes for both groups. Sample size was estimated assuming a 5% type 1 error rate (alpha) and 90% (1- beta) power. The 0.6% expected group difference was based on results of a meta-analysis of five small studies that reported a weighted average decrease in HbA1c of 0.66% associated with periodontal treatment [17].
The estimated SD of the change in HbA1c was derived from three sources: from the range of values reported in 10 small intervention studies [17], from the SD noted in an unpublished pilot study conducted by the study chair, and from a Veteran’s Administration study of 132 participants [18]. The reported or calculated SDs for changes in these trials ranged from 0.9 – 2.0%. Based on a two-tailed, two-sample t-test and the above considerations, we estimated needing baseline and 6–month data from 468 participants. Assuming a 20% attrition rate, we planned to randomize 600 participants (300 in each study arm).
2.14.2 Futility analysis plan
One futility analysis was specified as part of the protocol to: 1) provide the probability, conditional on the interim observed data, that the final data would demonstrate that periodontal treatment lowers HbA1c level significantly more than no treatment; and 2) allow early stopping of the trial for futility in the absence of any treatment effect on the primary outcome. The analysis was planned to be conducted after the first 300 randomized participants (50% of the recruitment goal) had completed their six-month visit, which was estimated to occur about 24 months after the start of enrollment. Predetermined guidelines for determining futility to continue the study were based on a two-sided, independent t-test and one-sided conditional power calculated using the B-value proposed by Lan and Wittes [41], again assuming a treatment group difference in HbA1c of 0.60% and a within-group standard deviation of 2.0%.
2.14.3 Primary analysis
The primary outcome, 6-month change in HbA1c, will be analyzed using use an intention-to-treat approach. A last-observation-carried-forward imputation method will be used for participants missing data from one or both follow-up visits. The primary analysis will be based on a linear regression model, with 6-month change in HbA1c as the response variable, treatment assignment as a factor and adjusting for clinical site as a covariate. The center effect was planned to be fixed. Clinical site was included as a covariate to address potential center differences in terms of the effect of periodontal therapy on the study outcomes, HbA1c and periodontal disease. In addition, the statistical analysis plan included ongoing monitoring of the balance in baseline characteristics between study arms within and between centers.
A secondary analysis will be conducted to evaluate baseline characteristics as potential covariates (e.g. age, gender, ethnicity, smoking status, BMI, diastolic blood pressure, BOP and duration of diabetes) in a multivariable regression model. Interactions between the above factors and treatment status also will be explored. Sub-group analyses will be performed to evaluate treatment effects on 6-month change in HbA1c in different levels of covariates (e.g. in each gender group). The same analytical strategy will be applied to the clinical measures of chronic periodontitis (PD, CAL, BOP). Additional secondary outcomes (e.g., fasting glucose, fasting insulin, HOMA2 insulin resistance and HOMA2 β-cell function) will also be analyzed using linear regression models. The association between each secondary outcome and the periodontal measurements will be estimated using Pearson or Spearman correlations.
4. Discussion
The exact mechanisms by which periodontal disease and its treatment may affect glycemic control in patients with T2DM have not been fully elucidated. A popular hypothesis involves inflammatory pathways common to the pathogeneses of periodontitis and insulin resistance [42]. Specifically, interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha), which are present in inflamed periodontal tissues [43], are known to adversely affect insulin signaling and action. [44, 45]. Both Il-6 and TNF-alpha may enter the systemic circulation in patients with periodontitis, where over time they may alter insulin sensitivity in target organs. Thus, it is biologically plausible that periodontitis may exacerbate glycemia in patients with T2DM and that treatment, by reducing levels of circulating cytokines, may improve glycemic control.
The DPTT is the largest and only controlled multi-center clinical trial designed to date to determine the effects of periodontal treatment on glycemic control in individuals with T2DM. Although a series of small trials suggest that non-surgical periodontal treatment results in modest reductions in HbA1c, data from large, multi-center trials, enrolling participants from diverse populations, are needed to better inform the dental and medical communities regarding the value of diagnosing and treating periodontitis in this patient population. The DPTT will help fill this important knowledge gap.
While the DPTT successfully built and sustained collaborations across professions (medicine, dentistry, epidemiology and biostatistics), it was not without its challenges. The same trial needs that fostered the development of integrated yet diverse study teams also challenged study personnel to operate in unfamiliar clinical settings and environments. Two challenges – recruitment and tracking medication use – are discussed below in more detail.
T2DM and periodontitis are prevalent conditions that are associated with one another. Thus, one might expect patients with T2DM to frequently present with periodontitis, and vice versa. The specific enrollment criteria for each disease, however, limited the pool of potentially eligible DPTT participants. While most screened participants had some level of periodontitis, enrollment was limited to those with moderate to advanced untreated periodontitis and no essential dental care needs. Over half of initially screened participants did not meet the dental or periodontal enrollment criteria. Those with essential dental care needs often needed to have extensively decayed or abscessed teeth extracted before being rescreened several months later. This slowed the enrollment process.
Recruitment also was challenging because T2DM and periodontitis are managed within different care systems. Few patients are “co-managed” across medicine and dentistry in a manner that regularly occurs within each profession. Thus, the trial was challenged to sustain collaborations between medical and dental provider networks to access the appropriate patient populations. Our original goal was to complete recruitment within 26 months. Even with the addition of two clinical sites, however, the recruitment period was extended for an additional 3 months.
Many participants were identified through primary care or specialty medical (e.g., diabetes) clinics. Physicians and their staff, however, are not routinely trained to recognize periodontal disease. At some Clinical Sites, patients recruited through medical clinics were screened by dental personnel directly in the medical clinics and at the time of their medical visits. Mostly, however, potentially eligible individuals were referred to dental clinics for screening, which increased the screening burden for these individuals.
Potential participants also were identified through dental record searches. Dental care providers, however, typically do not record HbA1c values for their T2DM patients. Thus, we could not identify through these records alone patients who were likely to qualify for the trial. In addition, many individuals identified through dental school records either had unmet essential dental care needs or had received at least some periodontal treatment. As a result, recruitment efforts within dental school clinics were not consistently fruitful.
Every Clinical Site struggled to optimize its recruitment efforts. Study personnel across sites regularly exchanged ideas through conference calls, emails and in-person meetings. Each site eventually relied on a mix of shared and unique recruitment strategies. A particularly effective approach was to encourage physicians at affiliated clinics to send study invitations to patients that she or he believed might be eligible for the trial. The IRB-approved introductory letters provided a brief overview of the study and listed several study phone numbers. Engaging physicians also may have helped reassure patients that their physician is concerned with their oral needs and that the trial was worthwhile. In contrast, the effectiveness of broad-based recruitment efforts (e.g., posted study fliers and brochures, radio advertisements) varied substantially by Clinical Site. Overall, 29% of all screened participants were randomized. Screening success, however, ranged from 17% to 51% across clinical sites, which likely reflected among-site differences in clinic patient characteristics and recruitment strategies.
Despite pre-trial training, Study Coordinators frequently had questions regarding drug names and dosing due to the wide variability of both generic and brand name medications with the same formulary. The problem was compounded by the number of available individual and combinatory hypoglycemic medications, and because the Coordinators were more experienced with dental than diabetes trials. To address this challenge, we instituted several protocols, outlined in Section 2.8.5, to assist the Coordinators in recording medication use and dosing changes. Although designed for computer systems, use of RxNorm enabled investigators to track and analyze drug information more efficiently and unambiguously within and between participant records. The CC also generated queries for medications and dosages that did not match the dosing contained in the trial’s drug glossary or that were inconsistent across study visits. Finally, the Study Coordinators received regular training updates on medication use during their bimonthly conference calls.
The DPTT was designed and initiated to address an important topic in “periodontal medicine” and diabetes control. Periodontal disease has been associated with a wide variety of conditions including diabetes [42], adverse pregnancy outcomes [46], cardiovascular disease [47], and certain cancers [48]. Much of the evidence for these links comes from observational studies, for which residual confounding by shared risk factors is a real concern. Interventional studies, such as the DPTT, are needed to better understand the nature of these associations. The DPTT should provide ample evidence to support or refute the hypothesis [47] that treatment of periodontal disease affects glycemic control in patients with T2DM. The DPTT’s approaches to building and training multi-professional study teams, and to applying broad and novel recruitment strategies, could be adopted by others studying new ways to manage patients with multiple chronic disorders.
Acknowledgments
Grant Support: Supported by NIH/NIDCR Grant UO1 DE018902 (to S Engebretson) and U01 DE018886 (to L Hyman). Clinical Trials.gov: NCT00997178
We thank the members of the DSMB (J Gunsolley (Chair), V Fonseca, J Jones, D Heitjan, J Megis), Rho Inc., and the NIDCR’s Program Officer (J Atkinson) and Medical Monitor (H Hamilton).
Appendix
The DPTT study team includes the following individuals.
Study Chair (Stony Brook University), S Engebretson (Study Chair), M Gelato, B Moonga, R Tenzler
Coordinating Center (Stony Brook University Medical Center): L Hyman (PI), E Schoenfeld (Co-PI), Li Ming Dong, Melissa Fazzari, W Hou, G Lerner, H Chen, S Lee, C Knuth, J Mendelsohn, G Pietrzak, C Hytner, L Snelling, S Ahmed, M Rodriguez, M Merin, J Merin, L Merill, L Seib.
University of Alabama at Birmingham: M Reddy (PI), C Lewis, N Geurs, P Vassilopoulos, A Abrahams, J Doobrow, M Geisinger, S Kukunooru, C Leavitt, J Pardo, R Abou Arraj, A Reganato, K Simmons, E Unger, J Bain, K Beaudry, M Nguyen, R Sauceda, J Bauerle, M Madigan, A Ntounis, M Kaur, A Stevens, S Goggin, L Pitman, K Trammel, C Peterson, S Haigh, J Jackson, E Finch, S Akers, V Grant, S Acharya, S McLean, J Turman, J Roche, C Bragg, R Rajanna, E Bolton.
University of Minnesota/Hennepin County Medical Center: B Michalowicz (PI), D DiAngelis (PI), E Seaquist, J Danielson, P Lenton, L Wolff, P Thibado, S Molletti, L Long-Simpson, Y Okorocha, B Hadfield, L Bartels, C Dunn, K Meyer, K Reibel, A Jordan, R Hedge, O Herrera, E Romero, S Mohamed, C Stull.
University of Texas Health Science Center at San Antonio: T Oates (PI), D Tripathy, P Alexander, D Lasho, H Gregory, G Huynh-Ba, J Jordan, S Pena, C Pacheco-Vera, M Carrera, A Munoz.
Stony Brook University: D Paquette (PI), S Engebretson (PI), M Gelato, T Sayasith, Y Gu, A Roth, A Urbankova, M Ryan, J Tuthill, J Hughes, S Grewal, R Tenzler, B Houshmand, V Iacono.
University of Texas Health Science Center at Houston: J Katancik (PI), B Wang (PI), P Orlander, S Eswaran, K Parthasarathy, R Weltman, M Wehmeyer, A Arastu, R Thomas, J Headley, A Cavender, NJ Harrison, T Dancsak, M Galpin, M Ruscheinksy
University of Minnesota Core Laboratory: M Tsai (PI), N Hanson, M Nowicki, V Le
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heron M. Deaths: leading causes for 2008. Natl Vital Stat Rep. 2012;60:1–94. [PubMed] [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the u.s. 2012 Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–8. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 (Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 5.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–57. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285:182–9. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 7.Gilmer TP, O’Connor PJ, Manning WG, Rush WA. The cost to health plans of poor glycemic control. Diabetes Care. 1997;20:1847–53. doi: 10.2337/diacare.20.12.1847. [DOI] [PubMed] [Google Scholar]
- 8.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA) Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 9.Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–40. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 10.Chavarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 2009;7:107–27. [PubMed] [Google Scholar]
- 11.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol. 1993;20:431–5. doi: 10.1111/j.1600-051x.1993.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 13.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 14.Sammalkorpi K. Glucose intolerance in acute infections. J Intern Med. 1989;225:15–9. doi: 10.1111/j.1365-2796.1989.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 15.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–9. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40 (Suppl 14):S113–34. doi: 10.1111/jcpe.12059. [DOI] [PubMed] [Google Scholar]
- 17.Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res. 2005;84:1154–9. doi: 10.1177/154405910508401212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JA, Miller DR, Wehler CJ, Rich SE, Krall-Kaye EA, McCoy LC, et al. Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol. 2007;34:46–52. doi: 10.1111/j.1600-051X.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 19.Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379:2252–61. doi: 10.1016/S0140-6736(12)60480-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2012;7:e42551. doi: 10.1371/journal.pone.0042551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol. 2013;84:S153–69. doi: 10.1902/jop.2013.1340017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 23.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE, et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–57. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis. 2005;11:293–8. doi: 10.1111/j.1601-0825.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 26.Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38:142–7. doi: 10.1111/j.1600-051X.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- 27.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–54. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamster IB, Lalla E, Borgnakke WS, Taylor GW. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139 (Suppl):19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 29.Mattila PT, Niskanen MC, Vehkalahti MM, Nordblad A, Knuuttila ML. Prevalence and simultaneous occurrence of periodontitis and dental caries. J Clin Periodontol. 2010;37:962–7. doi: 10.1111/j.1600-051X.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 30.Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 31.Ramfjord SP. The Periodontal Disease Index (PDI) J Periodontol. 1967;38(Suppl):602–10. doi: 10.1902/jop.1967.38.6.602. [DOI] [PubMed] [Google Scholar]
- 32.Pihlstrom BL, Wolff LF, Bakdash MB, Schaffer EM, Jensen JR, Jr, Aeppli DM, et al. Salt and peroxide compared with conventional oral hygiene. I. Clinical results. J Periodontol. 1987;58:291–300. doi: 10.1902/jop.1987.58.5.291. [DOI] [PubMed] [Google Scholar]
- 33.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 34.Faveri M, Gursky LC, Feres M, Shibli JA, Salvador SL, de Figueiredo LC. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: a randomized, placebo-controlled clinical trial. J Clin Periodontol. 2006;33:819–28. doi: 10.1111/j.1600-051X.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 35.Feres M, Gursky LC, Faveri M, Tsuzuki CO, Figueiredo LC. Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy. J Clin Periodontol. 2009;36:857–67. doi: 10.1111/j.1600-051X.2009.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sgolastra F, Petrucci A, Gatto R, Monaco A. Effectiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planing in the treatment of aggressive periodontitis: a systematic review and meta-analysis. J Periodontol. 2012;83:731–43. doi: 10.1902/jop.2011.110432. [DOI] [PubMed] [Google Scholar]
- 37.Preshaw PM. Antibiotics in the treatment of periodontitis. Dent Update. 2004;31:448, 50, 453–4, 456. doi: 10.12968/denu.2004.31.8.448. [DOI] [PubMed] [Google Scholar]
- 38.Armitage GC. Effect of periodontal therapy on general health--is there a missing component in the design of these clinical trials? J Clin Periodontol. 2008;35:1011–2. doi: 10.1111/j.1600-051X.2008.01327.x. [DOI] [PubMed] [Google Scholar]
- 39.Michalowicz BS, Gustafsson A, Thumbigere-Math V, Buhlin K. The effects of periodontal treatment on pregnancy outcomes. J Periodontol. 2013;84:S195–208. doi: 10.1902/jop.2013.1340014. [DOI] [PubMed] [Google Scholar]
- 40.Lindhe J, Haffajee AD, Socransky SS. Progression of periodontal disease in adult subjects in the absence of periodontal therapy. J Clin Periodontol. 1983;10:433–42. doi: 10.1111/j.1600-051x.1983.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 41.Lan KK, Wittes J. The B-value: a tool for monitoring data. Biometrics. 1988;44:579–85. [PubMed] [Google Scholar]
- 42.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–66. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 44.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 45.Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–8. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 46.Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol. 2007;196:135.e1–135.e7. doi: 10.1016/j.ajog.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520–44. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 48.Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38:83–95. doi: 10.1016/j.jdent.2009.10.007. [DOI] [PubMed] [Google Scholar]