Abstract
Purpose: Renal ischemia reperfusion (IR) is an important cause of renal dysfunction. It contributes to the development of acute renal failure (ARF). The purpose of this study was to investigate the anti-inflammatory effect of erythropoietin (EPO) and melatonin (MEL), which are known anti-inflammatory and antioxidant agents, in IR-induced renal injury in rats.
Methods: Male Wistar Albino rats were unilaterally nephrectomized and subjected to 45 min of renal pedicle occlusion followed by 24 h reperfusion. MEL (10mg/kg, i.p) and EPO (5000U/kg, i.p) were administered prior to ischemia. After 24 h reperfusion, blood samples were collected for the determination of total antioxidant capacity (TAC), malondialdehyde (MDA) and serum creatinine levels. Also, renal samples were taken for Immunohistochemical evaluation of Bcl2 and TNF-α (tumor necrosis factor-α) expression.
Results: Ischemia reperfusion increased creatinine, TAC, MDA levels and TNF-α expression, also, IR decreased Bcl2 expression. Treatment with EPO or MEL decreased creatinine, MDA levels, and increased TAC level. Also, MEL up-regulated Bcl2 expression and down-regulated TNF-α expression compared with EPO.
Conclusion: Treatment with EPO and MEL had a curative effect on renal IR injury. These results may indicate that MEL protects against inflammation and apoptosis better than EPO in renal IR injury.
Keywords: Erythropoietin, Inflammation, Melatonin, Renal ischemia reperfusion
Introduction
Renal ischemia reperfusion injury (IRI), which occurs during kidney transplantation, partial nephrectomy, and elective urological operations, is a common cause of acute renal failure (ARF). Ischemia insult, during renal transplantation, is responsible for primary graft dysfunction.1 Reperfusion (re-establishing blood flow) of ischemic renal tissue is highly damaging and initiates a series of cellular events that lead to necrotic and apoptotic cell death. Several mechanisms contribute to the pathophysiology of ischemia reperfusion injury, such as reactive oxygen species (ROS), ATP depletion and increased neutrophil infiltration.2 Therefore, increased generation of inflammatory cytokines and ROS in the reperfusion phase is believed to play a pivotal role. The excessive production of reactive oxygen and nitrogen species (RNS) after reperfusion results in the expression of genes for pro-inflammatory mediators, the lipid peroxidation of the cellular membranes and oxidative DNA damage, with the subsequent generation of toxic metabolites causing apoptotic cell death.3
Lipid peroxidation is related to IR injury-induced tissue damage and malondialdehyde (MDA) is an indicator of the rate of lipid peroxidation.4 Several anti-inflammatory and antioxidant agents have been explored to be effective in reducing renal ischemia-reperfusion injury.5,6
Erythropoietin (EPO) is a hypoxia-inducible hematopoietic factor, a key protein in red blood cell production, which is predominantly expressed in the kidney. EPO has multiple protective effects, including antioxidant, anti-inflammatory, angiogenic, and anti-apoptotic effects.7 The biological effects of erythropoietin are mediated by binding to its specific cell surface receptor (EPOR), and the presence of functional EPOR in renal mesangial and tubular epithelial cells has pointed to a potential role for erythropoietin in the kidney.8 One important effect of erythropoietin is the reduction in apoptosis and oxidative stress.9 It is also revealed that renal EPO level lowered after renal ischemia reperfusion.10
Melatonin (N-acetyl-5-methoxytryptamine) is the major product of the pineal gland that functions as a regulator of sleep, circadian rhythm, and immune function.11 Melatonin (MEL) and its metabolites have potent antioxidant/anti-inflammatory properties and have been proved to be highly effective in a variety of disorders linked to inflammation and oxidative stress.12 MEL not only neutralizes RNS and ROS species, but also acts through stimulation of several anti-oxidative systems and stabilizing cell membranes.13 It modulates the gene expression of several protective enzymes and reduces apoptosis and lipid peroxidation.14
Therefore, ROS and inflammation have been shown to contribute to the cellular damage induced by ischemia-reperfusion. The aim of the present study was to examine the potential effects of EPO and MEL on renal IR injury. For this purpose, we measured the plasma levels of MDA, total antioxidant capacity (TAC), creatinine (Cr), and using immunohistochemistry (IHC), expression of anti-apoptotic Bcl2 and inflammatory mediator TNF-α were assessed in renal sections after ischemia reperfusion (IR) in rats subjected to renal IR injury.
Materials and Methods
Animals
In this study, 40 male Wistar- Albino rats (weighing 200 - 300g) were obtained from the experimental animal research center, Medical Faculty, Tabriz University, Iran. The animals were housed in a room temperature (21±2 °C) and humidity (60±5%) controlled room in which a 12-12 h light-dark cycle was maintained. They had free access to standard water and food. The study was approved by the University Ethics Committee.
Surgery and Experimental protocol
Rats were anaesthetized with 75 mg/kg ketamine hydrochloride and 8 mg/kg xylazine, intraperitoneal injection. Right nephrectomy was performed and then, the left renal pedicle (artery and vein) was occluded by placing a microvascular clamp for 45 min to induce ischemia and then subjected to reperfusion for 24h.
The animals were divided into four groups of 10 animals each (n=10).
The sham group of animals underwent only nephrectomy without occlusion.
IR group (ischemic control)
MEL + IR group
EPO + IR group
MEL (10 mg/kg; i.p) or vehicle (1% alcohol in saline) was administered 10 min prior to ischemia. MEL (Sigma, St. Louis, MO, USA) was dissolved in absolute ethanol and then diluted in saline to give a final alcohol concentration of 1% ethanol. EPO (Neorecormon, Roche, Mannheim, Germany) was administered as a 5000 U/kg single dose, intraperitoneally 10 min before ischemia.
Biochemical analysis
The blood samples and left kidney tissues of the rats were obtained after 24 h reperfusion in each group for determination of plasma levels of TAC, MDA and creatinine. The blood samples were centrifuged at approximately 4000g for 10 min at 4°C. The Cr level in the serum was determined to assess the renal function, using the Autoanalyser (Alcyon 300 USA).
Malondialdehyde assessment
Plasma MDA levels were measured using the thiobarbituric acid reactive substances (TBARS) method.15
Total antioxidant capacity assessment
Plasma TAC was determined using Randox total antioxidant status kit in which ABTS (2, 2-Azino-di [3-ethylbenzthiazolin sulphanatel]) is incubated with a peroxidase and hydrogen peroxide to produce the radical cation ABTS+. This has a stable blue green color, which is determined at 600 nm. Antioxidants in the added sample cause suppression of this color production to a degree which is proportional to their concentration.16
Immunohistochemistry
Tissue samples preserved in 10% buffered formalin were dehydrated, embedded in paraffin and sectioned at 5 μm. Sections were mounted on slides and deparaffinized in xylene (3 ˟ 10 min) and ethanol (100% ethanol, 2 ˟ 5 min; 96%, 5 min; 70%, 5 min), then boiled with 10 mM citrate buffer in the microwave for 10 min, cooled down in citrate buffer for 20 min and rinsed with deionized water and PBS (phosphate buffered saline). To quench the endogenous activity, sections were incubated for 30 min in 0.1% H2O2 in methanol. They were then washed in PBS for 5 min and incubated with diluted normal rabbit serum (Abcam, Cambridge, UK) for 20 min. After blotting excess serum, the sections were incubated with the primary antibody in the dilution of 1/2000 for TNF-α (rabbit polyclonal to TNF-α, Abcam), the dilution of 1/100 for Bcl-2 (rabbit polyclonal to Bcl-2, Abcam). For negative control, slides were incubated with diluted normal rabbit serum (Abcam) for 1 hr in room temperature. After the sections were rinsed in PBS, a supersensitive biotinylated secondary antibody (Goat polyclonal to Rabbit IgG - H&L (HRP); Vector Laboratories Inc., Burlingame, CA, USA) was applied for 30 min. Slides were then rinsed in PBS again and incubated for 30 min with Vectastain ABC Reagent (Vector Laboratories Inc.), washed in PBS and incubated in peroxidase substrate solution (Vektor DAB Peroxide Substrate; Vector Laboratories Inc.) until desired stain intensity develops. Under microscope, a color reaction (brown) can be seen and the reaction can then be stopped with water.
Immunohistochemical staining for TNF-α and Bcl-2 was examined using light microscopy by an investigator blinded with respect to the animal group at a magnification of ˟40. In each group, 8 representative kidney sections were investigated, 20 view fields were counted per kidney section. To assess tubular staining for TNF-α and Bcl-2 expression, the following scoring system was used: grade 0: no expression; grade 1: minimal expression, grade 2: mild expression; grade 3: moderate expression; grade 4: severe expression.17
Statistical analysis
All the data are presented as mean ± standard deviation (M±SD). Significance testing between groups was performed using one-way analysis of variance (ANOVA) with SPSS Version 19 and multiple comparison post hoc test to determine significant differences between groups. A P-value of less than 0.05 was considered statistically significant.
Results
The effect of EPO and MEL on renal ischemia reperfusion injury was investigated in 45 minute of renal ischemia followed by 24 hour reperfusion. Biochemical analysis results are outlined in Table 1, and using immunohistochemistry (IHC), expression of anti-apoptotic Bcl2 and inflammatory mediator TNF-α were assessed in renal sections after IR.
Table 1. Biochemical measurements after 24 h of reperfusion .
| Groups | Sham group | IR group | MEL+IR group | EPO+IR group |
| Cr (mg/dl) | 0.74±0.13 | 1.32±0.88a | 1.14±0.20 | 0.93±0.21 |
| MDA(nmol/ml) | 2.19±0.69 | 2.64±1.35 | 2.01±0.61 | 2.46±0.55 |
| TAC (mmol/l) | 0.78±0.08 | 0.87±0.23 | 1.15±0.25b | 1.28±0.21b |
| aSignificantly increased when compared with sham group, P<0.05. | ||||
| bSignificantly increased when compared with IR group, P<0.05. | ||||
| Cr, creatinine; MDA, malondialdehyde; TAC, total antioxidant capacity; EPO, erythropoietin; MEL, melatonin; IR, ischemia reperfusion. | ||||
Effects of ischemia reperfusion
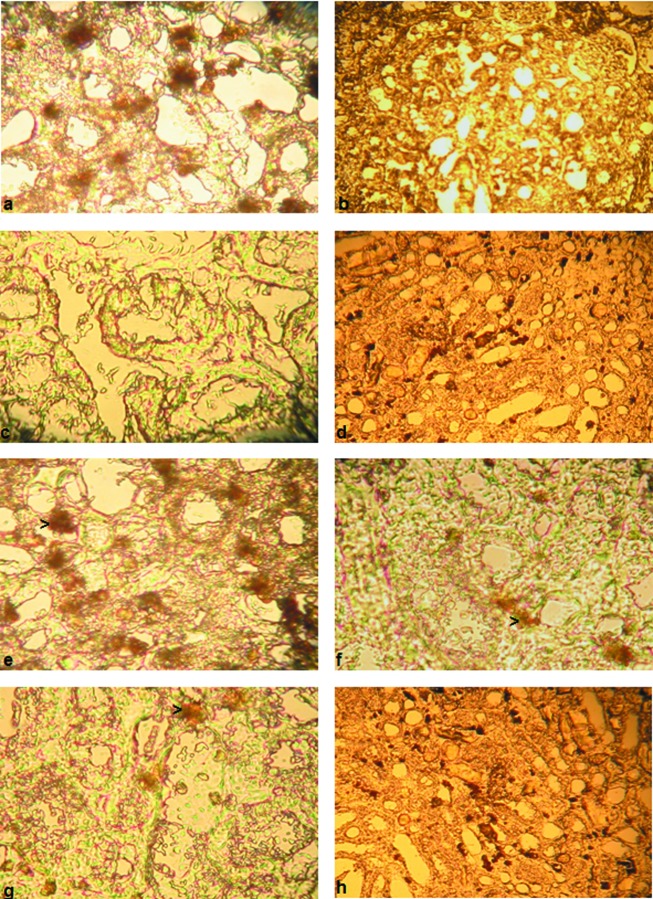
Serum creatinine level was significantly higher in the animals from IR group compared with those from sham group (P<0.05). The levels of MDA and TAC in the IR group were higher than those in the sham group, but the difference was not statistically significant (P > 0.05). In the sham group, there was prominent augmentation of Bcl2 expression and absence of TNF-α expression (Figure 1 a, b). Renal IR caused an absence of Bcl2 expression and great increase in TNF-α expression compared with the sham group (Figure 1 c, d).
Figure 1 .
(a, b) Bcl2 and TNF-α expression in renal tubules of the sham group. (c) Expression of Bcl2 after renal IR. The lesser degree of Bcl2 expression in tubular epithelial cells of IR group. Magnification × 40. (d) Expression of TNF-α after renal IR. Increased TNF-α expression in tubular epithelial cells of IR group. Magnification ×20. (e) Expression of Bcl2 after MEL administration. Increased Bcl2 expression in tubular epithelial cells of MEL group compared to IR group. Magnification × 40. (f) Expression of TNF-α after MEL administration. Decreased TNF-α expression in tubular epithelial cells of MEL group compared to IR group. Magnification ×40. (g) Expression of Bcl2 after EPO administration. Minimal increased Bcl2 expression in tubular epithelial cells of EPO group compared to IR group. Magnification × 40. (h) Expression of TNF-α after EPO administration. Lesser decreased TNF-α expression in tubular epithelial cells of EPO group compared to IR group. Magnification ×20.
Effects of melatonin on renal ischemia reperfusion
MDA and serum creatinine levels in the MEL + IR group were lower than those in IR group, but the difference was not statistically significant (P>0.05). The TAC level in the MEL + IR group was significantly higher than that in the IR group (P=0.01).
MEL administration resulted in severe increase of Bcl2 expression and marked reduction of TNF-α expression compared with IR group (Figure 1 e, f).
Effects of erythropoietin on renal ischemia reperfusion
MDA and serum creatinine levels in the EPO + IR group were lower than those in the IR group, but the difference was not statistically significant (P > 0.05).The TAC level in the EPO + IR group was significantly higher than that in the IR group (P=0.000).
EPO administration resulted in minimal Bcl2 expression and moderate TNF-α expression, nearly at the same level of IR group (Figure 1 g, h).
Discussion
Renal IR is a common result of clinical procedures such as organ procurement, vascular surgery, or renal transplantation. Furthermore, renal IR injury is a leading cause of ARF, which is associated with high mortality rates. ARF is characterized by increased vascular resistance in the kidney, a low rate of filtration through the glomeruli, and tubular necrosis. These deleterious effects have been attributed to ROS generation during renal reperfusion.18 ROS contributes to lethal cell damage. IR injury has been attributed to ROS-mediated lipid peroxidation.19
We found that renal IR insignificantly increased the plasma level of TAC. Increased total antioxidant capacity indicates a cellular defensive response to overproduction of ROS after renal IR. ROS generation within cells is countered by sophisticated extracellular and intracellular antioxidant defense systems. These can be divided into enzymatic and non-enzymatic antioxidant systems, which effectively combine different antioxidant activities such as those that prevent ROS formation and distribution, maintain antioxidant enzyme levels and activities, scavenge ROS, and repair ROS –mediated cellular injury. 20
Our results demonstrated that EPO and MEL significantly increased the level of TAC. This effect of EPO and MEL may be due to their antioxidant properties. Consistent with our finding, Kurcer et al.21 found that MEL ameliorated the functional and structural alterations in renal IR rats, decreased the total oxidative stress and increased the TAC. On the other hand, Dimitrijevic et al.22 demonstrated that increase in TAC accompanied increasing durations of EPO treatment and higher levels of TAC were also verified in the group with the longest duration of EPO treatment in hemodialysis patients.
Lipid peroxidation, as a free radical generating system, has been proposed to be closely related to IR induced tissue injury, and MDA is a good indicator of the degree of lipid peroxidation. In the present experiment, the level of MDA is increased by IR, which reflects increased lipid peroxidation due to increased oxidative stress. Erythropoietin decreased the level of MDA, which shows that it decreased the amount of oxidative stress and subsequently lipid peroxidation. Consistent with our findings, Ates et al.23 demonstrated that EPO decreased the level of MDA after right nephrectomy, clamping of the left renal pedicle, and reperfusion in rats. Our results show that melatonin causes a reduction in MDA production, indicating a reduction in lipid peroxidation and cellular damage. This protective effect of MEL may be in part by scavenging the very reactive ONOO-and OH.24
IR injury of the kidney results in both glomerular and tubular dysfunctions.25 In our study, IR significantly increased creatinine level, suggesting an impaired glomerular function which was greatly reduced after EPO and MEL treatment. It is shown that the administration of EPO before ischemia attenuated the deterioration in renal function as a result of IR injury.23 Also, it is shown that the administration of MEL retards deterioration of renal function and structure.26
In the present study, Immunohistochemical assessment showed that IR caused increase in pro-inflammatory cytokine such as TNF-α. It has been reported that renal IR causes synthesis of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6.27,28 EPO treatment did not obviously modify the TNF-α expression in kidney tubules when compared with the IR group. Sølling et al.29 reported that EPO did not modify the inflammatory response in a porcine model of endotoxemia. MEL severely attenuated the TNF-α expression, this effect of MEL may be due to its anti-inflammatory properties. Consistent with this finding, kireev et al.30 indicated that MEL administration lowered the expression of TNF-α and IL-1β in hepatic ischemia/reperfusion. In our study, renal IR resulted in decreased expression of Bcl2 protein at 24h reperfusion, indicating that down-regulation of Bcl2 protein could contribute in apoptotic cell death in renal IR. Previous studies conducted in similar animal models of renal IR injury have shown that ischemia leads to an increase in the Bax/Bcl2 ratio suggesting that the fine balance between the activity of pro-apoptotic and anti-apoptotic Bcl2 family members can determine cell survival and modulate the induction of apoptosis.31 MEL severely increased Bcl2 expression in renal tissue. This effect of MEL may be due to its powerful anti-apoptotic properties. Tunon et al.32 reported that some anti-apoptotic effects of MEL were related to a reduced expression of Bax and also to the diminished cytochrome C release to the cytosol, to the increased expression of Bcl2 and Bcl-xL, and to the inhibition of caspase-9 activity in an animal model of fulminant hepatic failure. In the EPO group, the Bcl2 expression did not show obvious changes in kidney when compared with IR group. Consistent with this finding, Johnson et al.33 showed that there was no apparent effect in the model of ischemia ARF on promotion of the anti-apoptotic proteins Bcl2 and Bcl-xL by EPO and darbepoetin.
Conclusion
In conclusion, ROS are considered to be principal components involved in the pathophysiological tissue alterations observed during renal IR. Antioxidant defense systems or TAC activities prevent ROS formation and scavenge ROS. The administration of EPO and MEL, which are potent anti-inflammatory and antioxidant agents, appears to have beneficial effects on IR-induced renal injury as indicated by higher levels of TAC activity, and lower degree of renal dysfunction. However, MEL pretreatment exerted more nephroprotective effect than EPO pretreatment. As MEL administration resulted in severe increase of Bcl2 expression and marked reduction of TNF-α expression compared with EPO group, MEL was probably effective to reverse renal IR by its potent anti-inflammatory and anti-apoptotic effects. These results may indicate that MEL protects against inflammation and apoptosis better than EPO in renal IR injury. However, further investigations are required to explore that the combination treatment of EPO and MEL has a synergistic effect of protection against IR-induced renal injury.
Acknowledgments
This study was financially supported by Drug Applied Research Center of Tabriz University of Medical Sciences. The paper was derived from Ph.D. thesis of Shokofeh Banaei entitled "Effect of erythropoietin and melatonin on renal ischemia-reperfusion injury in rats".
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ploeg RJ, Van Bockel JH, Langendijk PT, Groenewegen M, Van Der Woude FJ, Persijn GG. et al. Effect of preservation solution on results of cadaveric kidney transplantation. The European Multicentre Study Group. Lancet. 1992;340(8812):129–37. doi: 10.1016/0140-6736(92)93212-6. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein CL, Ling H, Schrier RW. The nature of renal cell injury. Kidney Int. 1997;51(5):1341–51. doi: 10.1038/ki.1997.183. [DOI] [PubMed] [Google Scholar]
- 3.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–38. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 4.Kacmaz A, Polat A, User Y, Tilki M, Ozkan S, Sener G. Octreotide improves reperfusion-induced oxidative injury in acute abdominal hypertension in rats. J Gastrointest Surg. 2004;8(1):113–9. doi: 10.1016/j.gassur.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Cau J, Favreau F, Zhang K, Febrer G, De La Motte GR, Ricco JB. et al. FR167653 improves renal recovery and decreases inflammation and fibrosis after renal ischemia reperfusion injury. J Vasc Surg. 2009;49(3):728–40. doi: 10.1016/j.jvs.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 6.Korkmaz A, Kolankaya D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren Fail. 2009;31(1):36–43. doi: 10.1080/08860220802546271. [DOI] [PubMed] [Google Scholar]
- 7.Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood. 1999;94(6):1864–77. [PubMed] [Google Scholar]
- 8.Westenfelder C, Biddle DL, Baranowski RL. Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int. 1999;55(3):808–20. doi: 10.1046/j.1523-1755.1999.055003808.x. [DOI] [PubMed] [Google Scholar]
- 9.Calo LA, Bertipaglia L, Pagnin E. Antioxidants, carnitine and erythropoietin. G Ital Nefrol. 2006;23 Suppl 34:S47–50. [PubMed] [Google Scholar]
- 10.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP. et al. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta. 2011;1812(1):77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC. et al. Melatonin--a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann N Y Acad Sci. 1994;738:419–20. doi: 10.1111/j.1749-6632.1994.tb21831.x. [DOI] [PubMed] [Google Scholar]
- 12.Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C. et al. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165(1-2):139–49. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V. et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 14.Reiter RJ, Guerrero JM, Garcia JJ, Acuna-Castroviejo D. Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin. Ann N Y Acad Sci. 1998;854:410–24. doi: 10.1111/j.1749-6632.1998.tb09920.x. [DOI] [PubMed] [Google Scholar]
- 15.Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 16.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84(4):407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 17.Bennett WM. The failed renal transplant: in or out? . Seminars in dialysis. 2005;18(3):188–9. doi: 10.1111/j.1525-139X.2005.18306.x. [DOI] [PubMed] [Google Scholar]
- 18.Carden DL, Granger DN. Pathophysiology of ischemia reperfusion injury. J Pathol. 2000;190(3):255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.McCord JM. The evaluation of free radicals and oxidative stress. Am J Med. 2000;108:652–9. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 20.Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. Br J Nutr. 2001;85 Suppl 2:S67–74. [PubMed] [Google Scholar]
- 21.Kurcer Z, Oguz E, Ozbilge H, Baba F, Aksoy N, Celik H. et al. Melatonin protects from ischemia/reperfusion-induced renal injury in rats: this effect is not mediated by proinflammatory cytokines. J Pineal Res. 2007;43(2):172–8. doi: 10.1111/j.1600-079X.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 22.Dimitrijevic ZM, Cvetkovic TP, Djordjevic VM, Pavlovic DD, Stefanovic NZ, Stojanovic IR. et al. How the duration period of erythropoietin treatment influences the oxidative status of hemodialysis patients. Int J Med Sci. 2012;9(9):808–15. doi: 10.7150/ijms.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ates E, Yalcin AU, Yilmaz S, Koken T, Tokyol C. Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J Surg. 2005;75(12):1100–5. doi: 10.1111/j.1445-2197.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- 24.Reiter RJ, Oh CS, Fujimori O. Melatonin Its intracellular and genomic actions. Trends Endocrinol Metab. 1996;7(1):22–7. doi: 10.1016/1043-2760(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 25.Paller MS. Pathophysiologic mechanisms of acute renal failure. In: Goldstein RS, editor. Mechanisms of Injury in Renal Disease and Toxicity. Ann Arbor: CRC Press; 1994. p. 3-13.
- 26.Quiroz Y, Ferrebuz A, Romero F, Vaziri ND, Rodriguez-Iturbe B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am J Physiol Renal Physiol. 2008;294(2):F336–44. doi: 10.1152/ajprenal.00500.2007. [DOI] [PubMed] [Google Scholar]
- 27.Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999;277(3 Pt 2):R922–9. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- 28.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol. 2003;285(1):F87–94. doi: 10.1152/ajprenal.00026.2003. [DOI] [PubMed] [Google Scholar]
- 29.Sølling C, Christensen AT, Nygaard U, Krag S, Frøkiaer J, Wogensen L. et al. Erythropoietin does not attenuate renal dysfunction or inflammation in a porcine model of endotoxemia. Acta Anaesthesiol Scand. 2011;55(4):411–21. doi: 10.1111/j.1399-6576.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 30.Kireev RA, Cuesta S, Ibarrola C, Bela T, Moreno Gonzalez E, Vara E. et al. Age-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatonin. J Surg Res. 2012;178(2):922–34. doi: 10.1016/j.jss.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 31.Gobe G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol. 2000;11(3):454–67. doi: 10.1681/ASN.V113454. [DOI] [PubMed] [Google Scholar]
- 32.Tunon MJ, San Miguel B, Crespo I, Jorquera F, Santamaria E, Alvarez M. et al. Melatonin attenuates apoptotic liver damage in fulminant hepatic failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2011;50(1):38–45. doi: 10.1111/j.1600-079X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006;69(10):1806–13. doi: 10.1038/sj.ki.5000356. [DOI] [PubMed] [Google Scholar]