Abstract
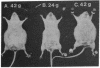
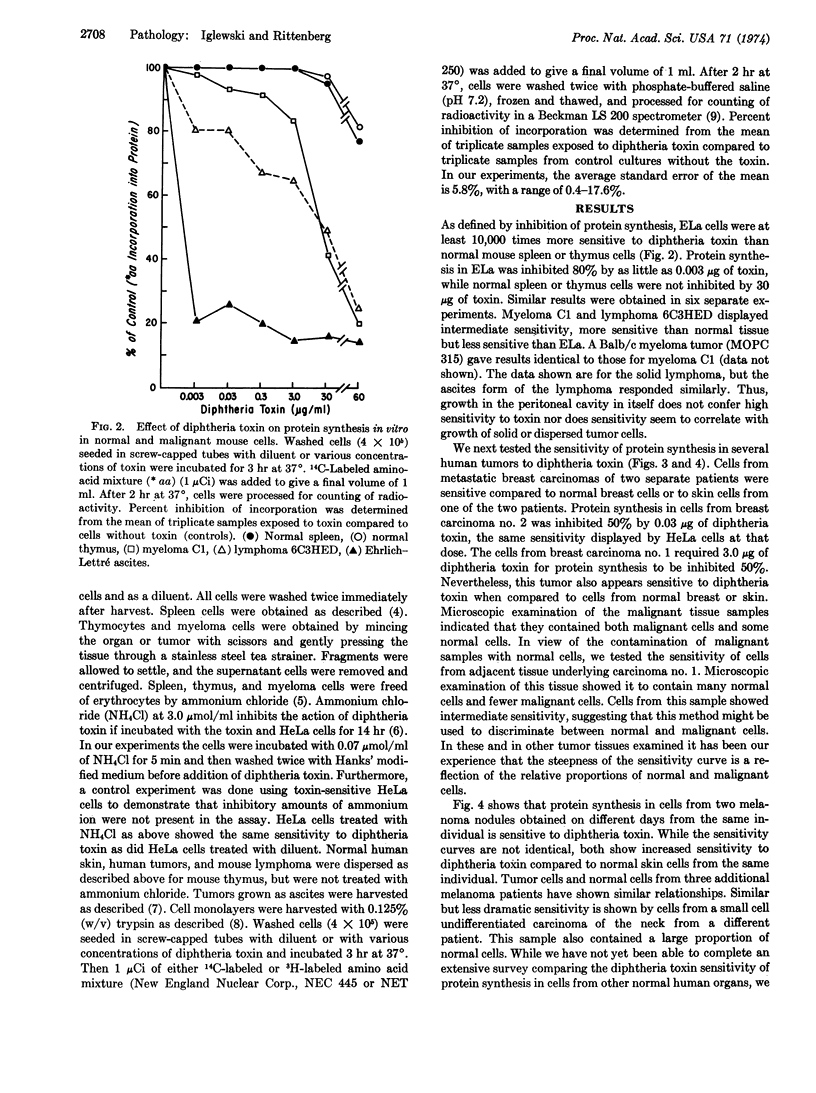
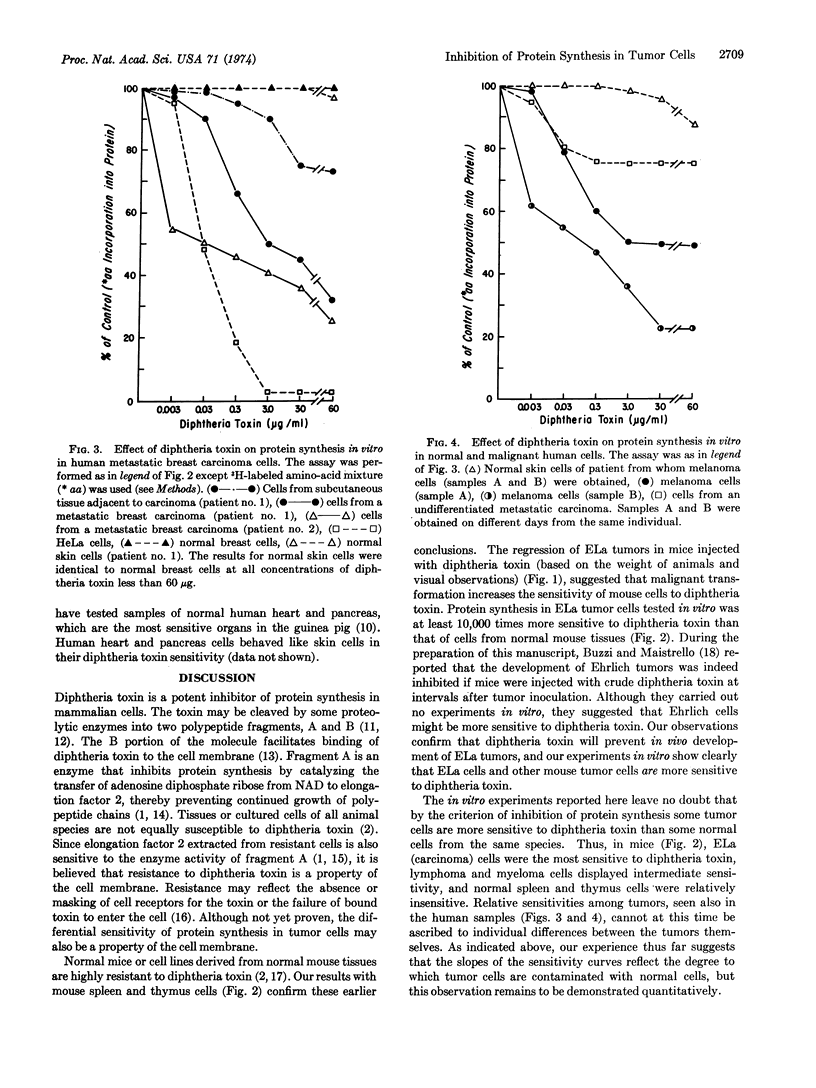
Purified diphtheria toxin is shown to inhibit protein synthesis in Ehrlich-Lettré ascites carcinoma cells in vitro. Protein synthesis in Ehrlich-Lettré cells is at least 10,000 times more sensitive to toxin than protein synthesis in normal mouse spleen or thymus cells. This sensitivity correlates with the observation that Ehrlich-Lettré tumors regress in mice injected with diphtheria toxin but not diphtheria toxoid. Using the criterion of inhibition of protein synthesis in vitro, we show that other mouse malignancies (lymphoma and myeloma) are also more sensitive to diphtheria toxin than normal spleen or thymus. Metastatic human breast carcinoma cells from two individuals, cells from two melanoma nodules removed at different times from a third patient, and cells from melanoma nodules from three additional individuals are shown to be more sensitive to diphtheria toxin than some normal human cells. The toxin sensitivity of protein synthesis in some of the malignant cells tested was so much greater than that of normal cells, that we have proposed that diphtheria toxin should be studied further since it might prove a useful anti-cancer agent in patients whose tumors are first shown to be highly sensitive to toxin in vitro.
Keywords: cancer, protein synthesis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonventre P. F., Imhoff J. G. Studies on the mode of action of diphtheria toxin. I. Protein synthesis in guinea pig tissues. J Exp Med. 1966 Dec 1;124(6):1107–1122. doi: 10.1084/jem.124.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bullock W. W., Rittenberg M. B. In vitro-initiated secondary anti-hapten response. II. Increasing cell avidity for antigen. J Exp Med. 1970 Nov;132(5):926–940. doi: 10.1084/jem.132.5.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi S., Maistrello I. Inhibition of growth of Erlich tumors in Swiss mice by diphtheria toxin. Cancer Res. 1973 Oct;33(10):2349–2353. [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- GABLIKS J., SOLOTOROVSKY M. Cell culture reactivity to diphtheria, Staphylococcus, tetanus and Escherichia coli toxins. J Immunol. 1962 Apr;88:505–512. [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr, Ames E. Studies on the mode of action of diphtheria toxin. V. Inhibition of peptide bond formation by toxin and NAD in cell-free systems and its reversal by nicotinamide. J Exp Med. 1967 Nov 1;126(5):923–939. doi: 10.1084/jem.126.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Kato I., Hayaishi O. Adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis by diphtheria toxin. J Biol Chem. 1971 Jul 10;246(13):4251–4260. [PubMed] [Google Scholar]
- Hotham-Iglewski B., Ludwig E. H. A cytocidal system in normal human serum active against Ehrlich Lettré ascites tumor cells. I. Identification of the components of the system and site of attachment. Cancer Res. 1967 Jan;27(1):181–184. [PubMed] [Google Scholar]
- Hotham-Iglewski B., Ludwig E. H. Effect of cortisone on activation of lysosomal enzymes resulting from mengovirus infection of L-929 cells. Biochem Biophys Res Commun. 1966 Jan 24;22(2):181–186. doi: 10.1016/0006-291x(66)90429-3. [DOI] [PubMed] [Google Scholar]
- Kim K., Groman N. B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J Bacteriol. 1965 Dec;90(6):1552–1556. doi: 10.1128/jb.90.6.1552-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The response of cultured mammalian cells to diphtheria toxin. II. The resistant cell: enhancement of toxin action by poly-L-ornithine. J Exp Med. 1968 Mar 1;127(3):541–554. doi: 10.1084/jem.127.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. 3. Inhibition of protein synthesis studied at the subcellular level. J Bacteriol. 1968 Jul;96(1):61–69. doi: 10.1128/jb.96.1.61-69.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]