Abstract
Purpose: Stem cell factor (SCF) plays an important role in the survival, proliferation and differentiation of hematopoietic stem cells and progenitor cells. Potential therapeutic applications of SCF include hematopoietic stem cell mobilization, exvivo stem/progenitor cell expansion, gene therapy, and immunotherapy. Considering the cost and problem in accessibility of this product in Iran, clears the importance of indigenizing production of rhSCF. In the present work, we describe the construction of the soluble rhSCF expression vector in pET-26b (+) with periplasmic localization potential.
Methods: Following PCR amplification of human SCF ORF, it is cloned in pET-26b (+) vector in NcoI and XhoI sites. The recombinant construct was transformed into BL21 (DE3) Ecoli strains.
Results: The construction of recombinant vector was verified by colony PCR and sequence analysis of pET26b-hSCF vector. Sequence analyses proved that human SCF ORF has been inserted into NcoI and XhoI site with correct orientation downstream of strong T7 promotor and showed no nucleotide errors.
Conclusion: The SCF ORF was successfully cloned in pET-26b (+) expression vector and is ready for future production of SCF protein.
Keywords: Human SCF, Cloning, pET-26b(+)
Introduction
Hematopoiesis is regulated by a number of cytokines that promote the survival, proliferation, and differentiation of hematopoietic stem cells and progenitor cells.1 Stem Cell Factor (SCF) plays an important role in hematopoiesis, spermatogenesis, and melanogenesis. Biological effects of SCF; as a hematopoietic cytokine; is triggered by binding to its ligand c-kit.1 SCF is encoded by the Sl locus on mouse chromosome and has been mapped to human chromosome 12q22-12q24. The soluble and transmembrane types of SCF are produced by alternative splicing that includes or excludes a proteolytic cleavage site in exon 6. Both the soluble and the transmembrane type of SCF are biologically active.2-8 Translation of mRNA including exon 6 encodes a proteolytic cleavage site, resulting in the production of soluble SCF (SCF248). In soluble form, the cleavage arises after Ala165. In contrast, the lack of exon 6 in human SCF220 results in production of the transmembrane form of human SCF. In SCF220, amino acids 149-177 are substituted by a Gly residue. The soluble form of SCF circulates as a noncovalently bonded dimer, is glycosylated, and has substantial secondary structure, containing regions of α helices and ß sheets. The molecular weight of the soluble type of SCF considered from its amino acid sequence is about 18.5 KD. Expression of SCF in Chinese hamster ovary (CHO) cells produce proteins of 28 to 40 KD, reflecting the presence of extensive and heterogeneous glycosylation.9 Even though an active dimeric form of SCF with 4 intermolecular disulfide bonds has been recognized during oxidation and refolding of recombinant SCF expressed in Escherichia coli, neither Chinese hamster ovary-expressed SCF nor native SCF dimers have been stated to contain intermolecular disulfide bonds, so it seems unlikely that this form of SCF plays a major role in vivo.10-11
Recombinant human SCF has major clinical potential through its synergy with other factors, to enhance hematopoietic stem cell mobilization.12,14 SCF is also useful in gene therapy as hematopoietic cells exposed to SCF either in vivo or in vitro are more efficiently transduced by retroviral vectors.2 Ex vivo expansion of hematopoietic stem cells and progenitor cells is another potential application for SCF.15-18 Considering the various applications of SCF and its high cost, production of human SCF as a recombinant protein is a necessity in our country. In the present work, we describe the construction of the soluble rhSCF expression vector in pET-26b(+) under the control of T7 promotor in bacterial host. This vector carries PelB signal sequence for potential periplasmic localization. In most cases, targeting protein production to the periplasmic space facilitates downstream processing, folding, and in vivo stability, enabling production of soluble and biologically active proteins at a reduced process cost.
Materials and Methods
Strains, plasmids and culture media
DH5-α and BL21 (DE3) Ecoli strains were used as the hosts for recombinant plasmid. BL21 (DE3) Ecoli is an E. coli B strain with DE3, a λ prophage carrying the T7 RNA polymerase gene. pET-26b(+) Vector (Novagen, USA) was used as the expression vector in experiments. pET-26b(+) (Cat. No. 69862-3) is a bacterial expression vector with the size of 5.5 kb containing PelB sequence for periplasmic localization. LB agar and Broth were used for culturing the strains. cDNA of soluble human SCF was provided from genecopea company.
Amplification of ORF SCF gene with PCR
ORF of human SCF gene was amplified by PCR using the following primers: SCF-E-Fwd 5΄CATCCATGGAAGGGATCTGCAGGAATCGT3΄ and SCF-E-Rev 5΄TATCTCGAGGGCTGCAACAGGGGG TAACAT3΄. The underlined bases designate NcoI and XhoI restriction sites. The PCR mixture consisted of 5 μL of 10 × PCR buffer, 3 mM MgCl2, 0.2 mM for each dNTP, 250 nM for each primer, 1 μL of template DNA, and 5 units of Pfu DNA polymerase (fermentas) in the final volume of 50 μL. The amplification consisted of 35 cycles on a thermocycler (Eppendorf) as follows: preliminary denaturation for 5 min at 95 °C followed by 10 cycles including denaturation for 30sec at 95 °C, annealing for 30 sec at 58 °C and extention for 30 sec at 72 °C, subsequently 25 cycle including denaturation for 30sec at 95 °C, annealing for 30 sec at 63˚C and extention for 30sec at 72 °C and final extention for 5min at 72 °C. PCR product fragment was electrophoresed on the 1% agarose gel and stained with ethidium bromide. After the PCR process, the amplified DNA fragments are size-separated by agarose gel electrophoresis and purified using the QIAquick Gel Extraction kit (QIAGEN).
Construction of the expression vector pET-26b(+)-hSCF
The PCR product and the pET-26b(+) vector were double-digested with the NcoI and the Xho1 restriction endonucleases for 12h at 37 °C. Then digested fragments were electrophoresed on the 0.8% agarose gel stained with ethidium bromide. Subsequently, fragments were purified using QIAquick Gel Extraction kit (QIAGEN) following manufacturer's instructions. Ligation was performed using T4 DNA ligase enzyme (Frementas) according to the manufacturer’s instructions. Briefly 100 ng of purified double-digested pET-26b(+) and 3 - 5 fold molar excess of insert were incubated with T4 DNA ligase enzyme and 10X T4 DNA ligase buffer at 22 °C for at least 6 hr. The recombinant vector were transformed into the competent DH5α by standard calcium chloride method. Transformants were selected on LB medium containing kanamycin (50 µg /mL). A single colony of E. coli cells carrying the ligated plasmid was grown in 3 ml LB medium containing kanamycin. Plasmid extraction was performed using the GeneJETTM Plasmid Miniprep kit (Fermentas). Subsequently clones containing ligated plasmid were screened by PCR and sequencing methods using the mentioned primers. Then the construct was transformed into BL21 (DE3) Ecoli strains and were selected on LB containing kanamaycin (50 µg /mL).
Results
Amplification of ORF SCF gene with PCR
ORF SCF gene without stop codon was amplified by SCF-E-Fwd and SCF-E-Rev primers which contains restriction sites. NcoI and XhoI restriction sites were introduced at the 5´ and 3´ of the ORF SCF gene respectively, therefore the coding sequence was preceded by a pelB signal sequence at the 5׳ region and a 6 His-tag at the 3׳ of the gene. Successful amplification of 495 bp of SCF ORF was visualized on 1% Agarose by UV transilluminator (Figure 1).
Figure 1 .
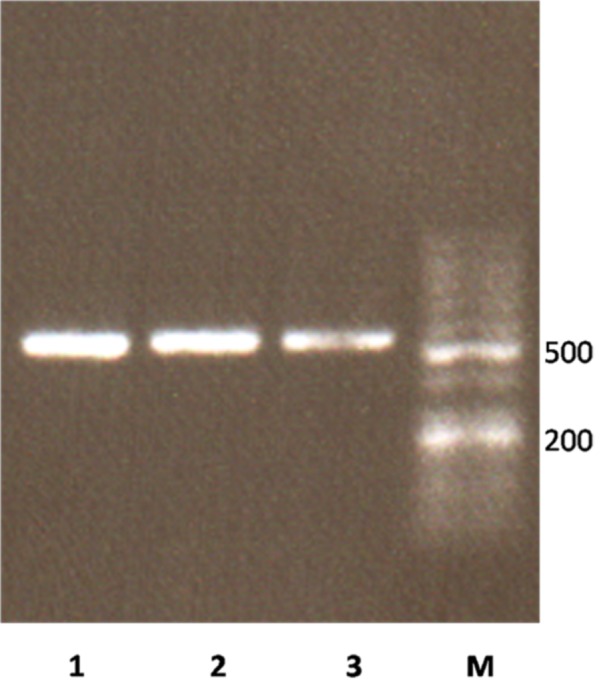
Agarose gel electrophoresis of amplified hSCF ORF. Lane M, 50bp DNA Ladder (Frementas); Lane 1-3, amplified ORF of hSCF gene
Construction of the expression vector pET-26b(+)-hSCF
Subsequent to digestion of PCR product and pET-26b(+) vector, in order to purify DNA fragments from gel, digested fragments were resolved onto ethidium-bromide stained 0.8% agarose gel and visualized by UV transilluminator (Figure 2). In electrophoresis of intact vector, two different bonds can be detected. The upper band belongs to coiled vector and lower belongs to supercoiled vector. Digested vector (Lane 2) is placed between these two bands. Following ligation, the construct was transformed into DH5-α E.coli cells and were selected on LB containing kanamaycin (50 µg /mL). Transformants were characterized by colony PCR screening using the mentioned primers and a single band of the expected size (495 bp) corresponding to human SCF ORF was detected by agarose gel electrophoresis (Figure 3). The recombinant pET26b-hSCF plasmid was extracted and sequence analysis of recombinant pET-26b(+)-hSCF plasmid with the mentioned primers confirmed that there are no amplification errors and experiment of cloning was accurate (Figure 4, 5). Then the construct was transformed into BL21 (DE3) Ecoli strains and were selected on LB containing kanamaycin (50 µg /mL). This approach allowed heterologous gene insertion between the T7 promoter sequence and the transcription termination sequence.
Figure 2 .
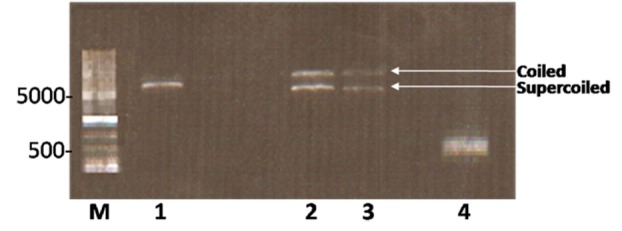
Agarose gel electrophoresis of digested pET-26b(+) and PCR product with Xho1 and Nco1 restriction enzymes. Lane M shows 1kbp DNA Ladder (Frementas); Lane 1 corresponds to digested pET-26b(+) vector; Lanes 2 and 3 correspond to undigested the pET-26b(+); Lane 4 illustrates digested PCR product.
Figure 3 .
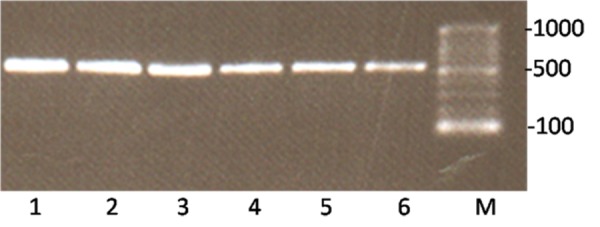
Colony PCR screening of cloned rhSCF. The clones on kanamycin plates were picked and screened by PCR. Lane M corresponds to 100bp ladder (Fermentas). Lane 1-5 represent bacterial clone containing amplified ORF of rhSCF.
Figure 4 .
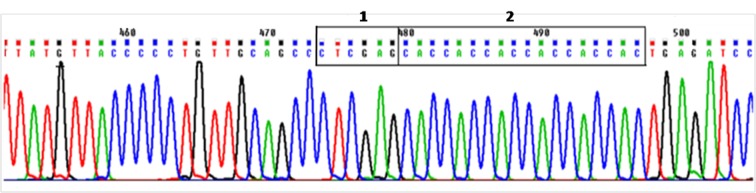
Sequencing analysis of recombinant pET-26b(+)-hSCF plasmid with SCF-E-Fwd primer. The box 1 shows restriction site of enzyme XhoI which located after ORF of SCF gene, the box 2 shows His- Tag that located after ORF of SCF gene and restriction site of enzyme XhoI.
Figure 5 .

Sequencing analysis of recombinant pET-26b(+)-hSCF with SCF-E-Rev primer. The box 1 shows restriction site of NcoI before ORF of SCF gene, the Box 2 shows initiation codon of SCF gene that located before PelB signal peptide(underlined).
Discussion
A number of elements are essential in the design of recombinant expression systems. The genetic elements of the expression plasmid include origin of replication (ori), transcriptional promoters, an antibiotic resistance marker, translation initiation regions (TIRs) as well as transcriptional and translational terminators.19,20 There are many promoters accessible for gene expression in E.coli, including those derived from gram positive bacteria and bacteriophages. An ideal promoter presents several preferable features: it is strong, it has a low basal expression level, it is easily transportable to other E. coli strains to simplify testing of an immense number of strains for protein products, and its induction is uncomplicated and cost-effective. Unlike systems based on E. coli promoters (e.g., lac, tac, pL), the pET System uses the bacteriophage T7 promoter to manage the expression of target genes. Since E.coli RNA polymerase does not distinguish the T7 promoter, there is actually no transcription of the target gene in the absence of a source of T7 RNA polymerase and the cloning step is thus effectively separated from the expression step. Many genes that have been hard to make in E. coli promoter-based systems have been stably cloned and expressed in the pET System.21-25
The periplasm presents some advantages for protein targeting. The target protein is thus drastic concentrated, and its purification is extremely less onerous. The oxidizing milieu of the periplasm promotes the correct folding of proteins, and the cleaving in vivo of the signal peptide during translocation to the periplasm is more probable to yield the genuine N terminus of the target protein. Protein degradation in the periplasm is also less extensive. The transport of a protein through the inner membrane to the periplasm generally requires a signal sequence.26-41 A wide diversity of signal peptides has been used successfully in E.coli for protein translocation to the periplasm. The pET-26b(+) vector produces recombinant protein with signal peptide pelB at the N-terminal for periplasmic secretion and a His-tag at the C-terminal for detection and purification.
In this study SCF gene was cloned correctly and colony PCR and sequence analysis of the recombinant pET-26b(+)-hSCF confirmed that there are no amplification errors and that cloning was accurate.
Conclusion
The SCF ORF was successfully cloned in pET-26b(+) expression vector and is ready for future production of SCF protein. The production of recombinant hSCF in Iran will facilitate clinical treatment of anemia (as it mobilizes hematopoietic stem cell) and gene therapy. In addition smooth the progress of ex vivo expansion of hematopoietic stem cells and progenitor cells.
Acknowledgments
We thanks “Staff Development and application of stem cell research” of “Vice President Strategic Technology Office of Science and Technology” for financial support.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res . 2008;14(7):1926–30. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- 2.Broudy VC. Stem cell factor and hematopoiesis. Blood . 1997;90(4):1345–64. [PubMed] [Google Scholar]
- 3.Broudy VC, Lin NL, Priestley GV, Nocka K, Wolf NS. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood . 1996;88(1):75–81. [PubMed] [Google Scholar]
- 4.Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood . 1998;91(4):1101–34. [PubMed] [Google Scholar]
- 5.Yin T, Li L. The stem cell niches in bone. J Clin Invest . 2006;116(5):1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Chen X, Focia PJ, He X. Structural basis for stem cell factor-KIT signaling and activation of class III receptor tyrosine kinases. EMBO J . 2007;26(3):891–901. doi: 10.1038/sj.emboj.7601545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graw J, Loster J, Neuhauser-Klaus A, Pretsch W, Schmitt-John T. Molecular analysis of two new Steel mutations in mice shows a transversion or an insertion. Mamm Genome . 1996;7(11):843–6. doi: 10.1007/s003359900247. [DOI] [PubMed] [Google Scholar]
- 8.Bedell MA, Copeland NG, Jenkins NA. Multiple pathways for Steel regulation suggested by genomic and sequence analysis of the murine Steel gene. Genetics . 1996;142(3):927–34. doi: 10.1093/genetics/142.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu HS, Clogston CL, Wypych J, Parker VP, Lee TD, Swiderek K. et al. Post-translational processing of membrane-associated recombinant human stem cell factor expressed in Chinese hamster ovary cells. Arch Biochem Biophys . 1992;298(1):150–8. doi: 10.1016/0003-9861(92)90106-7. [DOI] [PubMed] [Google Scholar]
- 10.Jones MD, Narhi LO, Chang WC, Lu HS. Refolding and oxidation of recombinant human stem cell factor produced in Escherichia coli. J Biol Chem . 1996;271(19):11301–8. doi: 10.1074/jbc.271.19.11301. [DOI] [PubMed] [Google Scholar]
- 11.Lu HS, Jones MD, Shieh JH, Mendiaz EA, Feng D, Watler P. et al. Isolation and characterization of a disulfide-linked human stem cell factor dimer. Biochemical, biophysical, and biological comparison to the noncovalently held dimer. J Biol Chem. 1996;271(19):11309–16. doi: 10.1074/jbc.271.19.11309. [DOI] [PubMed] [Google Scholar]
- 12.Costa JJ, Demetri GD, Harrist TJ, Dvorak AM, Hayes DF, Merica EA. et al. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J Exp Med . 1996;183(6):2681–6. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver A, Ryder D, Crowther D, Dexter TM, Testa NG. Increased numbers of long-term culture-initiating cells in the apheresis product of patients randomized to receive increasing doses of stem cell factor administered in combination with chemotherapy and a standard dose of granulocyte colony-stimulating factor. Blood . 1996;88(9):3323–8. [PubMed] [Google Scholar]
- 14.Nervi B, Link DC, Dipersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem . 2006;99(3):690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 15.Gammaitoni L, Bruno S, Sanavio F, Gunetti M, Kollet O, Cavalloni G. et al. Ex vivo expansion of human adult stem cells capable of primary and secondary hemopoietic reconstitution. Exp Hematol . 2003;31(3):261–70. doi: 10.1016/s0301-472x(02)01077-9. [DOI] [PubMed] [Google Scholar]
- 16.Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res . 2003;16(3):287–96. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 17.Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res . 2008;14(7):1926–30. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- 18.Bowie MB, Kent DG, Copley MR, Eaves CJ. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood . 2007;109(11):5043–8. doi: 10.1182/blood-2006-08-037770. [DOI] [PubMed] [Google Scholar]
- 19.Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol . 1999;10(5):411–21. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 20.Jonasson P, Liljeqvist S, Nygren PA, Stahl S. Genetic design for facilitated production and recovery of recombinant proteins in Escherichia coli. Biotechnol Appl Biochem . 2002;35(Pt 2):91–105. doi: 10.1042/ba20010099. [DOI] [PubMed] [Google Scholar]
- 21.Khlebnikov A, Keasling JD. Effect of lacY expression on homogeneity of induction from the P(tac) and P(trc) promoters by natural and synthetic inducers. Biotechnol Prog . 2002;18(3):672–4. doi: 10.1021/bp010141k. [DOI] [PubMed] [Google Scholar]
- 22.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol . 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 23.Swartz JR. Advances in Escherichia coli production of therapeutic proteins. Curr Opin Biotechnol . 2001;12(2):195–201. doi: 10.1016/s0958-1669(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 24.Vethanayagam JG, Flower AM. Decreased gene expression from T7 promoters may be due to impaired production of active T7 RNA polymerase. Microb Cell Fact . 2005;4(1):3. doi: 10.1186/1475-2859-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jana S, Deb JK. Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol . 2005;67(3):289–98. doi: 10.1007/s00253-004-1814-0. [DOI] [PubMed] [Google Scholar]
- 26.Rietsch A, Beckwith J. The genetics of disulfide bond metabolism. Annu Rev Genet . 1998;32:163–84. doi: 10.1146/annurev.genet.32.1.163. [DOI] [PubMed] [Google Scholar]
- 27.Bessette PH, Aslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci U S A . 1999;96(24):13703–8. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann K, Hoffmann S, Neudecker P, Suhr M, Becker WM, Rosch P. High-yield expression in Escherichia coli, purification, and characterization of properly folded major peanut allergen Ara h 2. Protein Expr Purif . 2003;31(2):250–9. doi: 10.1016/s1046-5928(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 29.Premkumar L, Bageshwar UK, Gokhman I, Zamir A, Sussman JL. An unusual halotolerant alpha-type carbonic anhydrase from the alga Dunaliella salina functionally expressed in Escherichia coli. Protein Expr Purif . 2003;28(1):151–7. doi: 10.1016/s1046-5928(02)00683-6. [DOI] [PubMed] [Google Scholar]
- 30.Stewart EJ, Aslund F, Beckwith J. Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins. EMBO J . 1998;17(19):5543–50. doi: 10.1093/emboj/17.19.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manting EH, Driessen AJ. Escherichia coli translocase: the unravelling of a molecular machine. Mol Microbiol . 2000;37(2):226–38. doi: 10.1046/j.1365-2958.2000.01980.x. [DOI] [PubMed] [Google Scholar]
- 32.Debarbieux L, Beckwith J. The reductive enzyme thioredoxin 1 acts as an oxidant when it is exported to the Escherichia coli periplasm. Proc Natl Acad Sci U S A . 1998;95(18):10751–6. doi: 10.1073/pnas.95.18.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonda S, Huber-Wunderlich M, Glockshuber R, Mossner E. Complementation of DsbA deficiency with secreted thioredoxin variants reveals the crucial role of an efficient dithiol oxidant for catalyzed protein folding in the bacterial periplasm. EMBO J . 1999;18(12):3271–81. doi: 10.1093/emboj/18.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shokri A, Sanden AM, Larsson G. Cell and process design for targeting of recombinant protein into the culture medium of Escherichia coli. Appl Microbiol Biotechnol . 2003;60(6):654–64. doi: 10.1007/s00253-002-1156-8. [DOI] [PubMed] [Google Scholar]
- 35.Ray MV, Meenan CP, Consalvo AP, Smith CA, Parton DP, Sturmer AM. et al. Production of salmon calcitonin by direct expression of a glycine-extended precursor in Escherichia coli. Protein Expr Purif . 2002;26(2):249–59. doi: 10.1016/s1046-5928(02)00523-5. [DOI] [PubMed] [Google Scholar]
- 36.Mavrangelos C, Thiel M, Adamson PJ, Millard DJ, Nobbs S, Zola H. et al. Increased yield and activity of soluble single-chain antibody fragments by combining high-level expression and the Skp periplasmic chaperonin. Protein Expr Purif . 2001;23(2):289–95. doi: 10.1006/prep.2001.1506. [DOI] [PubMed] [Google Scholar]
- 37.Mergulhao FJ, Monteiro GA, Larsson G, Bostrom M, Farewell A, Nystrom T. et al. Evaluation of inducible promoters on the secretion of a ZZ-proinsulin fusion protein in Escherichia coli. Biotechnol Appl Biochem . 2003;38(Pt 1):87–93. doi: 10.1042/BA20030043. [DOI] [PubMed] [Google Scholar]
- 38.Couprie J, Vinci F, Dugave C, Quemeneur E, Moutiez M. Investigation of the DsbA mechanism through the synthesis and analysis of an irreversible enzyme-ligand complex. Biochemistry . 2000;39(22):6732–42. doi: 10.1021/bi992873f. [DOI] [PubMed] [Google Scholar]
- 39.Paal M, Heel T, Schneider R, Auer B. A novel Ecotin-Ubiquitin-Tag (ECUT) for efficient, soluble peptide production in the periplasm of Escherichia coli. Microb cell fact . 2009;8:7. doi: 10.1186/1475-2859-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luirink J, Sinning I. SRP-mediated protein targeting: structure and function revisited. Biochim Biophys Acta . 2004;1694(1-3):17–35. doi: 10.1016/j.bbamcr.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Stirnimann CU, Grutter MG, Glockshuber R, Capitani G. nDsbD: a redox interaction hub in the Escherichia coli periplasm. Cell Mol Life Sci . 2006;63(14):1642–8. doi: 10.1007/s00018-006-6055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


