1 Abstract
Most facets of mammalian physiology and behavior vary according to time-of-day thanks to an endogenous “circadian” clock. Therefore, it is not surprising that many aspects of pharmacology and toxicology also oscillate according to the same 24-hour clocks. Daily oscillations in abundance of proteins necessary for either drug absorption or metabolism result in circadian pharmacokinetics; and oscillations in the physiological systems targeted by these drugs result in circadian pharmacodynamics. These clocks are present in most cells of the body, but organized in hierarchical fashion. Interestingly, some aspects of physiology and behavior are controlled directly via a “master clock” in the suprachiasmatic nuclei of the hypothalamus, while others are controlled by “slave” oscillators in separate brain regions or body tissues. Recent research shows that these clocks can respond to different cues, and thereby show different phase relationships. Therefore, full prediction of chronopharmacology in pathological contexts will likely require a systems biology approach considering “chrono-interactions” among different clock-regulated systems.
Keywords: circadian rhythms, drug metabolism, chronotherapy, cancer, peripheral oscillators, systems biology
2 Introduction
As a result of living on a planet whose principal source of light and heat is only periodically present, organisms on Earth rapidly adapted physiological systems to exploit these variations for maximum fitness. Collectively, these clocks are named “circadian” (Latin circa diem – about a day). In mammals, circadian clocks influence all major organ systems, and this influence translates directly into disease pathology that also varies with time of day. Historically, it was early recognized that rhythmic physiology resulted in rhythmic disease symptoms. Hippocrates already noticed ca. 400 BC that daytime sleepiness is indicative of disease, and nighttime sleeplessness can indicate pain and suffering (1). By medieval times, reports existed of daily variations in diseases such as bronchial asthma (2). For over thirty years, it has been known that drug absorption and distribution is subjected to diurnal variation in rodents and humans. A twenty-four hour change in drug bioavailability has therefore been established for hundreds of drugs in rodents and humans. For example, acetaminophen (3) or theophylline (4) show different pharmacokinetics in the morning compared to evening. These changes are the results of several time-dependent modifications of physiological and molecular aspects that influence drug absorption and distribution.
Considering the wide scope of circadian (patho-) physiology, it is logical that the pharmacodynamics and pharmacokinetics (PK/PD) of many drugs would be circadian, and therefore that drug efficacy and safety profiles would also vary with time of day. Nevertheless, this variation is only seldom considered by clinicians, drug developers, or regulators. In part, this apathy may stem from a lack of insight into the molecular mechanisms governing this control. However, two decades of intensive research have uncovered a wealth of information not only about basic mechanisms of circadian clocks, but also about how they interact with physiology and disease. Below, we review this knowledge on cellular and systems levels, and then consider its implications for pharmaceutical intervention.
3 Molecular fundamentals of circadian clocks
The basic unit of circadian timekeeping is the cell: even in very complex organisms, most cells contain autonomous circuitry for circadian oscillations. Generally speaking, this mechanism is comprised of negative feedback loops of transcription and translation: activation of a repressor gene results in its later repression by its own protein product, and the instability of this repressor insures this repression is short-lived, so that a new cycle can begin. In mammals, the principal activators within this system are the CLOCK and BMAL1 proteins and their homologs, which dimerize and bind to cis-acting E-box elements (with the simple consensus DNA sequence CAANTG) to activate transcription of a large number of circadian genes. Among these genes are loci encoding the PERIOD and CRYPTOCHROME families of repressor proteins (PER1-3 and CRY1-2), whose products multimerize and suppress the CLOCK:BMAL1 activating complex. Also among the genes activated by CLOCK:BMAL1 is the Rev-Erbα gene, which encodes a nuclear orphan receptor protein that together with its sister protein REV-ERBβ represses Bmal1 transcription in a parallel but interlocked loop. The ROR family of transcriptional activators likely competes with the REV-ERB family of repressors for the same binding sites, adding further cooperativity to the transition mechanism. Numerous reviews have been written about this basic oscillatory circuitry (5).
At each of these steps, additional precision and regulatory finesse is achieved through interaction with a wide range of auxiliary proteins: kinases that phosphorylate clock proteins to modigy their stability or activity (6); chromatin modifying proteins that phosphorylate, acetylate, or deacetylate histones and in some cases clock proteins to regulate chromatin structure and transcriptional activation potential (7); and RNA-binding proteins that serve as scaffolds for coactivating and corepressing activities (8). This “basic” clock mechanism is summarized in Figure 1. A parallel and independent circadian mechanism independent of transcription also exists in parallel to the “canonical” transcription-translation-based clock in mammalian cells. Evidence of this oscillator exists in the form of diurnal variation in oxidation states of hemoglobin and antioxidant molecules (9). Both the mechanism and the physiological relevance of these posttranslational clocks remain unknown in mammals, though posttranslational clocks based on phosphorylation are well studied in bacteria (10).
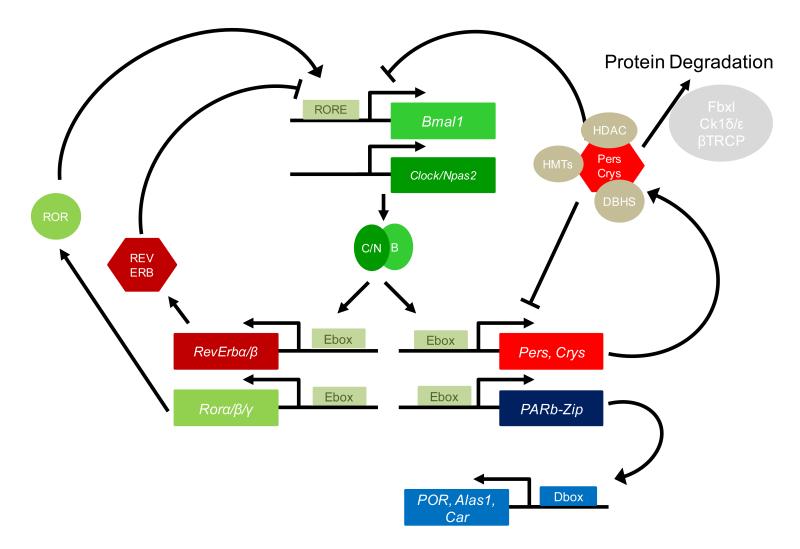
Figure 1. The canonical mammalian circadian oscillator and output relevant for xenobiotic metabolism.
Two interlocked feedback loops composed of activators (green) and repressors (red) that drive gene expression of output genes such as those important for xenobiotic metabolism. Components of these loops make extensive use of auxiliary factors including histone methyltransferases (HMTs), histone deacetylases (HDACs), DHBS family RNA-binding proteins (beige), and kinases and proteasome machinery (grey). Important output genes, involved in transcriptional control of the detoxification metabolism (blue). See text for details.
3.1 Hierarchical Organization of Clocks
The basic timekeeping mechanism of circadian oscillators is cell-autonomous, and self-sustained clocks exist in most cells of the body. However, under most circumstances these clocks are organized into a hierarchy: a “master clock” tissue within the suprachiasmatic nuclei (SCN) of the hypothalamus receives light input via the retina, and communicates timing signals to “slave” oscillators of similar molecular mechanism in cells from other tissues. Multiple redundant signals have been described. These include direct signals like innervation by the autonomous nervous system and hormones like glucocorticoids, and indirect signals emanating from SCN-controlled rhythmic behavior, such as timing of food intake and small rhythmic changes in body temperature from activity (11).
Under most circumstances, entrainment signals from the SCN to clocks in peripheral tissues act in concerted fashion, resulting in somewhat coherent phase among different organs. The exact phase of circadian clocks varies somewhat from organ to organ, perhaps because of tissue-specific differences in clock gene expression, or perhaps due to local differences in accessibility to entrainment signals. These differences become particularly acute under certain perturbation. For example, during an altered lighting cycle caused by time zone travel or shiftwork, the SCN will shift its phase much more quickly (within a day or two) than peripheral clocks (which can take a week or more), creating a situation in which clocks in different organs exhibit gross differences in “internal clock time” (12). Similarly, systematic manipulation of external cues such as feeding time to different phases of the light-dark cycle result in a phase change for peripheral clocks, but not for the SCN (13).
This hierarchical clock structure has two important implications for chronopharmacology. First, if clocks in different tissues govern different aspects of drug activity and metabolism – a topic that we explore in detail below – then these different phases must be considered in calculating the timing for optimal drug efficacy. The situation is further complicated because recent research suggests that these phase relationships are altered by age: older rodents show later SCN phases but earlier peripheral clock phases (14). Secondly, increasing evidence suggests that chronic circadian dysphasing by itself has significant negative consequences for health; either for rodents subjected to laboratory conditions of chronic jetlag or shiftwork, or in humans subjected to similar stresses. Documented changes include cancer susceptibility, inflammation, and altered metabolism (15-17). Thus, increasing evidence suggests that basal physiology may differ in individuals with clock disruption. Such an observation is particularly relevant to pharmacology because many diseases ranging from psychiatric and neurodegenerative disorders to cancer are themselves associated with mild to severe clock disruption (18; 19). The question of how disease specific to a peripheral organ might affect clockwork within this tissue has not been studied at all, but could also be highly pertinent to pharmacology.
3.2 Circadian control of cellular physiology
Whereas SCN clocks are entrained by light, and peripheral clocks are entrained by indirect and hormonal cues, individual aspects of cellular physiology are in turn directed by both local and central clocks through a variety of mechanisms. One fundamental mechanism is via transcription: in total, about ten percent of all transcripts in each tissue are regulated in circadian fashion (20; 21). In large part, this regulation occurs through the same cis-acting promoter elements that direct the rhythmic transcription of clock genes themselves, for example the E-boxes that serve as platforms for activation by CLOCK and BMAL1, and the RRE-elements that respond to REV-ERB proteins. Regulation of additional genes can occur through cascades of clock-regulated transcription factors. Among the best-studied are the PAR-bZIP family of factors: DBP, TEF, and HLF. The D-elements to which they bind control circadian expression of several families of genes, including liver metabolic regulators critical to circadian control of pharmacokinetics for a wide variety of drugs (22). Modeling studies suggest that simple combinations of these three elements – E-boxes, D-boxes, and RRE-elements, each with maximal occupancy at a certain time of day – can direct circadian transcription in any phase, and are responsible for a large portion of circadian transcription directed by cellular clocks (23).
Nevertheless, this mechanism represents only a portion of circadian transcription in living animals. Experiments in mice lacking functional clocks in specific tissues show that only a portion of circadian gene expression is abolished by such manipulations, while another portion persists because it is systemically driven (24). A portion of this transcription likely arises through rhythmic activity of the hypothalamic-pituitary-adrenal axis, and another portion through circadian stimulation of action/SRF signaling by unknown ligands (25). Additional contributions likely arise from heat shock signaling and immune signaling, also regulated by time of day. In all four cases, specific externally-activated transcription factors bind to cis-acting elements to drive transcription of certain genes. For example, rhythmic glucocorticoid production results in rhythmic activation of glucocorticoid receptor, which binds to cognate GREs (glucocorticoid receptor elements) to activate or repress transcription (26). Likewise, circadian body temperature variation results in rhythmic occupancy of heat shock elements (HSEs) (27). The result is circadian transcription of specific genes due to cell-extrinsic influences, and independent of the circadian clockwork present in that cell or tissue.
In addition to circadian transcription recent research has unearthed extensive evidence of circadian posttranscriptional regulation in mammals – including translational control (28), control of transcription termination and/or elongation (29), and to a lesser extent circadian control of splicing (30). Thus, the actual number of transcripts showing circadian abundance is significantly greater than the number of genes transcribed in circadian fashion (31-33), and the number of proteins that are expressed in circadian fashion is greater than the number of transcripts where this question has been addressed (34). Major signaling molecules like cAMP show circadian variations that both control clock output and play a role within the clock (35), and recent links between clocks and sirtuins suggest a similar influence of redox potential (36). Finally, a significant fraction of histone post-translational modifications vary in circadian fashion at a large number of loci (31; 32). Altogether, through a myriad of different mechanisms, a significant amount of cellular physiology is regulated by the circadian clock.
One case of such regulation meriting special attention is the circadian regulation of the cell cycle and DNA repair. Given the central importance of cell cycle deregulation to cancer – a disease treated separately below – it is easy to understand why circadian control of cell division in adult animals could be of central importance to clinical pharmacology. In fact, multiple studies have documented circadian or diurnal regulation of cell division, both in vivo during rodent liver regeneration (37), and in vitro in cultured cells (38). Even in humans, skin blister transcriptional profiling suggests a similar link (39). Moreover, multiple direct connections have been established between the circadian clock and cell cycle checkpoints, including via the checkpoint proteins WEE1 (37), p21-WAF (40), and CHK1/2 (41), and by control of circadian transcription of the p16-Ink4a locus through the clock-associated NONO protein. In the latter case, this circadian interaction has been demonstrated to be directly important for tissue regeneration (42). Related to circadian cell cycle control, extensive regulation of DNA damage repair by the circadian clock has also been documented (43), and this control would directly influence susceptibility to cancer.
4 From circadian control of physiology to chronopharmacology
As demonstrated above, at a cellular level large portions of cellular physiology – from transcription and translation to intracellular signaling cascades – can show daily variations in activity. This cellular diurnal variation translates directly into diurnal physiological variation in most organ systems, which in turn provides the mechanistic rationale for circadian variation in PK/PD.
4.1 Neurotransmitters and circadian behavior
Nearly all behaviors show diurnal patterns of activity. In most cases, these oscillations have been shown to manifest themselves independently of external environment or the sleep-wake cycle. For example, long-term memory shows a direct dependence upon the circadian oscillator: not only do rodents and humans learn better at certain times of day than another, but mice with a functional circadian system learn better than those without (44). Similarly, anxiety show a clear diurnal pattern that is modulated both by sleep-wake and by the circadian oscillator, and anxiety behaviors are elevated in mice lacking the Period clock genes (45). Even perception of multiple different types of pain varies in circadian fashion in both humans and animal models (46).
The likely basis for these circadian variations is that virtually all major neurotransmitter systems show either marked circadian variations or clock interactions. For example, circadian variations in opioid receptor abundance, as well as in the abundance of natural opioids themselves, have been reported numerously over the past two decades (46). The serotonergic system shows clear ultradian variations corresponding to sleep state, but these faster oscillations interact markedly with the circadian clock, and serotonergic signals appear necessary for integration of circadian information by the basal forebrain in controlling sleep timing (47). In the cholinergic system, numerous circadian variations have also been documented. For example, after a sustained attention task with daily repetition, daily increase in prefrontal cholinergic neurotransmission is observed even in the absence of the task (48). In general, the cholinergic system has been documented to play a critical role in this type of circadian “time–stamping” of behavior. It is sustained by circadian release of acetylcholine during the active phase of many mammals, accompanied by increase in choline acetyltransferase and decrease in acetylcholinesterase activity. Globally, availability of muscarinic acetylcholine receptors shows an inverse pattern to acetylcholine availability, with increased abundance during the quiescent phase of the 24-hour day, irrespective of activity per se (49).
Examination of the dopaminergic system also shows a diurnal pattern of dopamine abundance within the rodent forebrain. Interestingly, this circadian expression appears necessary for oscillation of the circadian clock gene Per2 in forebrain neurons, suggesting a role for dopamine in mediating circadian information to this brain region (50).
Multiple other neurotransmitters show circadian abundance that strongly interacts with the sleep-wake cycle. For example, adenosine shows circadian variations within the brain that are believed to be sleep-wake-dependent (51). More broadly, purinergic signaling shows a strong circadian component, and interacts directly with the circadian machienry through ATP, cAMP, and AMP (52). The hypocretin/orexin system also has circadian variation that regulates in particular REM sleep (53). Circadian release of GABA and glutamate – the principal inhibitory and excitatory neurotransmitters of the brain, respectively – in turn not only control behavior, but also hypothalamic hormone release to regulate many aspects of physiology (54).
4.2 Circadian hormones, cellular clocks, and the control of metabolism, digestion, and cardiac function
Beyond the neurotransmitters whose circadian output is directly or indirectly regulated by the SCN, numerous other hormones show diurnal regulation that significantly regulates physiology and pharmacology. Melatonin, a circadian hormone of the pineal gland, influences various aspects of retinal (55) and cardiovascular function (56), as well as affecting local clocks in diverse brain regions (57). Circadian regulation of the adrenal gland results in diurnal secretion of glucocorticoid hormone, which in turn strongly influences metabolism, and in fact directly regulates 60% of the liver transcriptome (58). Circadian regulation of gastrin, ghrelin, and somatostatin, as well as direct regulation by autonomous clocks within the gastrointestinal tract, mediate circadian influences upon digestive function (59).
More generally, autonomous circadian clocks not only within the GI tract, but also in numerous other tissues, have considerable influences upon physiology and metabolism. For example, ablation of clocks in pancreatic islets results in diabetes because of defects in coupling of β-cell stimulus to insulin secretion (60), and local clockwork controls expression of multiple ion channels and kinases in heart that influence cardiac function and triglyceride metabolism (61; 62). Recent transcriptome studies have identified widespread local circadian regulation not only in heart, but also in skeletal muscle and fat, showing that clocks in these tissues directly regulate physiology (63).
4.3 Circadian immune regulation
A second prominent pharmacological target with strong circadian regulation is the immune system. Diurnal variations in white blood cell count and susceptibility to endotoxic shock have long been documented. However, recent research shows that cell-autonomous clocks within immune cells themselves direct variation in a large number of circadian immune parameters. For example, the response of T-cells to stimulation varies in circadian fashion (64), and macrophages in turn stimulate immune responses in equally diurnal fashion with their own clocks (65). By contrast, far fewer reports exist of circadian B-cell activity, and indeed the oscillations documented in circadian gene expression in peripheral blood mononuclear cells is much lower in amplitude than that observed in other tissues such as the liver.
The consequences of pervasive circadian regulation of immune function are numerous, and range far beyond the aforementioned diurnal variation in infective susceptibility. For example, a pronounced circadian oscillation of blood clotting has long been known, and is supported by circadian variation in factors ranging from platelet aggregation and adhesion (66) to actual expression of clottin factors like PAI-1 (67). Circadian clocks also regulate circulation of many immune cells such as hematopoetic stem cells (68). Finally, circadian immune regulation results in diurnal variations in related immune parameters like inflammation, which plays a strong role in circadian variation in many diseases (69).
5 Circadian pharmacokinetics: oscillations in jejunal, hepatic, and renal systems
5.1 Rhythmic gastric and intestinal absorption
Drug transport and diffusion is highly dependent on gastric pH that regulates drug ionization and hydrophobicity. In most animal species including man (70), gastric pH present a strong circadian pattern influencing drug solubility. At the same time, gastric emptying after a meal (71) and gastrointestinal mobility (72) present a higher speed during the day than at night, increasing in this way absorption during the day. Interestingly, at least for the colon, this rhythmic motility seems to be regulated by the circadian clock, as it is severely perturbed in mice without a clock (73). Finally, the increased blood flow to the gastrointestinal tract in the beginning of the day also contributed to increased drug distribution in daytime in humans (74) (Fig. 2).
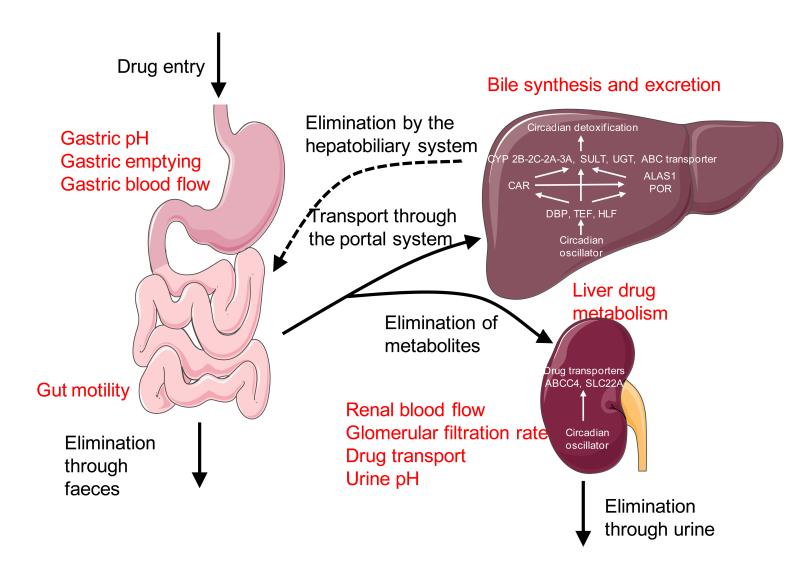
Figure 2. Modulation of drug pharmacokinetic by the circadian clock.
All rhythmic parameters influencing drug transport and metabolism are highlighted in red. Characteristic functions of the circadian clock on these process are indicated in the respective organs.
5.2 Rhythmic liver drug metabolism
Xenobiotic detoxification is organized as a multistep system consisting of three groups of proteins assuming distinct and successive functions (75). The phase I proteins functionalize drugs (possibly for inhibition or activation) by the oxidase, reductase, and hydroxylase activities of the microsomal P450 cytochrome (CYP P450) family of enzymes. The phase II proteins conjugate drugs to a hydrophilic molecule in order to increase solubility. They help to make lipophilic compounds hydrophilic enough to facilitate their excretion into urine, bile, and faeces. These reactions are achieved mainly by sulfotransferases, UDP-glucuronotransferases, glutathione S-transferases, and N-acetyltransferases. Finally, phase III transporters – mainly ABC transporters – transport xenobiotics outside the cell. Inversely, transporters of the solute carrier family (Slc) are involved in cellular import.
In addition to these three classes of enzymes, other proteins globally regulate the activity of most of the phase I enzymes of the P450 oxydoreductase family. For example, Aminolevulinic Acid Synthase (ALAS1) is the rate-limiting enzyme in synthesis of heme, the prosthetic group of all CYP P450 enzymes, and is therefore required for CYP P450 synthesis (76). Moreover, the monooxygenase reaction catalyzed by CYP P450 enzymes requires electrons that are extracted from NAD(P)H and transferred via the flavin group of the CYP P450 oxydoreductase (POR) enzyme to the heme group (77).
Importantly for pharmacokinetics, expression of all of these proteins is carefully coordinated to favor efficient liver detoxification. This control is achieved through the complex transcriptional regulation of these genes in a manner that is cell-type specific, daytime dependent, and inducible by xenobiotics themselves. Transcriptional induction involves a heterogeneous class of transcription factors collectively named “xenobiotic receptors”. The three main xenobiotic receptors are the nuclear receptors constitutive androstane receptor (CAR) and pregnane X receptor (PXR) (78), and the PAS-domain helix-loop-helix transcription factor aryl hydrocarbon receptor (AhR) (79). Mainly expressed in the liver and the small intestine, these xenobiotic receptors are associated with chaperone proteins in the cytoplasm. In response to xenobiotics – either through direct binding or by way of signal transduction – these proteins accumulate in the nucleus where they activate transcription of phase I, II and III genes.
Historical transcriptome analysis of mouse liver revealed that genes coding for enzymes involved in the three phases of xenobiotic detoxification represent an important part of the rhythmically expressed genes (20). Recent evidence suggests that these genes are not direct targets of BMAL1, but rather suggest regulation by circadian clock-controlled transcription factors (80). Studies in genetically engineered mice revealed the pivotal role of the PARbZip transcription factors to achieve this regulation. Their importance is highlighted by deletion studies: mice devoid of these three genes are born with a normal mendelian ratio and no apparent phenotypes, but fewer than 20% of them are still alive after one year (81).
Gene expression analysis revealed that PARbZip-deficient mice show a general decrease of the expression of genes coding for enzymes involved in xenobiotic detoxification of all phases in liver and kidney (22). PARbZip proteins probably regulate the expression of some of these enzymes through rhythmic binding to their promoters, for example Cyp3a4 (82) and Mdrla (MultiDrug Resistance 1a or Abcb1a) (83). However, another important mode of activity also exists: PARbZip transcription factors also regulate expression of CAR (22), which is strongly decreased with a dampened rhythm in the liver and small intestine of PARbZip deficient mice. As a consequence, induction of phase I, II, and III enzymes is very low throughout the day in these animals. Thus, the time-dependent induction of Cyp2b10 mRNA by phenobarbital is strictly compromised in PARbZip-deficient mice. These mice are also susceptible to toxicity from cyclophosphamide and mitoxantrone, two drugs used for the chemotherapeutic treatment of cancer. This phenotype is shared by mice deficient for the clock genes Clock and Bmal1 themselves, since these mice also present low level of expression of PARbZip transcription factors (84). It is interesting to note that the time-dependent toxicity of pesticide in Drosophila involves similar mechanisms, through the regulation of the CAR ortholog DHR96 by the PARbZip ortholog Pdp1 (85) and the rhythmic expression of phase II enzymes (86).
Despite the importance of PARbZip transcription factor in circadian pharmacokinetics, other clock-regulated genes are also involved in rhythmic drug detoxification. For example, RORα- and RORγ-knockout mice present a deficiency in expression of several phase I and phase II enzymes, probably also as a result of a perturbed expression of the CAR xenobiotic receptor (87). In addition, mRNA coding for the other xenobiotic receptors Pxr (88) and Ahr, and its heterodimerization partner Arnt (89), also present a rhythmic pattern of expression. In the latter case, AhR’s main target Cyp1a1 is not only rhythmically expressed (89), but also induced in a time-dependent manner by the AhR ligand 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) (90). This time-dependent induction of Cyp1a1 requires a functional circadian clock (91). Finally, acetaminophen time-dependent toxicity seems to be a result of rhythmic expression of the CYP2E1 (92), due itself to the rhythmic inhibition of the HNF1 nuclear receptor by CRY1 on the Cyp2e1 promoter (93). Whereas the relative importance of all these systems on global rhythmic drug detoxification in mouse liver is not yet clearly demonstrated, there is no doubt that the circadian clock is a major actor in this arena (Fig. 2).
5.3 Rhythmic elimination by the hepatobiliary system
Although most metabolized drugs are finally excreted into plasma and subsequently urine, several of them are first excreted through the hepatobiliary system into the gut and are subject to a second round of hepatic metabolism or fecal excretion. The hepatobiliary transport system is required not only for bile formation, but also elimination of various endo- and xenobiotics including cholesterol, phospholipids, and drugs (94). Depending on the nature of the molecule, a broad range of liver-specific export systems are involved. Bile is formed by excretion of bile salts (BS) and non-bile salt organic anions via ABC transporters. Monovalent BS are excreted via the bile salt export pump (BSEP or ABCB11), while divalent BS and anionic conjugates of endo- or xenobiotics are excreted via the conjugate export pump (MRP2 or ABCC2). The phospholipid export channel (MDR2 or ABCB4) allows the excretion of phosphatidylcholine (PC), which forms micelles in bile together with BS and cholesterol. Cationic metabolized drugs are excreted by the multidrug export channel (MDR1 or ABCB1). Other export pumps include the two-half transporter ABCG5/8 for cholesterol and the breast cancer resistance protein (BCRP or ABCG2) for anionic conjugates.
Excretion of bile acids, lipids, and xenobiotics into the bile follows a stringent circadian rhythm, at least in rodents (95), and clock involvement has been documented at multiple different steps. First, bile acid synthesis follows a stringent diurnal rhythm in both rodent (96) and human (97). Conversion of cholesterol into bile acids involved the rate-limiting cholesterol-7α-hydroxylase (CYP7A1), whose rhythmic expression is directly regulated mainly through REV-ERBα (98-100). In addition, most of the genes encoding transporters involved in bile secretion are expressed according to a circadian pattern in the liver, even if the mechanism is not clearly described for the moment (20). As a consequence, it has been observed that the biliary excretion of drugs, for example ampicillin (101) or flomoxef, presents a diurnal pattern in rats (102) and patients under percutaneous transhepatic biliary drainage (103) (Fig. 2).
5.4 Rhythmic elimination by the kidney
Most water-soluble drugs or drug metabolites are eliminated by urine through the kidney. The rate of drug elimination in the urine depends on several intrinsic variables related to the kidney function including the renal blood flow (RBF), the glomerular filtration rate (GFR), the capacity of the kidney to reabsorb or to secrete drugs across the epithelium, the urine flow, and the urine pH, which influences the degree of urine acidification. Interestingly, all these variables present a circadian behaviour in different mammalian models.
Around 20% of the RBF is converted into the urine through glomerular filtration. In the proximal tubule, many ionized drugs can be secreted in the urine from the remaining unfiltered blood via various active transports. Finally, filtered and secreted drugs can be passively or actively reabsorbed out of the urine into the blood. Because, the RBF is a key determinant of glomerular filtration and secretion, it is intimately associated with the elimination of most ionized drugs through urine. The RBF has been demonstrated to follow a circadian oscillation with a peak during the active phase (104). Although this rhythm is probably partially entrained by circadian arterial blood pressure and the cardiac output, the rhythmic RBF could also be generated by an intrinsic renal mechanism. For example, Cry1/Cry2 knockout (105) exhibit disrupted activity of the reninangiotensin-aldosterone system, one of the major mechanisms regulating RBF.
Rhythmic oscillations of the GFR are synchronized with those of RBF, but they are not fully determined by it, as GFR rhythm persists during continuous bed rest and in condition of inverted blood pressure (104). Rhythmic GFR is also maintained in transplanted human kidneys, indicating that sympathetic innervation is not required for this rhythm (106). These data indicate that GFR is generated by an intrinsic renal mechanism but the mechanism responsible for this functional rhythmicity remains unknown.
Renal reabsorption and secretion of water-soluble drugs depends on the expression of membrane transporters of the ABC and Slc families that facilitate diffusion of polar molecules through the apical and/or basolateral membranes of tubular cells. Most of drug reabsorption/secretion takes place in the proximal tubule of the kidney which is enriched in various transporters with a preferential affinity for small organic anions (107). It has been shown that several of these transporters present a robust diurnal expression in the more distal nephron segments, namely in the distal nephron and the collecting duct (108). Moreover, expression of MRP4 (ABCC4) and OAT2 (Slc22A7) are significantly reduced in the kidney of PARbZip knockout mice, providing direct evidence for circadian clock-controlled tubular reabsorption/secretion (22).
Drug ionization, which is mainly determined by urine pH, determines drug solubility and the rate of drug reabsorption in the nephron. Human urine pH may range from 4.5 to 8 and is controlled by a complex system of reabsorption/secretion/production of bicarbonate and secretion of protons. It usually exhibits lower values in the morning. The most important transporter involved in renal proton secretion is the sodium-proton exchanger 3 (NHE3 or Slc9A3) expressed in the proximal tubule. Expression of Nhe3 mRNA and protein in the kidney exhibit a robust circadian rhythm in rodent, with the maximal expression in the middle of the active phase (109). Interestingly, this circadian expression is significantly blunted in Cry1/Cry2 knockout mice, indicating that the circadian clock can influence the renal drug disposal via the control of urine acidification (Fig. 2).
6 Chronobiological implications for drug treatment
To what extent has the knowledge presented above translated to effective pharmaceutical interventions? The most obvious examples for successful chronotherapy are ones with obvious time-of-day-dependent symptoms. Treatment of bronchial asthma has been tuned to exhibit maximum plasma levels at the time of highest occurrence of dyspneas, and therefore alleviate symptoms most effectively (Fig. 3). Similarly, blood pressures shows a sharp peak in the early morning, importantly coinciding with the peak for cardiovascular events (110), and an extended trough during the night. Both healthy normotensive and patients suffering from essential hypertension exhibit this variation (111). The L-type calcium channel blocker Verapamil, for example, uses extended an release formulation to have therapeutically effective plasma levels in the early morning after bedtime oral administration (112). In addition, such delayed release drugs have been beneficial for hypertensive patients that do not show a nocturnal dip in blood pressure, so called “non-dippers” (113). Non-dipping is a risk factor for congestive heart failure even in clinically normotensive subjects (114; 115).
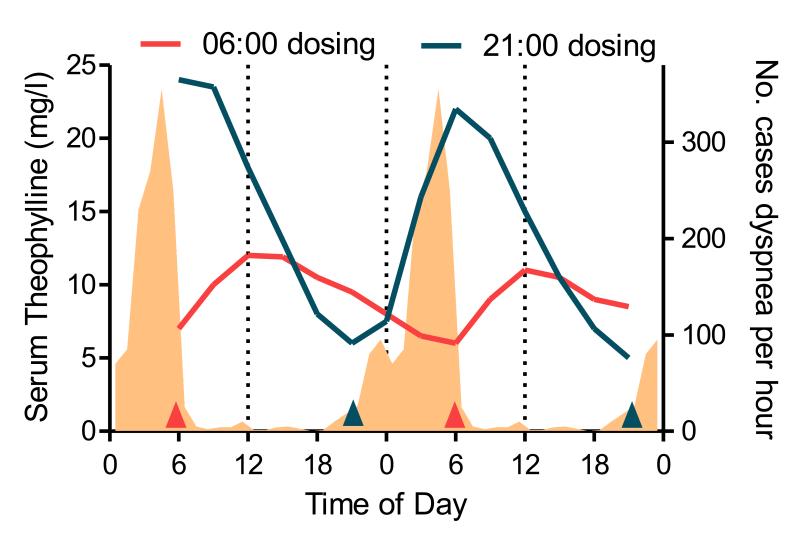
Figure 3. Time of day dependent variation in pharmacokinetics.
Average 3-hourly steady state serum concentrations of theophylline from eight asthmatic children dosed with Theo24© before breakfast (06:00, red) or at bedtime (21:00, blue) as indicated by arrows. Shaded area represents the occurrence of dyspnea. (Adapted from Smolensky et al 1987 and Dethlefsen & Repges (1985) c.f. Smolensky et al. (1987))
As mentioned above, not only PK/PD parameters are modulated by time-of–day, but also drug metabolism. For example, the over the counter acetaminophen (analgesic N-acetyl-para-aminophenol, APAP) is a leading cause of drug-induced liver failure in the United States (116). APAP is exclusively metabolized by the CYP P450 system of the liver (117), and toxicity is dependent on generation of N-acetyl-p-benzoquinone imine (NAPQI) by CYP2E1 (93). APAP toxicity is time-of-day dependent (3; 92), but liver specific ablation of the clock in mice blunts this rhythm (84).
6.1 Cancer
While the chronotherapeutic approach of the examples above is based on relatively few well-established variables, in the case of chemotherapy and associated cancer treatments the predictions for optimal treatment schedules become highly complex. On the one hand, chemotherapeutics should be dosed high enough to be toxic to the cancer, but on the other hand the dose should be low enough to prevent serious damage to healthy tissue or organs. That means pharmacokinetics and dynamics operate in a tight therapeutic range. Under these pre-conditions, the variations introduced by the circadian system on multiple levels can be crucial. This is further complicated by the possibility that not only the healthy tissue has a clock but also the tumor. In vivo, this has been shown measuring the incorporation of P32 in tumors of terminally ill breast cancer (118). These results are in line with newer in vitro data from various human and mouse cancer cell lines like the human U2 osteosarcoma (119). This is an important factor because most cancer drugs are toxic only to dividing cells or have a mechanism of action which is particularly effective in one phase of the cell cycle, which is – at least in healthy tissues – gated by the clock. The topoisomerase I inhibitor irinotecan, for example, is most effective in S-phase, while akylating agents cross-link DNA at any phase of the cell cycle (120). In case of an arrhythmic tumor, as is the case in the mouse xenograft Glasgow osteosarcoma model, a further interesting complication emerged: seliciclib, a cyclin dependent kinase inhibitor, seemed to induce rhythmic gene expression in tumors and might slow down tumor progression additionally by this mode of action (121). Given the known disturbance of circadian behavior in multiple human cancers, additional efficacy might be achieved harmlessly by this type of clock resynchronization.
Overall, in several different experimental rodent models it has been shown that efficacy and side effects of anticancer therapies vary up to 10-fold depending on time-of-day. However, these parameters are model- and drug-specific. Common to most, is that efficacy is based on the mechanism of action, metabolism, and toxicity, and the best treatment schedule has to take into account all those parameters. The therapeutic index of the alkylating agent cyclophosphamide, for example, is significantly better if the compound is administered during the first part of the active dark phase (122). This rhythm has been suggested to be dependent on CLOCK:BMAL1 binding in B-cells (122), changes in CYP P450 enzyme activity and even more importantly higher reduced glutathione levels at night might contribute, as has been described for other alkylating agents such as cisplatin. In contrast, 5-fluorouracil’s first and rate limited step in metabolism is dependent on the availability of dihydropyrimidine dehydrogenase (DPD), and certain 5-FU metabolites then block the activity and de novo synthesis of thymidilate synthase (TS), which is important for DNA synthesis (123). DPD and TS expression are high and low, respectively, during the first part of the light phase. Therefore, 5-FU exhibits best tolerability and efficacy 180° out of phase with cyclophosphamide and most other alkylating agents. Leucovorin (LV) is an inhibitor of TS and often co-administered with 5-FU. This adds to the effectiveness of 5-FU and interestingly changes the DPD to TS ratio in the same direction as observed at the optimal time of day established in animal experiments.
Interestingly, there are further common traits between irinotecan and 5-FU. For both, added value of chronotherapeutic treatment regimes is gender-specific in experimental animal models. In the case of 5-FU this has even been observed in clinical populations (124). While chronomodulated delivery of 5-FU improves survival for male patients compared to conventional treatment, the opposite was true for female study participants. The authors speculate that since disruption of the rest/activity rhythm during chemotherapy has been shown to predict overall survival for metastatic colorectal cancer (125), the men could exhibit more robust circadian rhythms. However, further investigations are needed.
6.2 Implications for drug discovery and development
Classically, the drug discovery process is preceded by the validation of a given target. The mechanism of action is established and molecular targets defined. Taking diurnal changes of relevant parameters into account might mean significantly higher costs because the same experiments might have to be conducted at multiple different times of day in order to assess if, for example, a certain type of receptor or protein is only expressed at a specific time of day. However, there are online resources that can be mined for information about the circadian expression of a given transcript or metabolite (126; 127). A special case presents in the quest for drugs against ageing related diseases. Similarly to human beings, rodent species typically used in these assays exhibit attenuated circadian rhythms. Thus, the PK/PD profile and target availability itself could change during the course of ageing.
Once the target is confirmed, and the lead optimization process started, the properties of the novel chemical entities are evaluated and selected. Typically, CYP P450 induction and inhibition in human and rodent primary hepatocytes is tested. This might introduce bias towards only one phase of the circadian cycle, since the cells that are used to evaluate the compounds contain a functional cell-autonomous clock that can influence drug metabolism as detailed above. The CACO-2 monolayer assay is an industry standard not only used to predict absorption after oral application through the intestinal barrier but also to assess interactions with important transporters such as P-gp (128). Interestingly, like the intestinal barrier itself, the human tumor derived CACO-2 cells have a functioning clock (129) that has been shown to directly control expression levels of Mdr1 (83).
As mentioned above, two of the most common reasons for novel chemical entities to fail in drug development or even marketed drugs to be withdrawn are liver toxicity and cardiac safety. In fact, QT prolongation often associated with blockade of the K+-channel encoded by human ether-à-go-go related gene (hERG) and a surrogate marker for torsade des pointes (130) was the single most common cause for withdrawal of marketed drugs in recent years (ICH webpage) (131). Therefore, an extensive battery of tests from in vitro channel function to in vivo electrophysiology in the freely moving dog or monkey has been established (ICH7b, 2005) that is performed before a so-called “thorough QT/QTc” (ICH E14, 2005) study in Phase I of development. Although new models have been developed that adjust for circadian variability due to changes in heart rate over the day (132; 133), the possibility that drug dependent QT-interval prolongation is directly influenced by time of day in patients has not yet been fully explored, but is not unsupported (134). Moreover, there is a clear rational for how the circadian clock would influence cardiac repolarization, namely krüppel-like factor 15 (135). Together these finding suggests that a time-of-day bias in testing drug induced QT–prolongation might lead to a misjudgment of risk to patients.
If, based on these considerations, drug developers would adopt a circadian testing policy, there is a further complication. In contrast to most pre-clinical animal models, there is a great deal of inter-individual variability in the circadian phenotype of people: period or phase and amplitude of clock controlled rhythms described above varies greatly in human populations (136). These are no small differences, and the lay terms “larks” and “owls” for people with an early and late activity phase, respectively, illustrate their magnitude. Moreover, diseases can even more severely alter rhythmic rest/activity and endocrine patterns. Some schizophrenic patients, for example, exhibit a nearly arrhythmic behavioral pattern (137), and not only are the cortisol levels in depressed patients elevated but their diurnal variation is blunted (138). Twin studies suggest that genetic traits are partially determining the chronotype (139). In fact, there are multiple loci that have been determined to contribute to differences in chronotype and sleep (140). Moreover, there are quite consistent age–dependent changes of chronotype (141), and recent results suggest relatively stable changes dependent on previous light history (142).
Inter-individual differences in clock phase are sizable and therefore probably clinically relevant. Albeit significant methodological advances have been made to determine the properties of an individual’s circadian clock in a simple test, this goal has not been possible so far. The ability to use human skin biopsies that are lentivirally transduced with circadian reporters to determine period is one cellular method in this direction (143), and hair follicles samples have also ben used (144). Alternatively, clock-controlled neuroendocrine signals such as the dim-light melatonin onset (DLMO) have been used to estimate clock phase (145). Due to large inter-individual variation in the levels of these hormones between individuals multiple sampling is necessary to establish meaningful results, which is also dependent on ambient light levels. To overcome these limitations, transcriptome and metabolome datasets have been used to establish timetable methods for internal time in mice and men (146-148). All of these methods, however, rely on at least two sampling times that are optimally 12 hours apart to compensate for inter-individual difference in the absolute levels of gene or metabolite expression, and “internal time” can be approximated with 2 hours’ precision. Thus, a feasible and accurate circadian test remains to be established.
Given aforementioned examples of arrythmicity in disease and inter-individual differences in clock phase, the circadian clock itself might be an interesting target for drug development. In fact, multiple pharmacological agents have been reported to phase-shift behavioral and biochemical rhythms in experimental animal models and people, and for example melatonin, melatonin agonists, or a combined melatonin agonist/5-HT2c antagonist have already received market approval, although not necessarily as phase shifting drugs. In addition to the melatonin receptors, several independent molecular targets have been identified to alter clock function. Amongst the first that was identified was casein kinase 1, and even sub-form-specific tool compounds have been described (149). As a target, however, kinase inhibitors are not without problem. Casein kinase 1 is, for example, involved in Wnt signaling and linked to cell proliferation and survival (150).
Furthermore, a number of compounds that target core molecular clock components or are believed to be important links between the clock and HPA axis have been identified and tested, including a Neuropeptide Y Y5 receptor (151), a tachykinin angagonist (152), an inverse agonist of RORα (153), and a corticotrophin releasing factor (CRF) antagonist (154). Most recently, several unbiased small molecule screens have been undertaken in an in vitro model of the circadian oscillator, and they have identified new molecular entities that target known clock components such as CRY, CK1, and REV-ERBα (155-157). Whether these in vitro data from U2OS cells will be able to translate in the in vivo situation will be very interesting.
More general, it remains to be proven if the clock does indeed present a “druggable” target, or if there are unsuspected mechanistic problems in altering circadian rhythms. Should such compounds exhibit safety profiles as favorable as melatonin, for example, they could prove useful in a wide spectrum of possible indications, ranging from sleep/wake problems in shiftworkers to amplitude-related problems in ageing related diseases. On the one hand such drugs could help to “boost” circadian rhythmicity beyond what can be achieved through behavioral measures, and on the other hand re-adjust specific rhythmic components to a favorable phase. This would be especially useful given the tendency of modern society towards a 24/7 lifestyle.
In this respect, more and more evidence has accumulated showing the sometimes disastrous effects of such clock disruption on health (15; 16; 18; 19; 158). Unfortunately, there seems to be a vicious cycle between cause and effect. Clock disruption over time can lead to various major pathologies and these in turn can feedback onto the clock and further abrogate rhythms. Interestingly, however, strengthening the clock by imposing strong timing cues can alleviate symptoms. In a mouse model of Huntington’s disease, for example, either the use of hypnotics and scheduled meals can normalize circadian gene expression rhythms and improve disease symptoms (159). Similarly, melatonin and bright light treatment have been shown to have a positive effect on institutionalized Alzheimer patients (160).
7 Conclusions
The recent findings that we have highlighted all yield insight into the growing field of chronopharmacology, and into the mechanistic basis for the variations in PK/PD that have been observed in a vast number of instances. However, many important questions remain unanswered. Most if not all of the circadian expression data at the genomic level on which these conclusions are based are available only for rodents. Considering the fact that expression and functional properties of drug metabolizing enzymes and drug transporters are highly species-specific (161), extrapolation of these results to humans is not a foregone conclusion. In order to translate research data into clinical application, significant progress in the characterization of circadian variations in protein expression and activity in human is absolutely necessary.
Although there has been much more awareness of the impact of the circadian clock on health, disease, and treatment in recent years, these findings seem to not have translated into clinics or regulatory agencies on a broad scale. Publicly available clinical trials databases such as clinicaltrials.gov list a historic total of 205 hits for the search term “circadian”. Twelve of these are cancer-related but none try to establish chronotherapeutic treatment regimes. The search term “chronotherapy” results in 14 hits. In contrast, the search term “cancer” produces 38331 results. Similar results were obtained from the EU clinical trials register. Given the fact that about 20% of the transcriptome, proteome and metabolome are under clock control (34; 162; 163), this seems disproportionate. In the case of regulatory authorities, none of the chronobiological effects upon PK/PD outlined here are mentioned in guidelines published by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). This is surprising, especially considering that unexpected hepatoxicity and cardiac side effects are the most common reasons for withdrawal of marketed drugs.
Finally, the large proportion of physiology regulated by the circadian clock suggests that the clock itself might present a possible pharmaceutical target to increase efficacy and reduce side effects. In order for such treatments to be effective, a more detailed knowledge will be required not only of how clocks control physiology, but also of how clocks in different organ systems contribute to different processes relevant to PK/PD.
8 Acknowledgements
Research support for the laboratory of Steven A. Brown comes from the Swiss National Science Foundation, the Velux Foundation, the Swiss Cancer League and the Clinical Research Priority Program Sleep and Health. The laboratory of Frédéric Gachon is supported by the European Research Council and the Leenaards foundation.
9 Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.Hippocrates, Laurentianus L, Galen . Hippocratis medici Sententiarum particula prima[-septima] Antonius Miscominus ex archetypo Laurentii … imprimi curauit; Florentiae: 1494. p. 196. (the first leaf blank) [Google Scholar]
- 2.Lemmer B. Discoveries of rhythms in human biological functions: a historical review. Chronobiology international. 2009;26:1019–68. doi: 10.3109/07420520903237984. [DOI] [PubMed] [Google Scholar]
- 3.Kamali F, Fry JR, Bell GD. Temporal variations in paracetamol absorption and metabolism in man. Xenobiotica; the fate of foreign compounds in biological systems. 1987;17:635–41. doi: 10.3109/00498258709043970. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H, Nakano S, Nagai K, Ogawa N. Time-Dependent Absorption of Theophylline in Man. Journal of Clinical Pharmacology. 1984;24:509–14. doi: 10.1002/j.1552-4604.1984.tb02760.x. [DOI] [PubMed] [Google Scholar]
- 5.Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. Coupling governs entrainment range of circadian clocks. Molecular systems biology. 2010;6:438. doi: 10.1038/msb.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reischl S, Kramer A. Kinases and phosphatases in the mammalian circadian clock. FEBS letters. 2011;585:1393–9. doi: 10.1016/j.febslet.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Brown SA. Circadian rhythms. A new histone code for clocks? Science. 2011;333:1833–4. doi: 10.1126/science.1212842. [DOI] [PubMed] [Google Scholar]
- 8.Kowalska E, Ripperger JA, Muheim C, Maier B, Kurihara Y, et al. Distinct roles of DBHS family members in the circadian transcriptional feedback loop. Molecular and cellular biology. 2012;32:4585–94. doi: 10.1128/MCB.00334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson CH, Mori T, Xu Y. A cyanobacterial circadian clockwork. Current biology: CB. 2008;18:R816–R25. doi: 10.1016/j.cub.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual review of physiology. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–5. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 13.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes and development. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, et al. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. Journal of Neuroscience. 2012;32:16193–202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golombek DA, Casiraghi L, Agostino PV, Paladino N, Duhart J, et al. The times are changing: Effects of circadian desynchronization on physiology and disease. Journal of physiology, Paris. 2013 doi: 10.1016/j.jphysparis.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, et al. Dysregulation of inflammatory responses by chronic circadian disruption. Journal of immunology. 2010;185:5796–805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PloS one. 2012;7:e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nature reviews. Neuroscience. 2010;11:589–99. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 19.Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Molecular medicine. 2012;18:1249–60. doi: 10.2119/molmed.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 21.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 22.Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell metabolism. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Ukai-Tadenuma M, Yamada RG, Xu H, Ripperger JA, Liu AC, Ueda HR. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 2011;144:268–81. doi: 10.1016/j.cell.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS biology. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber A, Esnault C, Aubert G, Treisman R, Pralong F, Schibler U. Blood-borne circadian signal stimulates daily oscillations in actin dynamics and SRF activity. Cell. 2013;152:492–503. doi: 10.1016/j.cell.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–41. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Reinke H, Saini C, Fleury-Olela F, Dibner C, Benjamin IJ, Schibler U. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes and development. 2008;22:331–45. doi: 10.1101/gad.453808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jouffe C, Cretenet G, Symul L, Martin E, Atger F, et al. The circadian clock coordinates ribosome biogenesis. PLoS biology. 2013;11:e1001455. doi: 10.1371/journal.pbio.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337:599–602. doi: 10.1126/science.1221592. [DOI] [PubMed] [Google Scholar]
- 30.McGlincy NJ, Valomon A, Chesham JE, Maywood ES, Hastings MH, Ule J. Regulation of alternative splicing by the circadian clock and food related cues. Genome biology. 2012;13:R54. doi: 10.1186/gb-2012-13-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–54. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Martelot G, Canella D, Symul L, Migliavacca E, Gilardi F, et al. Genome-wide RNA polymerase II profiles and RNA accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles. PLoS biology. 2012;10:e1001442. doi: 10.1371/journal.pbio.1001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife. 2012;1:e00011. doi: 10.7554/eLife.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, et al. Circadian Orchestration of the Hepatic Proteome. Curr Biol. 2006;16:1107–15. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–53. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell metabolism. 2011;13:125–37. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–9. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 38.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Sporl F, Korge S, Jurchott K, Wunderskirchner M, Schellenberg K, et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10903–8. doi: 10.1073/pnas.1118641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem. 2008;283:4535–42. doi: 10.1074/jbc.M705576200. [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Wood PA, Hrushesky WJ. Mammalian TIMELESS is required for ATM-dependent CHK2 activation and G2/M checkpoint control. J Biol Chem. 2010;285:3030–4. doi: 10.1074/jbc.M109.050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, et al. NONO couples the circadian clock to the cell cycle. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1592–9. doi: 10.1073/pnas.1213317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS letters. 2010;584:2618–25. doi: 10.1016/j.febslet.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, et al. Hippocampal-dependent learning requires a functional circadian system. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15593–8. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. The European journal of neuroscience. 2013;37:242–50. doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Junker U, Wirz S. Review article: chronobiology: influence of circadian rhythms on the therapy of severe pain. Journal of oncology pharmacy practice. 2010;16:81–7. doi: 10.1177/1078155209337665. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto H, Nakamaru-Ogiso E, Hamada K, Hensch TK. Serotonergic integration of circadian clock and ultradian sleep-wake cycles. Journal of Neuroscience. 2012;32:14794–803. doi: 10.1523/JNEUROSCI.0793-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paolone G, Lee TM, Sarter M. Time to pay attention: attentional performance time-stamped prefrontal cholinergic activation, diurnality, and performance. Journal of neuroscience. 2012;32:12115–28. doi: 10.1523/JNEUROSCI.2271-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behavioural brain research. 2011;221:466–80. doi: 10.1016/j.bbr.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 50.Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. Journal of neuroscience. 2010;30:14046–58. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porkka-Heiskanen T, Alanko L, Kalinchuk A, Stenberg D. Adenosine and sleep. Sleep medicine reviews. 2002;6:321–32. doi: 10.1053/smrv.2001.0201. [DOI] [PubMed] [Google Scholar]
- 52.Adenosine: a key link between metabolism and brain activity. xvi. Springer; 2013. p. 679. [Google Scholar]
- 53.Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, Scammell TE. Orexin neurons are necessary for the circadian control of REM sleep. Sleep. 2009;32:1127–34. doi: 10.1093/sleep/32.9.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, et al. SCN outputs and the hypothalamic balance of life. Journal of biological rhythms. 2006;21:458–69. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 55.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Experimental eye research. 2012;103:82–9. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. Journal of pineal research. 2010;49:14–22. doi: 10.1111/j.1600-079X.2010.00773.x. [DOI] [PubMed] [Google Scholar]
- 57.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin--a pleiotropic, orchestrating regulator molecule. Progress in neurobiology. 2011;93:350–84. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45:1478–88. doi: 10.1002/hep.21571. [DOI] [PubMed] [Google Scholar]
- 59.Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. Journal of physiology and pharmacology. 2011;62:139–50. [PubMed] [Google Scholar]
- 60.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko ML, Shi L, Tsai JY, Young ME, Neuendorff N, et al. Cardiac-specific mutation of Clock alters the quantitative measurements of physical activities without changing behavioral circadian rhythms. Journal of biological rhythms. 2011;26:412–22. doi: 10.1177/0748730411414170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2010;285:2918–29. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bray MS, Young ME. The role of cell-specific circadian clocks in metabolism and disease. Obes Rev. 2009;10(Suppl 2):6–13. doi: 10.1111/j.1467-789X.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 64.Fortier EE, Rooney J, Dardente H, Hardy MP, Labrecque N, Cermakian N. Circadian variation of the response of T cells to antigen. Journal of immunology. 2011;187:6291–300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- 65.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, et al. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21407–12. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujimura A, Ohashi K, Ebihara A. Daily variations in platelet aggregation and adhesion in healthy subjects. Life sciences. 1992;50:1043–7. doi: 10.1016/0024-3205(92)90099-b. [DOI] [PubMed] [Google Scholar]
- 67.Kluft C, Jie AF, Rijken DC, Verheijen JH. Daytime fluctuations in blood of tissue-type plasminogen activator (t-PA) and its fast-acting inhibitor (PAI-1) Thrombosis and haemostasis. 1988;59:329–32. [PubMed] [Google Scholar]
- 68.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 69.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nature reviews. Immunology. 2013;13:190–8. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore JG, Englert E., Jr. Circadian rhythm of gastric acid secretion in man. Nature. 1970;226:1261–2. doi: 10.1038/2261261a0. [DOI] [PubMed] [Google Scholar]
- 71.Goo RH, Moore JG, Greenberg E, Alazraki NP. Circadian variation in gastric emptying of meals in humans. Gastroenterology. 1987;93:515–8. doi: 10.1016/0016-5085(87)90913-9. [DOI] [PubMed] [Google Scholar]
- 72.Kumar D, Wingate D, Ruckebusch Y. Circadian variation in the propagation velocity of the migrating motor complex. Gastroenterology. 1986;91:926–30. doi: 10.1016/0016-5085(86)90696-7. [DOI] [PubMed] [Google Scholar]
- 73.Hoogerwerf WA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, et al. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol. 2010;298:G143–50. doi: 10.1152/ajpgi.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemmer B, Nold G. Circadian changes in estimated hepatic blood flow in healthy subjects. Br J Clin Pharmacol. 1991;32:627–9. doi: 10.1111/j.1365-2125.1991.tb03964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 76.Furuyama K, Kaneko K, Vargas VPD. Heme as a Magnificent Molecule with Multiple Missions: Heme Determines Its Own Fate and Governs Cellular Homeostasis. Tohoku J Exp Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 77.Pandey AV, Fluck CE. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol Ther. 2013;138:229–54. doi: 10.1016/j.pharmthera.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Chai X, Zeng S, Xie W. Nuclear receptors PXR and CAR: implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin Drug Metab Toxicol. 2013;9:253–66. doi: 10.1517/17425255.2013.754010. [DOI] [PubMed] [Google Scholar]
- 79.Guyot E, Chevallier A, Barouki R, Coumoul X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov Today. 2012 doi: 10.1016/j.drudis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 80.Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-Wide and Phase-Specific DNA-Binding Rhythms of BMAL1 Control Circadian Output Functions in Mouse Liver. PLoS biology. 2011;9:e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, et al. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes and development. 2004;18:1397–412. doi: 10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takiguchi T, Tomita M, Matsunaga N, Nakagawa H, Koyanagi S, Ohdo S. Molecular basis for rhythmic expression of CYP3A4 in serum-shocked HepG2 cells. Pharmacogenet Genomics. 2007;17:1047–56. doi: 10.1097/FPC.0b013e3282f12a61. [DOI] [PubMed] [Google Scholar]
- 83.Murakami Y, Higashi Y, Matsunaga N, Koyanagi S, Ohdo S. Circadian Clock-Controlled Intestinal Expression of the Multidrug-Resistance Gene mdr1a in Mice. Gastroenterology. 2008;135:1636–44.e3. doi: 10.1053/j.gastro.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 84.Dallmann R, Debruyne JP, Weaver DR. submitted.
- 85.Beaver LM, Hooven LA, Butcher SM, Krishnan N, Sherman KA, et al. Circadian Clock Regulates Response to Pesticides in Drosophila via Conserved Pdp1 Pathway. Toxicol Sci. 2010;115:513–20. doi: 10.1093/toxsci/kfq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beaver LM, Klichko VI, Chow ES, Kotwica-Rolinska J, Williamson M, et al. Circadian Regulation of Glutathione Levels and Biosynthesis in Drosophila melanogaster. PloS one. 2012;7:e50454. doi: 10.1371/journal.pone.0050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang HS, Angers M, Beak JY, Wu X, Gimble JM, et al. Gene expression profiling reveals a regulatory role for RORa and RORy in phase I and phase II metabolism. Physiol Genomics. 2007;31:281–94. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y-KJ, Yeager RL, Klaassen CD. Circadian Expression Profiles of Drug-Processing Genes and Transcription Factors in Mouse Liver. Drug Metab Dispos. 2009;37:106–15. doi: 10.1124/dmd.108.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang P, Ceccatelli S, Rannug A. A study on diurnal mRNA expression of CYP1A1, AHR, ARNT, and PER2 in rat pituitary and liver. Environ Toxicol Pharmacol. 2002;11:119–26. doi: 10.1016/s1382-6689(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 90.Qu X, Metz RP, Porter WW, Cassone VM, Earnest DJ. Disruption of period gene expression alters the inductive effects of dioxin on the AhR signaling pathway in the mouse liver. Toxicol Appl Pharmacol. 2009;234:370–7. doi: 10.1016/j.taap.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanimura N, Kusunose N, Matsunaga N, Koyanagi S, Ohdo S. Aryl hydrocarbon receptor-mediated Cyplal expression is modulated in a CLOCK-dependent circadian manner. Toxicology. 2011;290:203–7. doi: 10.1016/j.tox.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Matsunaga N, Nakamura N, Yoneda N, Qin T, Terazono H, et al. Influence of Feeding Schedule on 24-h Rhythm of Hepatotoxicity Induced by Acetaminophen in Mice. J Pharmacol Exp Ther. 2004;311:594–600. doi: 10.1124/jpet.104.069062. [DOI] [PubMed] [Google Scholar]
- 93.Matsunaga N, Ikeda M, Takiguchi T, Koyanagi S, Ohdo S. The molecular mechanism regulating 24-hour rhythm of CYP2E1 expression in the mouse liver. Hepatology. 2008;48:240–51. doi: 10.1002/hep.22304. [DOI] [PubMed] [Google Scholar]
- 94.Trauner M, Wagner M, Fickert P, Zollner G. Molecular Regulation of Hepatobiliary Transport Systems: Clinical Implications for Understanding and Treating Cholestasis. J Clin Gastroenterol. 2005;39:S111–S24. doi: 10.1097/01.mcg.0000155551.37266.26. [DOI] [PubMed] [Google Scholar]
- 95.Nakano A, Tietz PS, LaRusso NF. Circadian rhythms of biliary protein and lipid excretion in rats. Am J Physiol. 1990;258:G653–9. doi: 10.1152/ajpgi.1990.258.5.G653. [DOI] [PubMed] [Google Scholar]
- 96.Duane WC, Gilberstadt ML, Wiegand DM. Diurnal rhythms of bile acid production in the rat. Am J Physiol. 1979;236:R175–9. doi: 10.1152/ajpregu.1979.236.3.R175. [DOI] [PubMed] [Google Scholar]
- 97.Duane WC, Levitt DG, Mueller SM, Behrens JC. Regulation of bile acid synthesis in man. Presence of a diurnal rhythm. J Clin Invest. 1983;72:1930–6. doi: 10.1172/JCI111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, et al. Regulation of Bile Acid Synthesis by the Nuclear Receptor Rev-erbα. Gastroenterology. 2008;135:689–98. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 99.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, et al. REV-ERBα Participates in Circadian SREBP Signaling and Bile Acid Homeostasis. PLoS biology. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma K, Xiao R, Tseng H-T, Shan L, Fu L, Moore DD. Circadian Dysregulation Disrupts Bile Acid Homeostasis. PloS one. 2009;4:e6843. doi: 10.1371/journal.pone.0006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mesnard-Ricci B, White CA. Chronokinetics of active biliary ampicillin secretion in rats. Chronobiology international. 1998;15:309–21. doi: 10.3109/07420529808998692. [DOI] [PubMed] [Google Scholar]
- 102.Hishikawa S, Sugimoto K, Kobayashi E, Kumagai Y, Fujimura A. Dosing-time-dependent variation in biliary excretion of flomoxef in rats. Chronobiology international. 2003;20:463–71. doi: 10.1081/cbi-120020421. [DOI] [PubMed] [Google Scholar]
- 103.Hishikawa S, Kobayashi E, Sugimoto K-i, Miyata M, Fujimura A. Diurnal variation in the biliary excretion of flomoxef in patients with percutaneous transhepatic biliary drainage. Br J Clin Pharmacol. 2001;52:65–8. doi: 10.1046/j.0306-5251.2001.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clinical Science. 1989;77:105–11. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- 105.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 106.Buijsen JGM, van Acker BAC, Koomen GCM, Koopman MG, Arisz L. Circadian rhythm of glomerular filtration rate in patients after kidney transplantation. Nephrol Dial Transplant. 1994;9:1330–3. [PubMed] [Google Scholar]
- 107.Sekine T, Miyazaki H, Endou H. Molecular physiology of renal organic anion transporters. Am J Physiol Renal Physiol. 2006;290:F251–61. doi: 10.1152/ajprenal.00439.2004. [DOI] [PubMed] [Google Scholar]
- 108.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16523–8. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, et al. Circadian clock genes directly regulate expression of the Na+//H+ exchanger NHE3 in the kidney. Kidney Int. 2005;67:1410–9. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 110.Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke; a journal of cerebral circulation. 1998;29:1873–5. doi: 10.1161/01.str.29.9.1873. [DOI] [PubMed] [Google Scholar]
- 111.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–7. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 112.White WB, Anders RJ, MacIntyre JM, Black HR, Sica DA. Nocturnal dosing of a novel delivery system of verapamil for systemic hypertension. Verapamil Study Group. The American journal of cardiology. 1995;76:375–80. doi: 10.1016/s0002-9149(99)80104-0. [DOI] [PubMed] [Google Scholar]
- 113.White WB, Mehrotra DV, Black HR, Fakouhi TD. Effects of controlled-onset extended-release verapamil on nocturnal blood pressure (dippers versus nondippers). COER-Verapamil Study Group. The American journal of cardiology. 1997;80:469–74. doi: 10.1016/s0002-9149(97)00397-4. [DOI] [PubMed] [Google Scholar]
- 114.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level--the “normotensive non-dipper” paradox. Chronobiology international. 2013;30:87–98. doi: 10.3109/07420528.2012.701127. [DOI] [PubMed] [Google Scholar]
- 115.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–66. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 116.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 117.Henderson CJ, Pass GJ, Wolf CR. The hepatic cytochrome P450 reductase null mouse as a tool to identify a successful candidate entity. Toxicol Lett. 2006;162:111–7. doi: 10.1016/j.toxlet.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 118.Stoll BA, Burch WM. Surface detection of circadian rhythm in 32P content of cancer of the breast. Cancer. 1967;21:193–6. doi: 10.1002/1097-0142(196802)21:2<193::aid-cncr2820210206>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 119.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guichard S, Hennebelle I, Bugat R, Canal P. Cellular interactions of 5-fluorouracil and the camptothecin analogue CPT-11 (irinotecan) in a human colorectal carcinoma cell line. Biochemical pharmacology. 1998;55:667–76. doi: 10.1016/s0006-2952(97)00541-8. [DOI] [PubMed] [Google Scholar]
- 121.Iurisci I, Filipski E, Sallam H, Harper F, Guettier C, et al. Liver circadian clock, a pharmacologic target of cyclin-dependent kinase inhibitor seliciclib. Chronobiology international. 2009;26:1169–88. doi: 10.3109/07420520903209942. [DOI] [PubMed] [Google Scholar]
- 122.Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3407–12. doi: 10.1073/pnas.0409897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X, Diasio RB. Regulation of human dihydropyrimidine dehydrogenase: implications in the pharmacogenetics of 5-FU-based chemotherapy. Pharmacogenomics. 2007;8:257–65. doi: 10.2217/14622416.8.3.257. [DOI] [PubMed] [Google Scholar]
- 124.Giacchetti S, Dugue PA, Innominato PF, Bjarnason GA, Focan C, et al. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Annals of oncology. 2012;23:3110–6. doi: 10.1093/annonc/mds148. [DOI] [PubMed] [Google Scholar]
- 125.Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. International journal of cancer. Journal international du cancer. 2012;131:2684–92. doi: 10.1002/ijc.27574. [DOI] [PubMed] [Google Scholar]
- 126.Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic acids research. 2013;41:D1009–13. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5541–6. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shah P, Jogani V, Bagchi T, Misra A. Role of Caco-2 cell monolayers in prediction of intestinal drug absorption. Biotechnology progress. 2006;22:186–98. doi: 10.1021/bp050208u. [DOI] [PubMed] [Google Scholar]
- 129.Pardini L, Kaeffer B, Trubuil A, Bourreille A, Galmiche JP. Human intestinal circadian clock: expression of clock genes in colonocytes lining the crypt. Chronobiology international. 2005;22:951–61. doi: 10.1080/07420520500395011. [DOI] [PubMed] [Google Scholar]
- 130.Raehl CL, Patel AK, LeRoy M. Drug-induced torsade de pointes. Clinical pharmacy. 1985;4:675–90. [PubMed] [Google Scholar]
- 131.Roden DM. Drug-induced prolongation of the QT interval. The New England journal of medicine. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 132.Chain AS, Krudys KM, Danhof M, Della Pasqua O. Assessing the probability of drug-induced QTc-interval prolongation during clinical drug development. Clinical pharmacology and therapeutics. 2011;90:867–75. doi: 10.1038/clpt.2011.202. [DOI] [PubMed] [Google Scholar]
- 133.Fijorek K, Patel N, Klima L, Stolarz-Skrzypek K, Kawecka-Jaszcz K, Polak S. Age and gender dependent heart rate circadian model development and performance verification on the proarrhythmic drug case study. Theoretical biology and medical modelling. 2013;10:7. doi: 10.1186/1742-4682-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watanabe J, Suzuki Y, Fukui N, Ono S, Sugai T, et al. Increased risk of antipsychotic-related QT prolongation during nighttime: a 24-hour holter electrocardiogram recording study. Journal of clinical psychopharmacology. 2012;32:18–22. doi: 10.1097/JCP.0b013e31823f6f21. [DOI] [PubMed] [Google Scholar]
- 135.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–9. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS biology. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. British journal of psychiatry. 2012;200:308–16. doi: 10.1192/bjp.bp.111.096321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, et al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–8. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 139.Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. Journal of sleep research. 2007;16:156–62. doi: 10.1111/j.1365-2869.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 140.Allebrandt KV, Amin N, Muller-Myhsok B, Esko T, Teder-Laving M, et al. A K-ATP channel gene effect on sleep duration: from genome-wide association studies to function in Drosophila. Mol Psychiatr. 2013;18:122–32. doi: 10.1038/mp.2011.142. [DOI] [PubMed] [Google Scholar]
- 141.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, et al. Epidemiology of the human circadian clock. Sleep medicine reviews. 2007;11:429–38. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 142.Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. The Journal of physiology. 2011;589:1095–102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pagani L, Semenova EA, Moriggi E, Revell VL, Hack LM, et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PloS one. 2010;5:e13376. doi: 10.1371/journal.pone.0013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akashi M, Soma H, Yamamoto T, Tsugitomi A, Yamashita S, et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15643–8. doi: 10.1073/pnas.1003878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiology international. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 146.Minami Y, Kasukawa T, Kakazu Y, Iigo M, Sugimoto M, et al. Measurement of internal body time by blood metabolomics. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9890–5. doi: 10.1073/pnas.0900617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kasukawa T, Sugimoto M, Hida A, Minami Y, Mori M, et al. Human blood metabolite timetable indicates internal body time. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15036–41. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ueda HR, Chen W, Minami Y, Honma S, Honma K, et al. Molecular-timetable methods for detection of body time and rhythm disorders from single-time-point genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11227–32. doi: 10.1073/pnas.0401882101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15240–5. doi: 10.1073/pnas.1005101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–35. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 151.Kalra PS, Dube MG, Xu B, Farmerie WG, Kalra SP. Evidence that dark-phase hyperphagia induced by neurotoxin 6-hydroxydopamine may be due to decreased leptin and increased neuropeptide Y signaling. Physiology and behavior. 1998;63:829–35. doi: 10.1016/s0031-9384(97)00545-3. [DOI] [PubMed] [Google Scholar]
- 152.Gannon RL, Millan MJ. The selective tachykinin neurokinin 1 (NK1) receptor antagonist, GR 205,171, stereospecifically inhibits light-induced phase advances of hamster circadian activity rhythms. European journal of pharmacology. 2005;527:86–93. doi: 10.1016/j.ejphar.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 153.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, et al. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethy l]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol. 2010;77:228–36. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gannon RL, Millan MJ. The corticotropin-releasing factor (CRF)(1) receptor antagonists CP154,526 and DMP695 inhibit light-induced phase advances of hamster circadian activity rhythms. Brain research. 2006;1083:96–102. doi: 10.1016/j.brainres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 155.Chen Z, Yoo SH, Park YS, Kim KH, Wei S, et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:101–6. doi: 10.1073/pnas.1118034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–7. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circulation research. 2010;106:447–62. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maywood ES, Fraenkel E, McAllister CJ, Wood N, Reddy AB, et al. Disruption of peripheral circadian timekeeping in a mouse model of Huntington’s disease and its restoration by temporally scheduled feeding. Journal of neuroscience. 2010;30:10199–204. doi: 10.1523/JNEUROSCI.1694-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. Journal of the American Geriatrics Society. 2008;56:239–46. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–94. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 162.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, et al. Coordinated Transcription of Key Pathways in the Mouse by the Circadian Clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 163.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2625–9. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]