Abstract
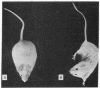
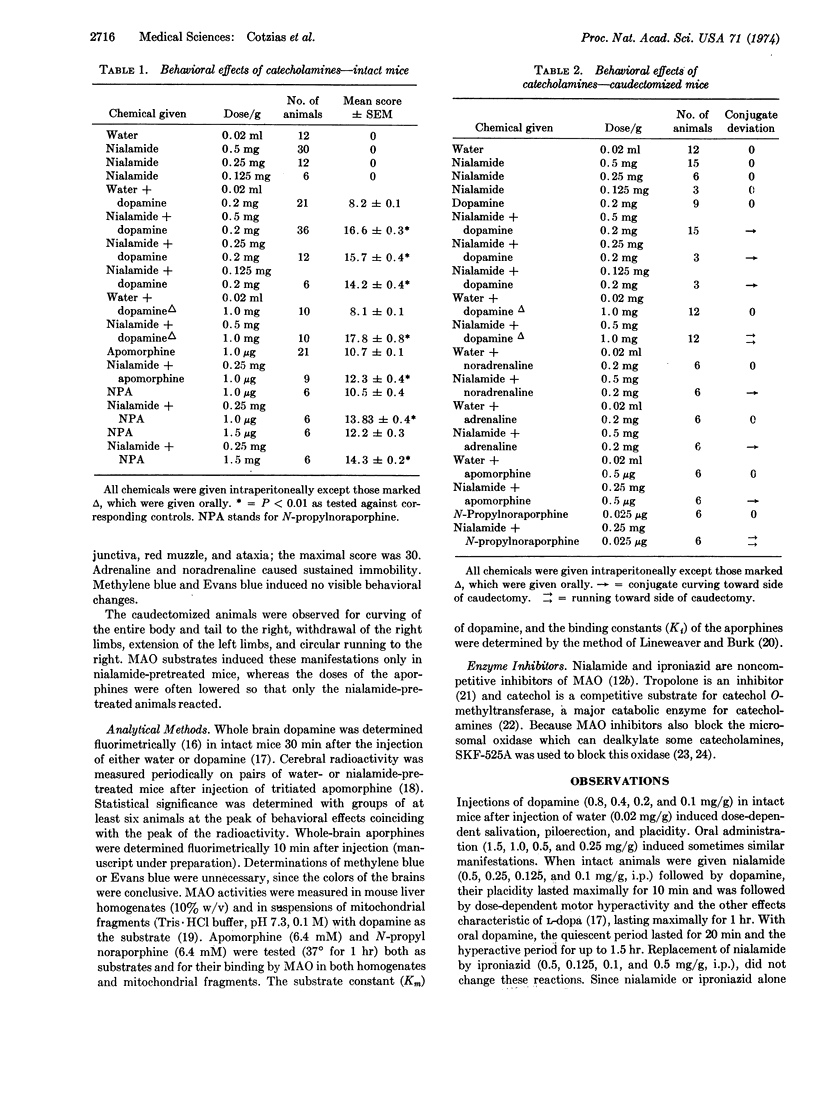
The brain uptake of amines that do not enter the brain or enter it poorly was promoted by noncompetitive inhibitors of monoamine oxidase, as shown by behavioral and chemical criteria. Mice pretreated with water or enzyme inhibitors other than those mentioned were placid after receiving dopamine (3,4-dihydroxyphenethylamine). Mice pretreated with monoamine oxidase inhibitors (nialamide or iproniazid) showed upon treatment with dopamine the brisk motor responses characteristic of treatment with its precursor, L-dopa (3,4-dihydroxyphenylalanine). After receiving dopamine, intact nialamide-pretreated mice showed marked increases of brain dopamine, in contrast to water-pretreated test mice or water-treated controls. In unilaterally caudectomized, nialamide-pretreated mice, dopamine induced marked lateral curving of the body toward the lesion followed by running in that direction. Noradrenaline or adrenaline induced curving in caudectomized mice, whereas intact ones remained placid.
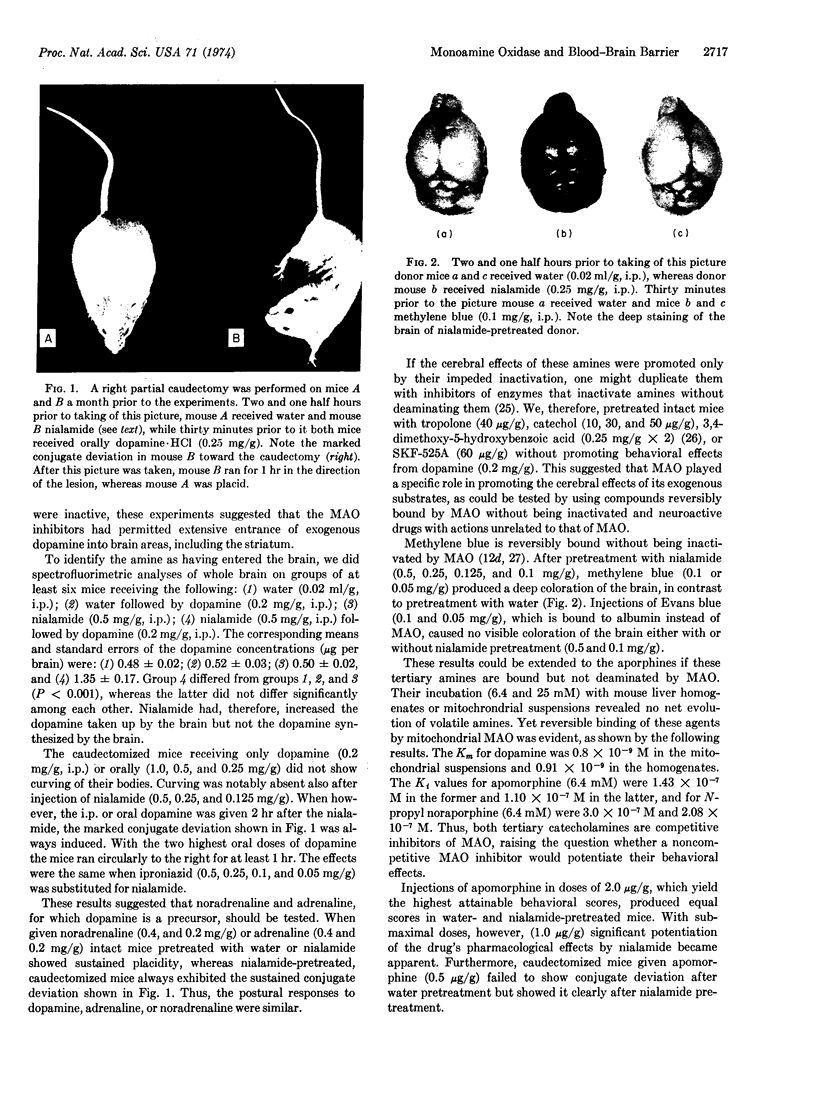
These catecholamines are bound and inactivated by monoamine oxidase. The cerebral uptakes of chemicals that are bound but not inactivated by monoamine oxidase were thereafter tested. Nialamide induced increased behavioral responses to apomorphine and to N-propyl noraporphine, increased cerebral concentrations of both, and a deep coloration of the brain from methylene blue (bound by monoamine oxidase) but not Evans blue (bound by albumin). Even large doses of nialamide, however, failed to affect the behavioral responses to oxotremorine, which has cholinergic rather than adrenergic or dopaminergic properties. Mitochondrial monoamine oxidase seems therefore to play a specific regulatory role in the transport of substances that it binds, either to inactivate or to release them.
Keywords: dopamine, noradrenaline, adrenaline, apomorphine, nialamide
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- AXELROD J., REICHENTHAL J., BRODIE B. B. Mechanism of the potentiating action of beta-diethylaminoethyl diphenylpropylacetate. J Pharmacol Exp Ther. 1954 Sep;112(1):49–54. [PubMed] [Google Scholar]
- Andén N. E., Rubenson A., Fuxe K., Hökfelt T. Evidence for dopamine receptor stimulation by apomorphine. J Pharm Pharmacol. 1967 Sep;19(9):627–629. doi: 10.1111/j.2042-7158.1967.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Methylation reactions in the formation and metabolism of catecholamines and other biogenic amines. Pharmacol Rev. 1966 Mar;18(1):95–113. [PubMed] [Google Scholar]
- BELLEAU B., BURBA J. OCCUPANCY OF ADRENERGIC RECEPTORS AND INHIBITION OF CATECHOL O-METHYL TRANSFERASE BY TROPOLONES. J Med Chem. 1963 Nov;6:755–759. doi: 10.1021/jm00342a028. [DOI] [PubMed] [Google Scholar]
- Baldessarini R. J., Chace K. V. Metabolism of L-dopa after inhibition of catechol-O-methyl transferase. Eur J Pharmacol. 1972 Jan;17(1):163–166. doi: 10.1016/0014-2999(72)90283-x. [DOI] [PubMed] [Google Scholar]
- Bertler A., Falck B., Owman C., Rosengrenn E. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol Rev. 1966 Mar;18(1):369–385. [PubMed] [Google Scholar]
- COTZIAS G. C., DOLE V. P. Metabolism of amines. I. Microdetermination of monoamine oxidase in tissues. J Biol Chem. 1951 Jun;190(2):665–672. [PubMed] [Google Scholar]
- COTZIAS G. C., DOLE V. P. Metabolism of amines. II. Mitochondrial localization of monoamine oxidase. Proc Soc Exp Biol Med. 1951 Oct;78(1):157–160. doi: 10.3181/00379727-78-19006. [DOI] [PubMed] [Google Scholar]
- Calne D. B., Reid J. L. Actions of levodopa on the blood pressure of conscious rabbits. Br J Pharmacol. 1973 Jun;48(2):194–197. doi: 10.1111/j.1476-5381.1973.tb06905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. B., Hanin I., Jenden D. J. Gas chromatographic evaluation of the effects of some muscarinic and antimuscarinic drugs on acetylcholine levels in rat brain. Biochem Pharmacol. 1970 Jun;19(6):2053–2059. doi: 10.1016/0006-2952(70)90302-3. [DOI] [PubMed] [Google Scholar]
- Christie J. E., Crow T. J. Behavioural studies of the actions of cocaine, monoamine oxidase inhibitors and iminodibenzyl compounds on central dopamine neurones. Br J Pharmacol. 1973 Jan;47(1):39–47. doi: 10.1111/j.1476-5381.1973.tb08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias G. C., Papavasiliou P. S., Fehling C., Kaufman B., Mena I. Similarities between neurologic effects of L-dipa and of apomorphine. N Engl J Med. 1970 Jan 1;282(1):31–33. doi: 10.1056/NEJM197001012820107. [DOI] [PubMed] [Google Scholar]
- Cotzias G. C., Papavasiliou P. S., Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969 Feb 13;280(7):337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- Cotzias G. C., Tang L. C., Miller S. T., Ginos J. Z. Melatonin and abnormal movements induced by L-dopa in mice. Science. 1971 Jul 30;173(3995):450–452. doi: 10.1126/science.173.3995.450. [DOI] [PubMed] [Google Scholar]
- Düby S. E., Cotzias G. C., Papavasiliou P. S., Lawrence W. H. Injected apomorphine and orally administered levodopa in Parkinsonism. Arch Neurol. 1972 Dec;27(6):474–480. doi: 10.1001/archneur.1972.00490180010004. [DOI] [PubMed] [Google Scholar]
- EHRINGER H., HORNYKIEWICZ O., LECHNER K. [The effect of methylene blue on monoamine oxidase and the catechol amine and 5-hydroxytryptamine metabolism of the brain]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1961;241:568–582. [PubMed] [Google Scholar]
- Ernst A. M. Relation between the action of dopamine and apomorphine and their O-methylated derivatives upon the CNS. Psychopharmacologia. 1965 May 21;7(6):391–399. doi: 10.1007/BF00402361. [DOI] [PubMed] [Google Scholar]
- Ginos J. Z., LoMonte A., Cotzias G. C., Bose A. K., Brambilla R. J. Synthesis of tritium- and deuterium-labeled apomorphine. J Am Chem Soc. 1973 May 2;95(9):2991–2994. doi: 10.1021/ja00790a043. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Lotti V. J. Action of various centrally acting agents in mice with unilateral caudate brain lesions. Life Sci I. 1971 Jul 15;10(14):781–789. doi: 10.1016/0024-3205(71)90032-4. [DOI] [PubMed] [Google Scholar]
- Missala K., Lal S., Sourkes T. L. O-methylation of apomorphine and the metabolic prolongation of apomorphine-induced stereotyped behaviour. Eur J Pharmacol. 1973 Apr;22(1):54–58. doi: 10.1016/0014-2999(73)90183-0. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Papavasiliou P. S., Cotzias G. C., Düby S. E., Steck A. J., Fehling C., Bell M. A. Levodopa in Parkinsonism: potentiation of central effects with a peripheral inhibitor. N Engl J Med. 1972 Jan 6;286(1):8–14. doi: 10.1056/NEJM197201062860102. [DOI] [PubMed] [Google Scholar]
- Spector S., Tarver J., Berkowitz B. Effects of drugs and physiological factors in the disposition of catecholamines in blood vessels. Pharmacol Rev. 1972 Jun;24(2):191–202. [PubMed] [Google Scholar]
- UDENFRIEND S., WEISSBACH H., BOGDANSKI D. F. Biochemical findings relating to the action of serotonin. Ann N Y Acad Sci. 1957 Mar 14;66(3):602–608. doi: 10.1111/j.1749-6632.1957.tb40750.x. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., WHITBY L. G., AXELROD J. The uptake of circulating [3H]norepinephrine by the pituitary gland and various areas of the brain. J Neurochem. 1961 Oct;8:55–64. doi: 10.1111/j.1471-4159.1961.tb13526.x. [DOI] [PubMed] [Google Scholar]