Abstract
Bioactive lipid mediators play crucial roles in promoting the induction and resolution of inflammation. Eicosanoids and other related unsaturated fatty acids have long been known to induce inflammation. These signaling molecules can modulate the circulatory system and stimulate immune cell infiltration into the site of infection. Recently, DHA- and EPA-derived metabolites have been discovered to promote the resolution of inflammation, an active process. Not only do these molecules stop the further infiltration of immune cells, they prompt non-phlogistic phagocytosis of apoptotic neutrophils, stimulating the tissue to return to homeostasis. After the rapid release of lipid precursors from the plasma membrane upon stimulation, families of enzymes in a complex network metabolize them to produce a large array of lipid metabolites. With current advances in mass spectrometry, the entire lipidome can be accurately quantified to assess the immune response upon microbial infection. In this review, we discuss the various lipid metabolism pathways in the context of the immune response to microbial pathogens, as well as their complex network interactions. With the advancement of mass spectrometry, these approaches have also been used to characterize the lipid mediator response of macrophages and neutrophils upon immune stimulation in vitro. Lastly, we describe the recent efforts to apply systems biology approaches to dissect the role of lipid mediators during bacterial and viral infections in vivo.
Keywords: Lipidomic analysis, Mass spectrometry, Systems biology, Lipid mediators, Proinflammatory, Anti-inflammatory, Pro-resolution, E. coli, Peritonitis, Borrelia burgdorferi, Lyme disease, Influenza
1. Introduction
Microorganisms form most of the biomass in the world. Some of these microbes live in the environment and are innocuous, but some have evolved virulence mechanisms to infect and replicate in mammalian hosts and cause disease. The human body contains many beneficial microflora, but is also subject to frequent challenges by pathogenic bacteria and viruses. Therefore, the ability to discriminately identify and eliminate virulent microbes is essential for the host. The immune system has evolved pattern recognition receptors (PRRs) to recognize microbial components. Upon recognizing a microbial threat, the immune system induces an inflammatory response to recruit leukocytes to the site of infection. The cardinal signs of inflammation include heat, redness, swelling, pain and loss of tissue function [1]. At a molecular level, these symptoms are caused by a signaling cascade, which is promoted by collaborations between cytokines, chemokines, and eicosanoids and related lipid mediators including prostaglandins and leukotrienes. Eicosanoids are a family of bioactive lipid mediators that regulate a wide variety of physiological as well as pathophysiological inflammatory responses [1,2]. These mediators are generated from arachidonic acid (AA) after its enzymatic release from membrane phospholipids via complex metabolic mechanisms involving over 50 unique enzymes [3]. In addition to arachidonic acid, the same enzymes can effectively metabolize other polyunsaturated fatty acids, such as linoleic and linolenic acids. While the induction of inflammation is a highly regulated process to control microbial infection, failure to resolve inflammation can lead to chronic disease or severe tissue damage. A class of anti-inflammatory/pro-resolution lipid mediators, including lipoxins, resolvins, protectins, and maresins, which are derived from AA, DHA and EPA, orchestrate the resolution phase of inflammation [4].
The lipid metabolism network contains multiple precursors, large enzyme families, and over one-hundred lipid species. The complexity of this network is magnified by several characteristics. Multiple enzymes can act on a single substrate, and conversely, multiple substrates can be modified by the same enzyme [1–3] allowing for crosstalk between the pathways. Inhibition or down-regulation of an enzyme within one pathway may “shunt” the substrate through another pathway [2,3,5]. Also, many lipid mediators are susceptible to lipid peroxidation, non-enzymatic oxidation, or other modifications. Often, the in vivo levels of a specific lipid mediator are most accurately inferred by measuring the abundance of a stable degradation product rather than the mediator itself. Lastly, transcellular biosynthesis, a process in which a substrate intermediate produced by one cell type is utilized by another to generate the final lipid mediator [3,4], requires the understanding of interactions between different cell types. For these reasons, a systematic approach is required to understand the entire lipidomic response network.
Although studying the lipid-mediated immune response(s) involves deconvoluting a complicated network, the resulting insights have significant potential for therapeutic and translational impact. Certain lipidomic metabolism pathways have been highly valuable targets for pharmacological interventions. Non-steroidal anti-inflammatory drugs (NSAIDs) have been widely used as over-the-counter analgesics that mainly target the cyclooxygenase pathway [4,6]. Besides the COX pathway, therapeutics have been developed that inhibit the lipoxygenase pathway. For example, the leukotriene receptor antagonists zafirlukast (Accolate) and montelukast (Singulair) have been shown to significantly improve the quality of life for asthmatic patients [7]. Moreover, statins, a class of therapeutics that reduce low-density lipoprotein (LDL) cholesterol levels in humans, also induce the generation of 15-epi-lipoxins, thus having an anti-inflammatory activity [8]. The use of statins and other immunoregulatory compounds may modulate inflammation during influenza [9] or other infections [10]. Lastly, the discovery of anti-inflammatory/pro-resolution lipid mediators that have the ability to modulate excessive inflammation in a wide range of animal disease models [8,11], including cystic fibrosis [12], sepsis [13] and colitis (IBD) [14], provides a potential new class of pharmacological compounds.
2. Role of eicosanoid and other bioactive lipid mediators in the induction and resolution of inflammation
Many lipid mediators have been studied individually to determine their functions in various biological contexts. Receptors recognizing lipid mediators may have one or multiple isoforms expressed differentially in distinct cell types; therefore, the effects of a lipid mediator are likely to be cell and tissue specific [15]. Due to the complexities and vast body of knowledge in the literature, we will not comprehensively discuss the characteristics, functions, and biogenesis of all the bioactive lipids but refer the reader to the many excellent reviews describing the various families of lipid mediators [3,4,16–18]. Instead, we will give an overview of the different classes of lipid mediators in the context of the immune response.
2.1. Arachidonic acid
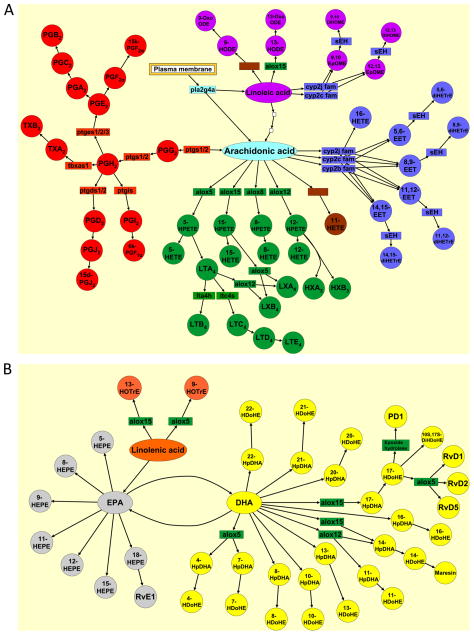
Arachidonic acid (C20:4ω6) is released from the plasma membrane by phospholipases. The three major metabolic pathways for enzymatic arachidonic acid biogenesis are the cyclooxygenase pathway, lipoxygenase pathway, and cytochrome P450 pathway (Fig. 1A). The cyclooxygenase pathway (COX-1 and COX-2) produces prostaglandins and thromboxanes. The lipoxygenase pathway (5-LOX, 12-LOX and 15-LOX) produces leukotrienes, and numerous hydroperoxy, hydroxy fatty acids (HPETEs and HETEs), hepoxilins and lipoxins. Finally, the cytochrome P450 pathway produces epoxides and corresponding dihydroxy metabolites of arachidonic acids (EETs and diHETrEs).
Fig. 1.
Lipid metabolism pathways. Rectangular boxes represent the enzyme catalyzing the reaction. Circles represent the lipid mediators within the pathway. (A) Omega-6 derived lipid metabolites from precursors, arachidonic acid and linoleic acid. (B) Omega-3 derived lipid metabolites from precursors, α-linolenic acid, DHA, and EPA. Red denotes COX pathway; green denotes LOX pathway; blue denotes CYP450 pathway; purple denotes linoleic acid-derived metabolites; orange denotes linolenic acid-derived metabolites; yellow denotes DHA-derived metabolites; and gray denotes EPA-derived metabolites.
The conversion from AA to prostaglandins begins with the catalytic enzymes COX-1 or COX-2. COX-1 is encoded by a constitutively expressed gene ptgs1 and COX-2 is encoded by an immediate early response gene ptgs2 [15]. COX-1/2 produces PGG2 and PGH2, which in turn are converted to various prostaglandins (PGE2, PGD2, PGI2, and PGF2α) or thromboxanes (TXA2 and TXB2) by their cognate synthases. Many prostaglandins have pro-inflammatory activity due to their vasomodulatory effects [19]. PGE2 is one of the most abundant prostaglandins produced in the body. It promotes many of the signs of inflammation due to its ability to augment arterial dilation and increase microvascular permeability; it also induces pain by acting on peripheral sensory neurons and on central sites within the spinal cord and the brain [20]. Recently, PGE2 has been shown to possess anti-inflammatory activity, up-regulating cAMP and inducing the secretion of IL-10, an anti-inflammatory cytokine [21]. PGD2 is synthesized in the central nervous system to regulate neurophysiological functions and is also produced by mast cells, which initiate acute allergic responses [16,22]. In addition to its pro-inflammatory activities, PGD2 can significantly attenuate inflammation in experimental models of pleuritis and colitis [22]. Moreover, PGD2 can be converted into its nonenzymatic degradation product 15d-PGJ2, which inhibits NFκb signaling and activates PPARγ, both contributing to anti-inflammatory effects [23]. PGE2 along with PGD2 also upregulates 15-LOX, giving rise to lipid mediator class switching and promoting the biosynthesis of pro-resolving mediators [24]. PGI2 regulates cardiovascular homeostasis and mediates the edema and pain that accompany acute inflammation [16]. PGI2 exerts its effects locally and is rapidly converted to its inactive hydrolysis product, 6-keto-PGF1α, by nonenzymatic processes [16]. Elevated PGF2α levels are reported in patients with chronic inflammatory diseases, such as rheumatoid arthritis, psoriatic arthritis, reactive arthritis, and osteoarthritis [16]. Because of its instability, the level of PGF2α in vivo is reflected by its major stable metabolite 15k PGF2α [16]. Lastly, thromboxane A2 (TXA2) is an unstable metabolite that degrades into the biologically inactive TXB2 [3]. TXA2 is produced predominantly by platelets and mediates platelet adhesion and aggregation, smooth muscle contraction and proliferation, and activation of endothelial inflammatory responses [16].
Within the LOX pathway, 5-LOX-derived leukotrienes (LTB4, LTC4 and LTE4) have chemoattractant activities and are potent mediators for immediate hypersensitivity, bronchoconstriction, smooth muscle contraction, and increased vascular permeability [25]. 5-HETE, another mediator produced by 5-LOX, has been shown to induce airway contraction and potentiate neutrophil transcellular migration [26]. In contrast to the 5-LOX-derived pro-inflammatory lipid mediators, 12- and 15-HETE, derived from 12-LOX and 15-LOX, have anti-inflammatory activity, blocking TNFα-induced IL-6 secretion from macrophages [27]. 12-LOX derived hepoxilins (HXA3 and HXB3) are highly unstable, but their stable analogs can inhibit macrophage influx as well as fibrosis in the lung [28]. Lipoxins (LXA4 and LXB4), derived from 15-LOX or 5-LOX/12-LOX, are the prototypic members of the endogenous anti-inflammatory/pro-resolution lipid mediators [29].
There are 57 human and 102 mouse functional enzymes within the CYP450 pathway [3]. 16-HETE, an ω-hydroxylated derivative of AA produced by CYP450 enzyme, can inhibit human PMN adhesion and aggregation, as well as decrease LTB4 synthesis [30]. Epoxy- and dihydroxy-derivatives of arachidonic acid (EET and diHETrE) have anti-inflammatory activities due to their ability to activate the peroxisome proliferator-activated receptor alpha (PPARα) pathway [31–33]. Specifically, 5,6 EET and 11,12 diHETrE prevent leukocyte adhesion to the vascular wall [34] and 14,15 EET prevents TNFα-induced IκBα degradation in human airway smooth muscle cells [35].
2.2. DHA and EPA
DHA (C22:6ω3) and EPA (C20:5ω3) are omega-3 fatty acids enriched in marine oil [36] (Fig. 1B). These fatty acids can be hydroxylated by auto-oxidation or other nonenzymatic routes and many of these metabolites may not have biological activities. In contrast, some hydroxylated DHA, such as 13-HDoHE and 17-HDoHE, have direct and potent effects on the anti-inflammatory and pro-resolution processes [37,38]. Furthermore, 18-HEPE, 17-HDoHE, and 14-HDoHE can be metabolized into potent mediators, such as resolvins, protectins, and maresins [39]. These lipid mediators can prevent further infiltration of immune cells to the site of infection as well as signal the non-phlogistic phagocytosis of apoptotic immune and epithelial cells, allowing return to homeostasis after microbial infection. These pro-resolution mediators exert their activities by binding to their cognate G-protein coupled receptors [40].
2.3. Linoleic and linolenic acid
Linoleic acid (C18:2ω6) and α-linolenic acid (C18:3ω3) are essential fatty acids [41]. Linoleic acid, an omega-6 fatty acid, can be converted to arachidonic acid (Fig. 1A), while α-linolenic acid, an omega-3 fatty acid, can be converted to EPA (Fig. 1B). Through the enzymatic activities of CYP450, epoxides of linoleic acid (9,10 EpOME and 12,13 EpOME) are generated. These epoxylated fatty acids are leukotoxins produced by activated neutrophils and macrophages. Elevated levels are associated with ARDS (acute respiratory distress syndrome) and cause pulmonary edema, vasodilation, and cardiac failure in animal models [42,43]. These mediators and their soluble epoxide hydrolase catalyzed mediators DiHOMEs are chemotactic to neutrophils and exert their toxicity by disrupting mitochondrial function [44,45]. Linoleic acid can also be metabolized into 9 or 13 hydroxylated linoleic acids, 9-HODE and 13-HODE. While, 9-HODE production can be catalyzed by multiple enzymes and non-enzymatic reactions, 13-HODE is generated by 15-LOX [46]. Interestingly, 9-HODE has been ascribed a pro-inflammatory role, activating G2A, a G protein-coupled receptor, which mediates intracellular calcium mobilization and JNK activation [46,47], while 13 HODE is an agonist for PPARγ and plays an anti-inflammatory role [48–52]. In contrast to linoleic acid, the 5-LOX-catalyzed α-linolenic acid metabolite 9-HOTrE has no known biological activity; similar to linoleic acid, the linolenic acid metabolite 13-HOTrE, catalyzed by 15-LOX, possesses anti-inflammatory activity as observed in inflammatory joint diseases [53].
3. Technological advances for mass spectrometry of lipids
Since the aforementioned precursors can be metabolized through enzymatic or non-enzymatic processes into over one-hundred different lipid species, the ability to accurately measure a large quantity of mediators simultaneously is essential for profiling the lipidome. Although ELISA can be used to quantify specific metabolites [54], these assays depend on the availability of specific antibodies against the desired target and multiplexing capacity is limited. While GC–MS is able to quantify multiple analytes this approach requires chemical derivatization, which is not suitable for all lipid species. The electrospray ionization (ESI) technology allows for volatilizing lipids from an aqueous sample without prior derivatization. In this approach, lipid species are extracted from samples using solid phase extraction to enhance specificity and sensitivity [55] and deuterated analogs of each analyte are added as internal standards [56]. High performance liquid chromatography (HPLC) and collision-induced decomposition, when combined with ESI-MS and multiple reaction monitoring (MRM), can isolate and identify individual lipid species by matching their MRM signal and LC retention time with those of the pure standards. Quantification is achieved using the stable isotope dilution method and comparison with quantitation standards [57]. Further details of the methodology, including comprehensive descriptions and explanations, have been previously published [56,58,59].
4. Lipidomic profiling of immune cells response to stimuli in vitro
Recent advances in mass spectrometry now allow for the simultaneous measurement of a large number of lipid mediators. Since the macrophage is a crucial innate immune cell type in promoting the regulation of inflammation, it has been utilized as an in vitro model system for interrogating the mechanisms of lipid mediator induction. Ligand binding of Toll like receptor 4 (TLR4) has been shown to activate macrophages to a primed state, and subsequent stimulation with zymosan (TLR2/6 ligand) promoted the release of arachidonic acid [60]. To understand the mechanism of eicosanoid metabolism, Kdo2-LipidA was used as a defined ligand for TLR4 to stimulate a mouse macrophage cell line, RAW264.7 cells [61]. Upon stimulation of TLR4, cyclooxygenase-2 (COX2) was induced transcriptionally. Free arachidonic acid was released, followed by increased levels of secreted COX-derived PGF2α, PGE2, PGD2, and 15-deoxy-Δ12,14-PGD2, and 15-deoxy-Δ12,14-PGJ2. A separate study determined that the eicosanoid production by RAW macrophages was similar even when the different TLR receptors (TLR1–7, TLR9) were stimulated by their cognate ligands [62]. In addition to TLR stimulation, intracellular Ca2+ levels also regulate the production of eicosanoids [63]. Ca2+ influx causes several enzymes, including phospholipase and 5-LOX to localize to the plasma membrane, thus activating lipid mediator metabolism. The interaction between TLR stimulation and Ca2+ influx has been explored in greater depth using combinations of TLR ligands and Ca2+ modulating ligands (PAF and UDP for transient Ca2+ spike and ionomycin and ATP for sustained Ca2+ influx). Data from lipidomic profiling demonstrated a synergy between TLR priming and sustained Ca2+ influx to produce COX- and LOX-derived metabolites [62]. A systems biology approach has been applied further to integrate the transciptomic and proteomic data with lipidomic profiling during TLR stimulation in macrophages [3,64].
In parallel to the multiple T lymphocyte helper cell subsets (Th1, Th2, Th17, Treg), macrophages also have the plasticity to polarize toward different subsets [65]. While M1 is the classical activated cell type that produces pro-inflammatory cytokines and has enhanced microbicidal activity to eliminate pathogens, there is a gradient of alternatively polarized states for M2 macrophages [66]. M2a macrophages, stimulated by IL-4/IL-13, combat parasites and induce allergic reactions. M2b macrophages, stimulated by immune complexes and TLR/IL-1R ligands, are known to produce IL-10 and serve an immunoregulatory function. Finally, M2c macrophages, stimulated by IL-10, are involved in immunoregulation, matrix deposition and tissue remodeling.
Since the induction and resolution of inflammation would likely require different polarized macrophage cell types, Dalli et al. investigated the lipidomic profiles of human phagocytes under various pro-resolving conditions [67]. One of the pivotal events during resolution of inflammation is the clearance of apoptotic neutrophils by macrophages. When co-cultured with apoptotic PMNs, human macrophages produced increased levels of pro-resolving mediators, such as resolvin D1, D2, and lipoxin B4, along with prostanoids, including PGE2. When comparing lipidomic profiles of M1 (polarized by IFNγ and LPS) versus M2 (polarized by IL-4) macrophages, the pro-inflammatory M1 macrophages produced more pro-inflammatory mediators, such as PGE2, PGF2α, TXB2, and the EPA-derived prostanoids. In contrast, M2 macrophages produced more pro-resolving mediators, such as resolvin D5 and E2, maresin 1, protectin D1, and lipoxins (LXA4, LXB4, and LXB5) [67]. In addition, this study demonstrated that during efferocytosis, a key step in the resolution of inflammation, apoptotic cells and microparticles donate precursors to macrophages that are utilized for the biosynthesis of pro-resolving lipid mediators.
These in vitro systems have yielded a tremendous amount of data and insights into the lipidomic response of a specific cell type under defined stimulating conditions. However, during a microbial infection, multiple cell types with specific expression patterns and activation statuses work cooperatively to ensure a controlled process of inducing and resolving inflammation.
5. Lipidomic profiling in infection models
5.1. Roles of lipid mediators in bacterial infections
Bacterial pathogens have evolved a plethora of virulence mechanisms that interact with the host to cause a wide array of diseases. Conversely, the mammalian immune system relies on extracellular (TLR) and intracellular (NOD-like receptor, NLR) receptors, to recognize pathogens by their microbial components [68]. Upon ligation of these receptors and activation of signaling cascades, the host immune system mounts a coordinated response to recruit neutrophils, macrophages, and monocytes to the site of infection to combat the pathogen. While the physiological activities of many lipid mediators in modulating the host response have been characterized individually, several recent studies have examined the lipid metabolism network as a whole in the context of bacterial infection in different animal models.
Lyme disease is a vector-borne disease caused by the bacterium Borrelia burgdorferi. Untreated infection can manifest in diverse pathologies, the most common of which is arthritis [69]. The mouse model recapitulates key aspects of the disease, including the accumulation of macrophages and neutrophils at the site of infection [70]. Moreover, infection in a susceptible mouse strain (C3H) leads to edema and inflammation in the tibiotarsal joints, eventually developing into severe arthritis [70]. In contrast, infection in a resistant mouse strain (DBA) only leads to a mild inflammatory response and disease. By conducting a comprehensive analysis of B. burgdorferi infection in C3H versus DBA mice, Blaho et al. defined distinct lipidomic profiles for each of these infections [71]. The most dramatic differences between the infections in each strain were the elevated levels of the pro-inflammatory leukotriene E4, as well as the anti-inflammatory/pro-resolving protectins, PD1, in the infection of the susceptible strain compared to the resistant strain. This study was significant both because it defined the lipidomic profiles during an active bacterial infection and because many metabolites were identified, including CYP450 and 12-LOX products, that had not been previously associated with the immune response to infection.
While B. burgdorferi is transmitted to humans by tick bites, other bacterial pathogens, such as Escherichia coli, Listeria mono-cytogenes, and Salmonella typhimurium, are ubiquitous and can be transmitted via contaminated food [72]. To understand the role of lipid mediators in promoting the induction and resolution of inflammation in response to E. coli, Chiang et al. conducted lipidomic analysis in a mouse model of peritonitis [73]. They compared bacterial inocula that lead to a self-limiting inflammatory response (105 CFU E. coli per mouse) to inocula that resulted in exacerbated inflammation and delayed resolution (107 CFU E. coli per mouse). The self-limiting infection was characterized by an increased level of pro-resolving, anti-inflammatory lipid mediators, including resolvins (RvD5) and protectins (PD1), compared to the delayed-resolving infection. This bias toward a pro-resolving profile was concurrent with the cellular profile of the infected tissues. The number of PMNs peaked early, at 12 h post self-limiting infection, followed by an influx of monocyte/macrophages. In contrast, in the infection with delayed resolution, the number of PMNs peaked at 24 h and was sustained through 48 h. Furthermore, the authors determined that the pro-resolving lipid mediators were able to enhance the phagocytic activities of the immune cells, prevent hypothermia, and improve survival of the infected animals. Besides limiting bacterial burden when added exogenously, these pro-resolving lipid mediators allowed the use of a lower dose of antibiotic to treat the infection (ciprofloxacin against E. coli in the peritonitis model and vancomycin against Staphylococcus aureus in the skin infection model). This is especially significant because antibiotic resistance of bacterial pathogens is an increasingly severe problem. Intracellular sensing of bacterial pathogens by NLRs has been identified as an important second signal after TLR ligation and occurs through activation of a multi-protein complex called the inflammasome [68]. The activated inflammasome, through caspases, induces processing of the pro-inflammatory cytokines IL-1 and IL-18 and triggers the pro-inflammatory cell death pathway known as pyroptosis [74]. Infection with S. typhimurium and Legionella pneumophila activate the inflammasome in vivo [75]. In order to study activation of the inflammasome in the absence of other potentially confounding microbial components, von Moltke et al. utilized FlaTox (a fusion protein of the L. pneumophila flagellin, FlaA, and the amino-terminal domain of Bacillus anthracis lethal factor) [76]. Interestingly, systemic activation of the inflammasome in mice led to an “eicosanoid storm”, characterized by rapid production of COX and LOX derived metabolites (prostaglandins, leukotrienes, but not lipoxins). The aberrant release of these pro-inflammatory eicosanoids led to hemoconcentration, fluid accumulation, diarrhea, hypothermia, and death within 30 min. These expeditious physiological phenotypes were due to the uniquely primed states of the resident peritoneal macrophages. In contrast, bone marrow derived macrophages did not produce the same lipid mediators upon FlaTox stimulation [76]. This study was the first to describe a link between eicosanoid production and inflammasome activation. In addition, while activation of the inflammasome causes secretion of pro-inflammatory IL1β/IL18 and pyroptosis as eventual consequences, this data suggests that caspase activation and rapid calcium mobilization may trigger early release of bioactive lipid mediators as a primary effector function.
The use of FlaTox allows for precise activation of the inflammasome without triggering an extraneous host response; while the selective nature of the activation makes FlaTox a useful tool, it results in a somewhat artificial system. Further animal experiments using Salmonella or Legionella will be important to determine the physiological consequences of inflammasome activation. Lipidomic analyses of these infections could confirm the induction of pro-inflammatory lipid mediators. In addition, these analyses could reveal whether anti-inflammatory/pro-resolving mediators, especially those derived from DHA or EPA, are also produced during normal infection to prevent catastrophic events, such as rapid hemoconcentration. Lastly, inoculation with purified, exogenous lipid mediators would determine if any anti-inflammatory/pro-resolution metabolites could ameliorate the pro-inflammatory response and prevent mortality.
Many bacterial pathogens cause acute infection with transient clinical symptoms; others have evolved to modulate the immune response to establish persistent or chronic infection [77]. Mycobacterium tuberculosis (Mtb) is a Gram-positive bacterium that causes the respiratory disease tuberculosis (TB), characterized by granuloma formation. An estimated one-third of the world population is infected with M. tuberculosis but remains asymptomatic [78]. These latent infections are persistent and can be reactivated to cause active TB. The vaccine strain, BCG, has not been particularly effective in providing immune protection [79]. To make matters worse, multi-drug resistant (MDR), extensively drug-resistant (XDR), and totally drug-resistant (TDR) strains of TB are beginning to emerge [80].
The impact of host-genetic variation on the immune response leading to the various outcomes after Mtb infection is not well understood. In a zebrafish M. marinum infection model, a mutation in the locus lta4h, encoding the enzyme involved in producing leukotriene B4, was shown to cause a hypersusceptibility phenotype in zebrafish [81]. The hypersusceptibility was due to an increased shunting of the leukotriene B4 (LTB4) precursor LTA4 to produce the anti-inflammatory lipoxin A4 (LXA4), resulting in dysregulated TNF signaling. To further dissect the mechanism, Tobin et al. measured the lipidomic profile of zebrafish during M. marinum infection and determined that excesses of either anti-inflammatory LXA4 or pro-inflammatory LTB4 induced macrophage necrosis [82]. The dysregulated cell death program allowed for exuberant extracellular growth of the bacterium. Perturbation of the crucial balance between pro- and anti-inflammatory responses to modulate the LTA4H-TNF axis was achieved by both genetic methods (overexpressing or knock-down of LTA4H) and pharmacological interventions (aspirin, 15-LOX inhibitor, LTB4 receptor antagonist, and dexamethasone). These disruptions influenced the levels of TNF production, altering the host susceptibility to infection. Importantly, this led the investigators to identify a human genetic polymorphism at the lta4h promoter that is associated with the beneficial or detrimental effects of therapeutics [82]. The host responds differentially to various pathogens to ensure successful containment of the infection by relying on lipid mediators to induce the appropriate pro- and anti-inflammatory programs. Some pathogens may have evolved virulence mechanisms to exploit this process. For example, a secreted 15-LOX homolog, LoxA, was discovered in P. aeruginosa that may be able to modulate the host response via biosynthesis of the anti-inflammatory 15-HETE [83].
These lipidomic profiling studies have yielded intriguing insights into the role of lipid mediators in promoting the induction and resolution of inflammation during bacterial infections. Moreover, these studies identified potential drug targets and treatments, either using pro-resolving lipid mediators or chemical compounds to influence lipid metabolism or signaling.
5.2. Role of lipid mediators in viral infections
A virus hijacks the host machinery to replicate itself. Mechanisms leading to activation of the immune response to viral pathogens have been the focus of intensive research. Host cells use various receptors including TLRs and intracellular sensors, such as RIG-I like receptors (RLRs), AIM2-like receptors (ALRs), and DExD/H box proteins DDXs/DHXs, to induce an antiviral type I interferon response against RNA and DNA virus infections [84,85]. Influenza virus is an enveloped, negative-sense, single-stranded RNA virus belonging to the family Orthomyxoviridae. Different strains of influenza virus can have varying levels of pathogenicity. For example, the notorious and highly pathogenic 1918 pandemic H1N1 strain is believed to induce a “cytokine storm”, which is responsible for devastating tissue damage and resulting fatality [86–88]. While seasonal influenza strains typically have low mortality rates, the recently emerging avian strains H5N1 and H7N9 appear to be highly pathogenic. There is significant evidence that different influenza strains induce qualitatively different host responses, potentially influencing the disease outcome.
Recently, multiple groups have investigated how lipid mediators can regulate inflammation during an influenza infection. Morita et al. profiled lipid mediators in the lungs of infected animals during early events (up to 48 h post infection) using a mouse influenza model [89]. When comparing infection with the mouse-adapted PR8/H1N1 strain to uninfected PBS controls, several pro-resolving mediators, including 12-HETE, 15-HETE, 17-HDoHE, and PD1 were found at a lower abundance in infected animals. This was significant because the same mediators were able to inhibit influenza infection in vitro when added exogenously. Using the 2009 H1N1 pandemic strain, a highly pathogenic H5N1 strain and its avirulent variant, the lipidomic profile indicated that production of PD1 is suppressed only in the H5N1 infection. Upon exogenous addition (i.v.), a biological isomer of PD1 was able to lower the mortality rate of animals during lethal influenza infection. Furthermore, the investigators discovered that this PD1 isomer prevents influenza replication by inhibiting RNA export from the nucleus. Interestingly, this beneficial effect of the protectin pathway was not linked to its anti-inflammatory/pro-resolution activities.
Using a similar approach, we have profiled the lipidome in the broncho-alveolar lavage of mice infected with PR8 (at either a sub-lethal or lethal dose) or a low pathogenicity strain, X31/H3N2, during the course of influenza infection (day 3–19 post infection) [90]. Surprisingly, metabolites within the COX pathway showed similar kinetics between all three infection conditions. Metabolites derived from the LOX and CYP450 pathways, and those derived from linoleic acid, DHA, and EPA, had distinct profiles in the two viral infections during the resolution phase of inflammation. By analyzing the metabolites based on their pro-or anti-inflammatory activities, we discovered a normal sequence of events during mild influenza infection with X31: the pro-inflammatory response peaked early and diminished, followed by an anti-inflammatory response later in the course of infection. In contrast, PR8 (at both a sublethal or lethal dose) induced overlapping pro- and anti-inflammatory responses. Furthermore, when we analyzed the metabolites derived from the LOX pathway, 5-LOX metabolites correlated with the pathogenic phase of the infection whereas 12/15-LOX metabolites were associated with the resolution phase. Lastly, some of our findings in the mouse model were validated by the lipidomic profiles of nasopharyngeal lavages from human influenza clinical samples obtained during the 2009–2011 influenza seasons.
5.3. Comparison between viral and bacterial infection
Although comparing the host-response to infection with different pathogens is a challenging task, this approach can identify common, fundamental immune mechanisms as well as highlight unique features of each pathogen that drive disease. We have compared the lipidomic profiles between the E. coli peritonitis model [73], Lyme arthritis model [71], and the influenza infection model [89,90] and determined the fold-change over uninfected or mock-infected controls for a selected number of representative metabolites (Fig. 2).
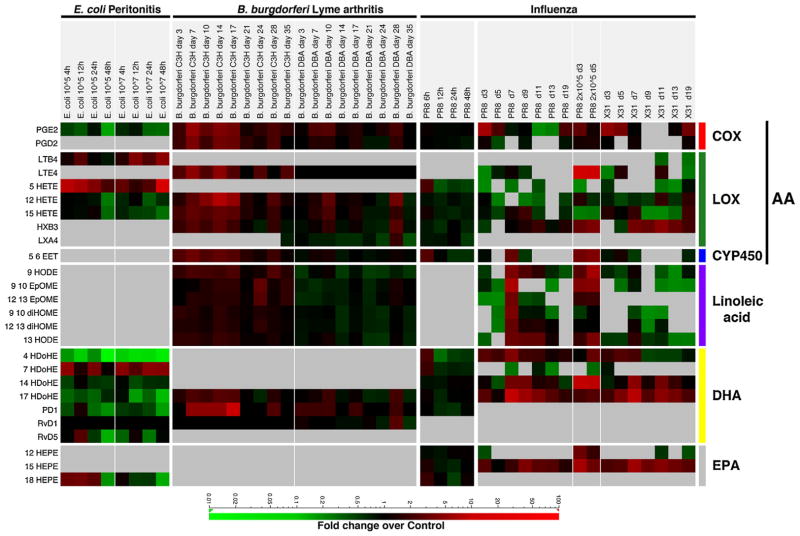
Fig. 2.
Comparative lipidomic analysis. Fold change of measured metabolites over uninfected or mock-infected controls represented as a heat map. Data included in the analysis: self-limiting E. coli infection (105 CFU) or delayed resolution (107 CFU) in the peritonitis model [73], B. burgdorferi infection of susceptible strain (C3H) or resistant strain (DBA) [71], influenza infection with highly pathogenic PR8 strain at early time points [89], and PR8 strain (sublethal at 200 PFU, lethal 2 × 105 PFU) or low pathogenicity strain, X31 (sublethal at 2 × 105 PFU) from day 3 to day 19 post-infection [90].
In all of these infection models, leukotriene B4 or E4 was associated with adverse disease outcomes, such as delayed resolution of E. coli infection, severe arthritis in B. burgdorferi infection of the susceptible mouse strain, and lethality in influenza infection with the high-pathogenicity strain PR8. This is not surprising because the sustained recruitment of PMNs would delay resolution of inflammation and lead to increased cellular damage from neutrophils. Conversely, the anti-inflammatory EPA-derived metabolites are associated with self-limiting E. coli and low pathogenicity X31 (influenza) infections, both of which are self-resolving. One interesting discrepancy between the infection models is the production of PD1. During E. coli and influenza infections, production of PD1 was down-regulated compared to uninfected controls. However, during B. burgdorferi infection, especially in the susceptible mouse strain, PD1 production was up-regulated. While linoleic acid-derived metabolites were only measured in the Lyme arthritis and influenza models, significantly increased levels were detected in the susceptible B. burgdorferi infection and high pathogenicity influenza infection (either sublethal or lethal). This concurrence between the two animal models suggests that linoleic acid-derived metabolites are associated with the pathogenic phase of infection.
We have shown that the ratio of 13:9 HODE is a potential biomarker for immune status during influenza infection [90]. The ratio of 13:9 HODE increased during the resolution phase of infection and was significantly elevated in the low pathogenicity infection, indicating a bias toward an anti-inflammatory, pro-resolution state. During B. burgdorferi infection, the ratio of 13:9 HODE was higher in the resistant mouse strain than in the susceptible strain at every time point, suggesting that the ratio may be useful as a biomarker for immune status in other infection models. We have also determined that infection with the high pathogenicity PR8 influenza virus was associated with a dysregulated immune program in which the pro-inflammatory and anti-inflammatory responses overlapped [90]. This was also recapitulated in the Lyme arthritis model, exemplified by the increased levels of LTE4, linoleic acid-derived metabolites (pro-inflammatory) and 12-HETE, 15-HETE, 5, 6-EET, PD1 (anti-inflammatory/pro-resolving). While the similarities and differences between the lipidomic profiles in the various infection models are intriguing, additional datasets and further experiments will be required to determine the breadth and biological significance of these observations.
6. Conclusion
The innate immune response is the first line of defense against microbial pathogens. The recruitment of mononuclear cells and PMNs is crucial in mounting an effective response. However, if the induction of inflammation is left unchecked, the immune response can become detrimental to the host. Lipid mediators allow a rapid response to pathogens due to the readily availability of the precursors and enzymes. Stimulation of innate immune cells in vitro with Toll ligands and other immune modulators induces rapid and robust production of a wide range of lipid mediators. Lipidomic analysis also revealed differences in activation status of macrophages [61], as well as various polarized subsets of macrophages [67]. The similarly dynamic lipidomic profiles observed in various infection models provided informative insights for the status and progress of inflammation. A common theme is that aberrant production of the pro-inflammatory or anti-inflammatory/pro-resolution lipid mediators often leads to a severe disease outcome or mortality. Importantly, several biomarkers, chemical interventions, and potential drug targets have been identified in these studies.
7. Future directions
While utilizing animal models has been fruitful in biomedical research, in the future, more emphasis will likely be placed on examining human clinical samples. The genetic uniformity and precisely defined experimental conditions available in an animal model is an advantage for basic research, but the clinical relevance of studying human biological samples is indisputable. Computational tools and systems biology approaches will be required to unravel the complexities of human genetic variability and uncertain environmental and epidemiological conditions. Many studies have undertaken the task of determining the lipidomic profiles in human samples, such as exhaled breath condensate from asthma patients [91], blood samples from patients undergoing abdominal aortic aneurysm surgery [92], and our study of the nasopharyngeal lavage from influenza-infected patients [90]. Undoubtedly, lipidomic analysis will be applied to understand the role of lipid mediators in other human diseases. From the animal models discussed above, we have learned that genetic deficiencies or hypomorphic variants may disrupt the lipid metabolism networks in complicated manners, often due to crosstalk between the enzymes and substrates. The integration of transcriptomic, proteomic, and lipidomic analysis that has been successfully applied in vitro [3] will be critical to overcome the challenges of human clinical samples. Confirmation of the relevance to human disease of specific metabolic pathways identified in animal studies will elucidate potential drug targets that can then be perturbed in animal models to determine their impact on the pathogenesis of disease. This continuous and iterative process, using both animal models and human clinical samples, will surely generate important insights into combating infectious diseases.
Acknowledgments
We thank Charles Serhan and Nan Chiang for providing additional data for comparative analysis and for their valuable advice. We thank Jesmond Dalli and Karsten Gronert for their helpful suggestions and insight. Lastly, we thank Alan Diercks and Emily Pierson for critical reading of the manuscript. This work was supported by National Institute of Allergy and Infectious Diseases Contract #HHSN272200800058C, “A Systems Biology Approach to Infectious Disease Research”.
Abbreviations
- AA
arachidonic acid
- ALR
AIM2-like receptor
- CFU
colony forming units
- COX
cyclooxygenase
- CYP450
cytochrome P450
- DDXs/DHXs
DExD/H box proteins
- DHA
docosahexaenoic acids
- diHETrE
dihydroxy-eicosatrienoic acids
- diHOME
dihydroxy-octadecenoic acids
- EET
epoxy-eicosatrienoic acids
- EPA
eicosapentaenoic acid
- EpOME
epoxy-octadecenoic acids
- ESI
electrospray ionization
- GM–MS
gas chromatography coupled with mass spectrometry
- HDoHE
hydroxy-docosahexaenoic acid
- HEPE
hydrox-eicosapentaenoic acids
- HETE
hydroxy-eicosatetraenoic acids
- HODE
hydroxy-octadecadienoic acids
- HOTrE
hydroxy-octadecatrienoic acids
- HPLC
high performace liquid chromatography
- HXA3/B3
hepoxilin A3/B3
- IκB
inhibitor of kappa B
- LC–MS
liquid chromatography coupled with mass spectrometry
- LOX
lipoxygenase
- LT
leukotriene
- LXA4/B4
lipoxin A4/B4
- MRM
multiple reaction monitoring
- NFκB
nuclear factor kappa B
- NLR
NOD-like receptor
- NSAIDs
non-steroidal anti-inflammatory drugs
- oxoODE
oxo-octadecadienoic acid
- PAF
platelet-activating factor
- PD1
protectin D1
- PG
prostaglandin
- PMN
polymorphonuclear cells
- PPAR
peroxisome proliferator-activated receptor
- PRRs
pattern recognition receptors
- RLR
RIG-I like receptor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TXA2/B2
thromboxane A2/B2
- UDP
uridine 5′-diphosphate
References
- 1.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–95. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 2.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812–23. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buczynski M, Dumlao D, Dennis E. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–38. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci USA. 2012;109:8517–22. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong CKS, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5:19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riccioni G, Vecchia Della R, Di Ilio C, D’Orazio N. Effect of the two different leukotriene receptor antagonists, montelukast and zafirlukast, on quality of life: a 12-week randomized study. Allergy Asthma Proc. 2004;25:445–8. [PubMed] [Google Scholar]
- 8.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2009;153:S200–15. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–35. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Wen X, Peng J, Lu Y, Guo Z, Lu J. Systematic review and meta-analysis on the association between outpatient statins use and infectious disease-related mortality. PLoS ONE. 2012;7:e51548. doi: 10.1371/journal.pone.0051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–84. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–92. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 13.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–7. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- 15.Harizi H, Corcuff J-B, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–9. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 18.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2008;50:S52–6. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtzman MJ. Arachidonic acid metabolism in airway epithelial cells. Annu Rev Physiol. 1992;54:303–29. doi: 10.1146/annurev.ph.54.030192.001511. [DOI] [PubMed] [Google Scholar]
- 20.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 21.Sha W, Brüne B, Weigert A. The multi-faceted roles of prostaglandin E2 in cancer-infiltrating mononuclear phagocyte biology. Immunobiology. 2012;217:1225–32. doi: 10.1016/j.imbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Joo M, Sadikot RT. PGD synthase and PGD2 in immune resposne. Mediators Inflamm. 2012;2012:503128. doi: 10.1155/2012/503128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57:703–8. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 24.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 25.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittleman DB, Casale TB. 5-Hydroxyeicosatetraenoic acid (HETE)-induced neutrophil transcellular migration is dependent upon enantiomeric structure. Am J Respir Cell Mol Biol. 1995;12:260–7. doi: 10.1165/ajrcmb.12.3.7873191. [DOI] [PubMed] [Google Scholar]
- 27.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, et al. 12/15-Lipoxygenase counteracts inflammation and tissue damage in arthritis. J Immunol. 2009;183:3383–9. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 28.Jankov RP, Luo X, Demin P, Aslam R, Hannam V, Tanswell AK, et al. Hepoxilin analogs inhibit bleomycin-induced pulmonary fibrosis in the mouse. J Pharmacol Exp Ther. 2002;301:435–40. doi: 10.1124/jpet.301.2.435. [DOI] [PubMed] [Google Scholar]
- 29.Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–20. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Bednar MM, Gross CE, Russell SR, Fuller SP, Ahern TP, Howard DB, et al. 16(R)-hydroxyeicosatetraenoic acid, a novel cytochrome P450 product of arachidonic acid, suppresses activation of human polymorphonuclear leukocyte and reduces intracranial pressure in a rabbit model of thromboembolic stroke. Neurosurgery. 2000;47:1410–8. discussion 1418–9. [PubMed] [Google Scholar]
- 31.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med. 2012;2012:605101. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray JA, Sugden MC, Zeldin DC, Greenwood GK, Samsuddin S, Miller-Degraff L, et al. The epoxygenases CYP2J2 activates the nuclear receptor PPARα in vitro and in vivo. PLoS ONE. 2009;4:e7421. doi: 10.1371/journal.pone.0007421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol. 2010;48:331–41. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. EET displays anti-inflammatory effects in TNF-alpha stimulated human bronchi: putative role of CPI-17. Am J Respir Cell Mol Biol. 2008;38:192–201. doi: 10.1165/rcmb.2007-0232OC. [DOI] [PubMed] [Google Scholar]
- 36.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–41. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–93. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory – pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–47. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–39. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- 42.Ishizaki T, Ozawa T, Voelkel NF. Leukotoxins and the lung. Pulm Pharmacol Ther. 1999;12:145–55. doi: 10.1006/pupt.1999.0179. [DOI] [PubMed] [Google Scholar]
- 43.Ishizaki T, Shigemori K, Nakai T, Miyabo S, Ozawa T, Chang SW, et al. Leukotoxin, 9,10-epoxy-12-octadecenoate causes edematous lung injury via activation of vascular nitric oxide synthase. Am J Physiol. 1995;269:L65–70. doi: 10.1152/ajplung.1995.269.1.L65. [DOI] [PubMed] [Google Scholar]
- 44.Totani Y, Saito Y, Ishizaki T, Sasaki F, Ameshima S, Miyamori I. Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP. Eur Respir J. 2000;15:75–9. doi: 10.1183/09031936.00.15107500. [DOI] [PubMed] [Google Scholar]
- 45.Sisemore M. Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Arch Biochem Biophys. 2001;392:32–7. doi: 10.1006/abbi.2001.2434. [DOI] [PubMed] [Google Scholar]
- 46.Obinata H, Izumi T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat. 2009;89:66–72. doi: 10.1016/j.prostaglandins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Hattori T, Obinata H, Ogawa A, Kishi M, Tatei K, Ishikawa O, et al. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol. 2007;128:1123–33. doi: 10.1038/sj.jid.5701172. [DOI] [PubMed] [Google Scholar]
- 48.Emerson MR, LeVine SM. Experimental allergic encephalomyelitis is exacerbated in mice deficient for 12/15-lipoxygenase or 5-lipoxygenase. Brain Res. 2004;1021:140–5. doi: 10.1016/j.brainres.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 49.Belvisi MG, Mitchell JA. Targeting PPAR receptors in the airway for the treatment of inflammatory lung disease. Br J Pharmacol. 2009;158:994–1003. doi: 10.1111/j.1476-5381.2009.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altmann R, Hausmann M, Spöttl T, Gruber M, Bull AW, Menzel K, et al. 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochem Pharmacol. 2007;74:612–22. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 51.Stoll LL, Morland MR, Spector AA. 13-HODE increases intracellular calcium in vascular smooth muscle cells. Am J Physiol. 1994;266:C990–6. doi: 10.1152/ajpcell.1994.266.4.C990. [DOI] [PubMed] [Google Scholar]
- 52.Fritsche KL. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot Essent Fatty Acids. 2008;79:173–5. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Schulze-Tanzil G, de SP, Behnke B, Klingelhoefer S, Scheid A, Shakibaei M. Effects of the antirheumatic remedy hox alpha – a new stinging nettle leaf extract – on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol. 2002;17:477–85. doi: 10.14670/HH-17.477. [DOI] [PubMed] [Google Scholar]
- 54.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powell WS. Extraction of eicosanoids from biological fluids, cells, and tissues. Methods Mol Biol. 1999;120:11–24. doi: 10.1385/1-59259-263-5:11. [DOI] [PubMed] [Google Scholar]
- 56.Deems R, Buczynski MW, Bowers Gentry R, Harkewicz R, Dennis EA. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 2007;432:59–82. doi: 10.1016/S0076-6879(07)32003-X. [DOI] [PubMed] [Google Scholar]
- 57.Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J Am Soc Mass Spectrom. 1998;9:527–32. doi: 10.1016/S1044-0305(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 58.O’Donnell VB, Maskrey B, Taylor GW. Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol. 2008;462:5–23. [PubMed] [Google Scholar]
- 59.Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aderem AA, Cohen DS, Wright SD, Cohn ZA. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986;164:165–79. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, et al. A mouse macrophage lipidome. J Biol Chem. 2010;285:39976–85. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–47. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 63.Aderem AA, Scott WA, Cohn ZA. Evidence for sequential signals in the induction of the arachidonic acid cascade in macrophages. J Exp Med. 1986;163:139–54. doi: 10.1084/jem.163.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabido E, Quehenberger O, Shen Q, Chang CY, Shah I, Armando AM, et al. Targeted proteomics of the eicosanoid biosynthetic pathway completes an integrated genomics-proteomics-metabolomics picture of cellular metabolism. Mol Cell Proteomics. 2012;11:M111, 014746–6. doi: 10.1074/mcp.M111.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 67.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discriminationof pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–52. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 71.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J Biol Chem. 2009;284:21599–612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falkow S, Isberg RR, Portnoy DA. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–63. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 73.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moltke von J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, Van Rooijen N, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–11. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young D, Hussell T, Dougan G. Chronic bacterial infections: living with unwanted guests. Nat Immunol. 2002;3:1026–32. doi: 10.1038/ni1102-1026. [DOI] [PubMed] [Google Scholar]
- 78.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 79.Kaufmann SHE. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol. 2012;33:373–9. doi: 10.1016/j.it.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Müller B, Borrell S, Rose G, Gagneux S. The heterogeneous evolution of multidrug-resistant Mycobacterium tuberculosis. Trends Genet. 2013;29:160–9. doi: 10.1016/j.tig.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tobin DM, Vary JCV, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–30. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci USA. 2004;101:2135–9. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loo Y-M, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–92. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21:413–20. doi: 10.1016/j.tim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–81. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuen KY, Wong SS. Human infection by avian influenza A H5N1. Hong Kong Med J. 2005;11:189–99. [PubMed] [Google Scholar]
- 89.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, et al. The lipid mediator protectin D1 Inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–25. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, et al. Lipidomic profiling of influenza infection identifies mediators that induce-and resolve inflammation. Cell. 2013;154:213–27. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pillai PS, Leeson S, Porter TF, Owens CD, Kim JM, Conte MS, et al. Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation. 2012;35:98–113. doi: 10.1007/s10753-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]