Abstract
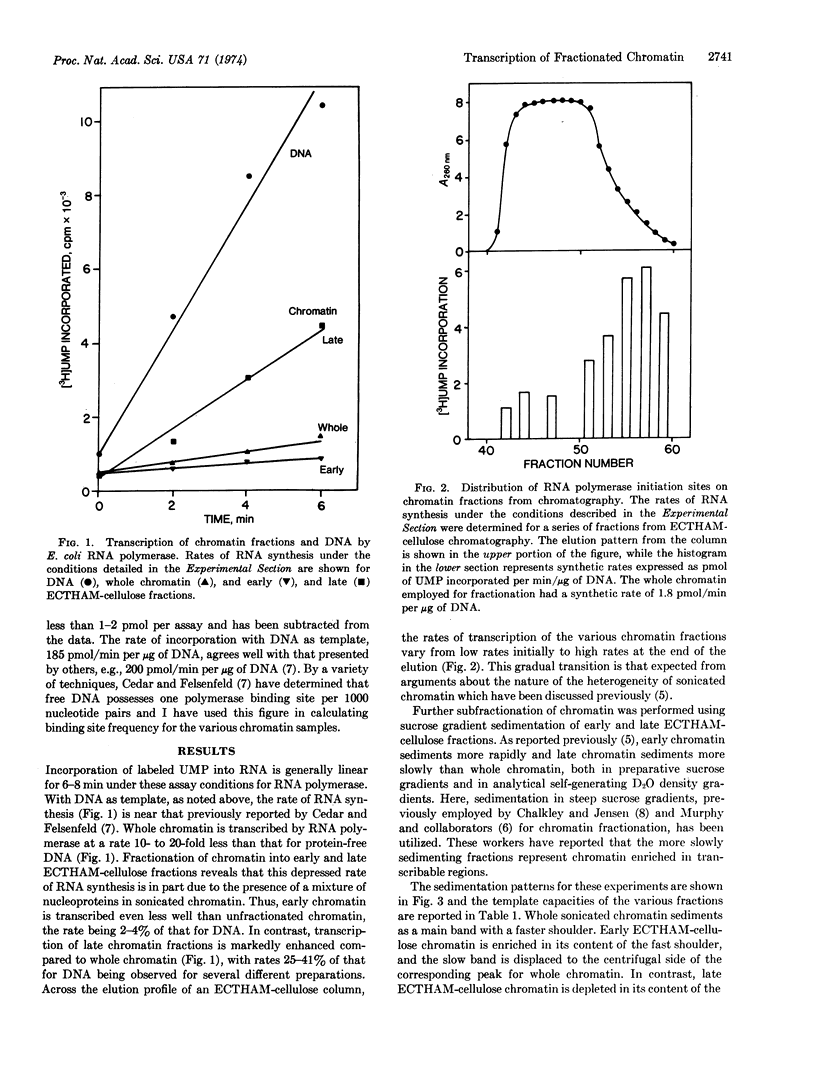
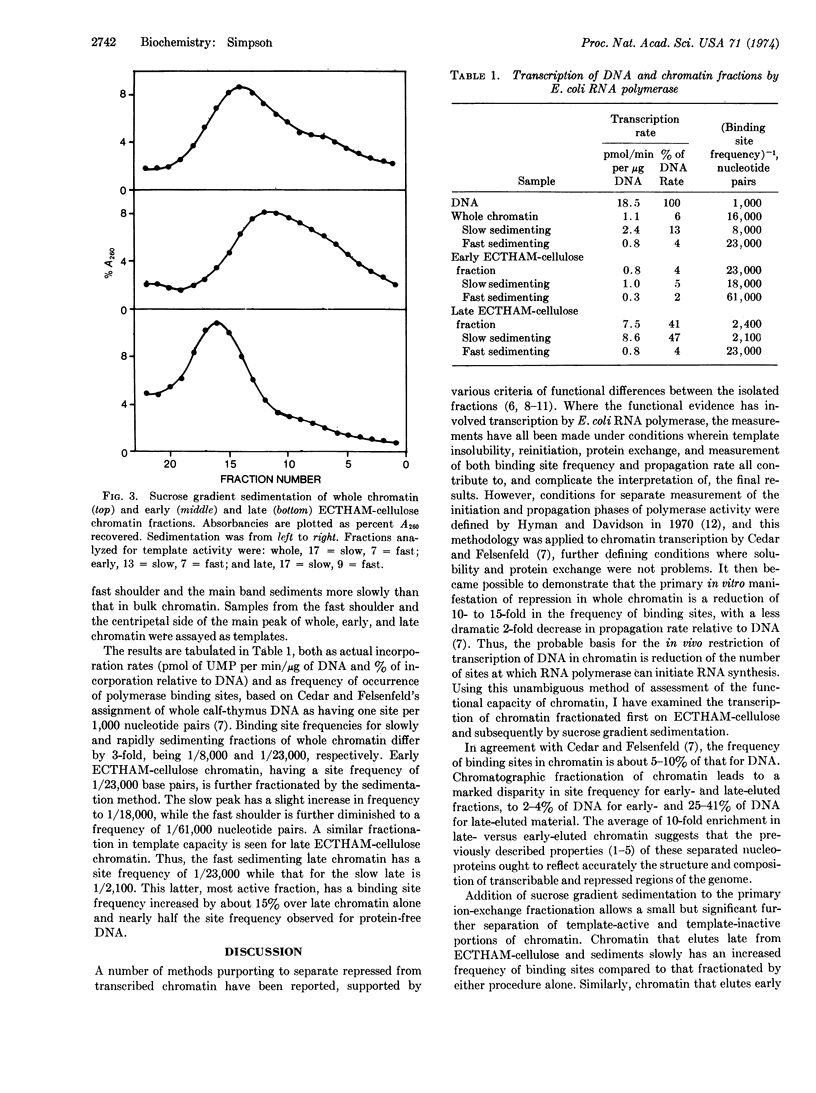
Calf-thymus chromatin was fractionated by ion-exchange chromatography on ECTHAM-cellulose and sucrose gradient sedimentation. [ECTHAM-cellulose is a cationic adsorbent prepared by coupling tris(hydroxymethyl)aminomethane to cellulose with epichlorohydrin.] The capacity of these fractionated chromatins to support RNA synthesis by DNA-dependent RNA polymerase of Escherichia coli was examined, using procedures that permit measurement of binding site frequency. Unfractionated calf thymus chromatin has 5-10% as many binding sites as protein-free DNA. By combination of the two fractionation methods, chromatin samples were obtained containing as few as 2% and as many as 47% of the number of binding sites found on protein-free DNA.
Keywords: transcription, ECTHAM-cellulose, sucrose gradient sedimentation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Chalkley R., Jensen R. H. A study of the structure of isolated chromatin. Biochemistry. 1968 Dec;7(12):4380–4388. doi: 10.1021/bi00852a034. [DOI] [PubMed] [Google Scholar]
- FRENSTER J. H., ALLFREY V. G., MIRSKY A. E. REPRESSED AND ACTIVE CHROMATIN ISOLATED FROM INTERPHASE LYMPHOCYTES. Proc Natl Acad Sci U S A. 1963 Dec;50:1026–1032. doi: 10.1073/pnas.50.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. W., Davidson N. Kinetics of the in vitro inhibition of transcription by actinomycin. J Mol Biol. 1970 Jun 14;50(2):421–438. doi: 10.1016/0022-2836(70)90202-0. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Fractionation of liver chromatin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2941–2944. doi: 10.1073/pnas.68.12.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Hall S. H., Shepherd J. H., Weiser R. S. Fractionation of mouse myeloma chromatin. Biochemistry. 1973 Sep 25;12(20):3843–3853. doi: 10.1021/bi00744a008. [DOI] [PubMed] [Google Scholar]
- Polacow I., Simpson R. T. Circular dichroism spectra of putative transcribed and repressed chromatin. Biochem Biophys Res Commun. 1973 May 1;52(1):202–207. doi: 10.1016/0006-291x(73)90974-1. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Simpson R. T., Sober H. A. Resolution of a spectrum of nucleoprotein species in sonicated chromatin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2317–2321. doi: 10.1073/pnas.69.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Polacow I. Protein-DNA interactions in extended and condensed chromatin. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1078–1084. doi: 10.1016/s0006-291x(73)80005-1. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Reeck G. R. A comparison of the proteins of condensed and extended chromatin fractions of rabbit liver and calf thymus. Biochemistry. 1973 Sep 25;12(20):3853–3858. doi: 10.1021/bi00744a009. [DOI] [PubMed] [Google Scholar]



