Abstract
Objective: We wish to implement a proteomics-based approach to pick and identify the proteins associated with curcumin enhancing efficacy of irinotecan inducing apoptosis of colorectal cancer LOVO cells, and further explore their synergy mechanism by bioinformatics. Methods: A colorectal cancer cell line (LOVO cell) treated by curcumin combined with irinotecan in different ways respectively was used as our comparative model. Protein spots were analyzed through MALDI-TOF/TOF. The location and function of differential protein spots were analyzed through UniProt database. Protein-protein interactions were examined through String software. Results: A total of 54 protein spots differentially expressed with 1.5-fold difference were picked, 11 of which were repeated. They mainly were involved in intracellular calcium pathways, cellular respiratory chain pathway and intracellular redox reaction pathways of LOVO cell. According to the function of various protein points, combining with varying curves of protein points in each treatment groups, we selected five interesting protein spots, 4 of which exists Protein-protein interactions, and they were close to the formation and reduction of disulfides in intracellular endoplasmic reticulum (ER). Conclusion: We selected preliminary but comprehensive data about differential expression protein spots of LOVO cell. Among these, the five interesting differential expression protein spots identified in this study may provide new insight into LOVO cell therapeutic biomarkers. Curcumin may suppress GSTM5 expression to enhance the lethal effect of irinotecan on LOVO cells, and maybe their combination via the affection of PDI and PRDX4 to disturb the formation and reduction of disulfides results in inducing apoptosis of LOVO cell.
Keywords: Curcumin, irinotecan, proteomics, colorectal cancer
Introduction
In recent years, the incidence of CRC has markedly increased in China, particularly in urban populations. And the statistics in recent years show that, as the people’s living standards improving, diet changed, the incidence of colorectal cancer in China is increasing year by year [1,2]. Chemotherapy is an important part of comprehensive treatment for patients with advanced colorectal cancer. From the late 1980s, chemotherapeutic regimen of fluorouracil in combination with leucovorin (FU/LV) became the standard first-line therapy for advanced colorectal cancer, but its response rate is only 25% [3-5], and the median survival is < 1 year [6,7]. Since irinotecan and oxaliplatin were introduced to advanced colorectal cancer treatment, incorporated in to FU/LV-based regimens, FOLFIRI and FOLFOX markedly improve response rate and prolong median survival over fluorouracil with leucovorin (FU/LV) [8-10], and have supplanted FU/LV as the standard systemic approach for advanced colorectal cancer.
Irinotecan (CPT-11) is semi-synthetic camptothecin derivative extracted from chinese unique plant Camptotheca acuminata, its antitumor mechanisms is that irinotecan (predominantly in the form of SN38) binds to the Top I-DNA complex, stabilizing it and preventing re-ligation, colliding with advancing replication forks results in the formation of double stranded DNA breaks. These breaks can activate cell cycle arrest in G2 phase, if unrepaired, it can cause cell death. The toxicity of irinotecan is especially stronger for the S phase of the cell than other phases [11]. With widely used in clinical application, it has found that the efficacy of irinotecan for the tumor cells was 49%, mainly due to their multidrug resistance [12].
Curcumin, the main chemical ingredient of turmeric, is polyphenolic compound, and its pharmacological effect includes anti-tumor, anti-inflammatory, anti-oxidation, anti-atherosclerotic, anti-HIV [13-15]. Previous studies have found that curcumin could enhance the efficacy of the chemotherapy drugs through different mechanisms. It has found that curcumin can inhibit the expression of the epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor (IGF-1R), resulted in enhancing killing effect of 5-FU and oxaliplatin to colon cancer HCT-116 and HT-29 cell lines [16]. It was also found that curcumin could enhance the efficacy of irinotecan inhibiting the growth of LOVO cells [17].
In this study, we planned to implement a proteomics-based approach to pick and identify the proteins associated with curcumin enhancing efficacy of irinotecan inducing apoptosis of colorectal cancer LOVO cell, and further explore their synergy mechanism by bioinformatics.
Materials and methods
Cell culture
Colorectal cancer LOVO cells (ATCC, USA) were used in this study. LOVO cells were cultured as monolayers in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, Brazil) and 1% penicillin/streptomycin. Cells were grown at 37°C in a humidified atmosphere containing 5% CO2.
Experimental groups and drug intervention
Experimental groups
Divided into five groups, each set up three parallel groups, grouped as follows:
Group 1: Control group (no drug intervention group).
Group 2: Irinotecan monotherapy group (treated by irinotecan for 48 h).
Group 3: Curcumin monotherapy group (treated by curcumin for 48 h).
Group 4: Mixed-administered group (treated by curcumin mixed with irinotecan together for 48 h).
Group 5: Sequential treatment group (first treated by curcumin for 48 h, then transformed into irinotecan for 48 h).
Drug intervention
LOVO cells at 5 × 106/dish were expanded to 15 cm cell culture dishes until the cells reached 90% confluence, then washed with sterile PBS for drug intervention. In our Preliminary works, we have been selected the half-inhibitory concentration (IC50) of curcumin and irinotecan on LOVO cells by the MTT assays for 48 h, which the half-inhibitory concentration of curcumin on LOVO cells for 48 h is 10.3 μg/ml, and of irinotecan is 21.2 μg/ml. Drug intervention according to the methods above.
Sample preparation for DIGE
Cells were first harvested, washed with PBS, and pelleted by centrifugation and stored at -80°C until use. Frozen cells samples were weighed (100 mg/mL lysis buffer) and thawed on ice. And then the samples were resuspended in Lysis Buffer (30 mM Tris-HCl, 7 M urea, 2 M thiourea, 4% CHAPS, at pH 8.5), and incubated on ice for 30 minutes. Suspensions were sonicated on ice during sonication to prevent sample heating and used 10 seconds bursts with 30 seconds pauses for 5 times. Lysates were then centrifuged at 12,000 g for 30 minutes. The suspension proteins were then precipitation with 2D Clean-up Kit (GE Healthcare) according to the manufacturer’s protocol and resuspended in Lysis Buffer. Concentration was determined by 2D Quant Kit (GE Healthcare) according to the instruction. Protein was aliquoted to required amounts, frozen or freeze dried. All reagents were supplied by Sigma Chemical Company unless otherwise noted.
Differential in-gel electrophoresis (DIGE)
For DIGE, 50 μg of proteins were minimally labelled with CyDyes at the ratio of 50 μg proteins: 400 pmol Cy3 or Cy5 protein-labeling dye (GE Healthcare) according to the manufacturer’s protocol. Cy3 and Cy5 were used for samples, Cy2 was used for the internal standard (a pool of all samples). Each labeled sample was mixed with rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 2% dithiothreitol, 2% Pharmalyte; GE Healthcare) and applied to a 24 cm immobilized pH gradient gel strip (immobilized pH gradient (IPG) strip pH 3 to 10 NL) for separation in the first dimension. First dimension isoelectric focusing was carried out at 20°C in IPGphor III (GE Healthcare) using the following protocol: 30 V for 12 hours; 1,000 V gradient for 2 hours; 8,000 V gradient for 2 hours; then at 8,000 V for 60,00 volt-hours followed by a 500 V hold. After isoelectric focusing, strips were equilibrated by agitating for 15 minutes in 50 mM Tris-HCl, pH 8.8, 6 M urea, 30% (v/v) glycerol, 2% (w/v) sodium dodecyl sulfate (SDS), 650 mM DTT and then agitating for 15 minutes in 50 mM Tris-HCl, pH 8.8, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 1.27 M iodoacetamide. The strips were next loaded onto a 24 × 24 cm 12% polyacrylamide gel using low fluorescence glass plates and subjected to an electric field in DALT Six (GE Healthcare; 15°C at a constant 3 W per gel, 12 hours in a running buffer containing 25 mM Tris, 192 mM glycine, and 0.1% (w/v) SDS). After the 2-DE run, gels were scanned on a Typhoon 9400 imager (GE Healthcare) and analyzed with DeCyder 2D Software V6.5 (GE Healthcare). Protein spots, which were found differentially expressed with 1.5-fold difference between groups were interesting spots. Samples for the spot picking gel were prepared without labeling by Cydyes, a preparative gel was run using 600 μg of pooled protein sample and stained with colloidal Coomassie blue G-250. Matched spots of interest were picked automatically from the preparative gel by Ettan Spot Picker (GE Healthcare).
Protein identification
The picked spots were destained with 50% acetonitrile (ACN)/100 mM NH4HCO3 for 10 min, dehydrated with 100% ACN for 10 min, and then dried using a centrifugal concentrator (TOMY SEIKO, Tokyo). Next, 2 μl of 25 ng/ml trypsin (Promega) diluted in 50 mM NH4HCO3 was added to each gel piece and incubated for 30 min at 4°C, and then 30 μl of 50 mM NH4HCO3 was added, and then the spots were incubated overnight at 37°C. We used two solutions to extract the resulting peptide mixtures from the gel pieces. First, 100 μl of 60% (v/v) ACN in 0.1% aqueous trifluoroacetic acid (TFA) was added to the gel pieces, which were then sonicated for 15 min. Next, we collected the solution and added 50 μl 100% ACN for the last extraction. Digested peptides were dried with Vacuum Pump and dissolved using 2 μl 50% acetonitrile/0.1% TFA, and aliquots of 0.5 μl were applied on the target disk and air-dried. Then 0.5 μL of matrix solution (CHCA saturated in 50% acetonitrile/0.1% TFA) was added to the dried samples and again allowed to dry. Samples on the MALDI target plates were then analyzed by ABI 4800 Proteomics Analyzer MALDI-TOF/TOF mass spectrometer (Applied Biosystems). For MS analyses, typically 800 shots were accumulated. MS/MS analyses were performed using air, at collision energy of 2 KV. MASCOT search engine (version 2.1, Matrix Science) was used to search all of the tandem mass spectra. GPS ExplorerTM software version 3.6.2 (Applied Biosystems) was used to create and search files with the MASCOT search for peptide and protein identification. Protein identities were obtained by using Mascot searching engine against Swiss-Prot non-redundant sequence databases selected for human taxonomy.
Bioinformatics
The theoretical isoelectric point (pI) and molecular weight (MW) of protein spots were calculated through MALDI-TOF/TOF. The sub-cellular location and function of the identified proteins were elucidated by UniProt knowledgebase (Swiss-prot/TrEMBEL). A protein-protein interactions network was done by STRING software9.05 through inputting IPI number http://string.embl.de.
Results
The main purpose of this study is to define synergy mechanism that curcumin combined with irinotecan pass though different approach effect on colorectal cancer LOVO cells in vitro, by using 2-D electrophoresis to filter out the differential expression of protein spots between the groups, and then identified protein function by MALDI-TOF/TOF.
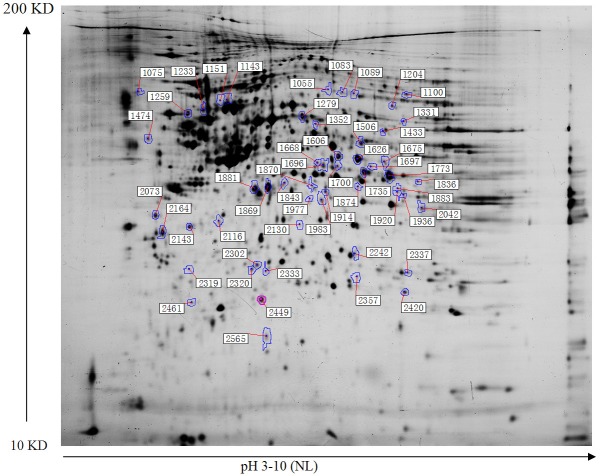
In our study, we adopted 2-D electrophoretic separation of proteome of LOVO cell in different groups, and then by DeCyder 2D Software V6.5, according to the screening principle that differential expression decreasing or increasing 1.5-fold, after the comparison between the different groups, we picked a total of 54 differentially expressed proteins, the position of each spot was shown in Figure 1, on this basis, we further identified all these 54 protein spots by MALDI-TOF/TOF, and found that 11 protein spots were repeating. Information about each spot was shown in Table 1. According to function of all these 43 protein spots, we found that the combination of curcumin and CPT-11 mainly affect intracellular calcium pathways, cellular respiratory chain pathway and intracellular redox reaction pathways of LOVO cell. Among them, proteins associated with intracellular calcium ion channel pathway include Calnexin, Nucleobindin-1, Annexin A1, Annexin A5, Calpain small subunit 1, Translocon-associated protein subunit delta (protein spots marked * shown in Figure 1); Related to intracellular respiratory chain pathway contain NADH-ubiquinone oxidoreductase 30 kDa subunit, NADH-ubiquinone oxidoreductase 23 kDa subunit, Ubiquinone biosynthesis methyltransferase COQ5, Flavoprotein subunit of complex II (spots marked & shown in Figure 1), and correlated to Intracellular redox reaction pathway cover Peroxiredoxin-4, Peroxiredoxin-2, Glutathione S-transferase Mu 5, Protein disulfide-isomerase (spots marked Φ shown in Figure 1), Searching by Uniprot Database, we initially understood the function of each protein spot, combined with our experimental purposes and protein expression changing curve, we picked five interesting protein spots, which included Glutathione S-transferase Mu 5 (GSTM5), Peroxiredoxin-4 (PRDX4), Calpain small subunit 1 (CAPNS1), Translocon-associated protein subunit delta (SSR4) and Protein disulfide-isomerase (P4HB), the positions of the five proteins in 2-D DIGE image were shown in Figure 2.
Figure 1.
Location of 54 picked protein spots in the 2-D gel.
Table 1.
Proteins with CV values were identified by MALDI-TOF/TOF
| Spot score no.a) | Protein name | Access. no.b) | Theoretical | Protein score | Protein (C.V%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Mass (Da) | pI | |||||
| 1055 | Cytokeratin-9 | P35527 | 62254.9 | 5.14 | 46 | 52.33 |
| *1075 | Calnexin | P27824 | 67982 | 4.47 | 75 | 99.941 |
| 1083 | Cytokeratin-9 | P35527 | 62254.9 | 5.14 | 50 | 79.665 |
| 1089 | Keratin, type I cytoskeletal 10 | P13645 | 59045.8 | 5.09 | 140 | 100 |
| Φ1100 | Flavoprotein subunit of complex II | P31040 | 73671.7 | 7.06 | 268 | 100 |
| 1143 | Gamma-actin | P63261 | 42107.9 | 5.31 | 636 | 100 |
| 1151 | Gamma-actin | P63261 | 42107.9 | 6.57 | 226 | 100 |
| 1204 | T-complex protein 1 subunit zeta | P40227 | 58443.8 | 6.23 | 53 | 88.825 |
| *1233 | Nucleobindin-1 | Q02818 | 53846.3 | 5.15 | 173 | 100 |
| &1259 | Protein disulfide-isomerase | P07237 | 57479.8 | 4.76 | 59 | 99.975 |
| 1279 | Cytokeratin-8 | P05787 | 53671.1 | 5.52 | 98 | 100 |
| 1331 | Keratin, type I cytoskeletal 10 | P13645 | 59045.8 | 5.09 | 122 | 100 |
| 1352 | Heterogeneous nuclear ribonucleoprotein H | P31943 | 49483.5 | 5.89 | 278 | 100 |
| 1433 | TBP-interacting protein | Q9Y265 | 50538.4 | 6.02 | 212 | 100 |
| 1474 | Cytokeratin-9 | P35527 | 62254.9 | 5.14 | 41 | 0 |
| 1506 | 26S proteasome non-ATPase regulatory subunit 11 | O00231 | 47719.1 | 6.08 | 38 | 0 |
| 1606 | Serpin B3 | P29508 | 44593.6 | 6.35 | 27 | 0 |
| 1626 | Choline-phosphate cytidylyltransferase A | P49585 | 42047 | 6.82 | 37 | 0 |
| 1668 | Actin regulatory protein CAP-G | P40121 | 38778.6 | 5.88 | 97 | 100 |
| 1675 | L-2-hydroxyglutarate dehydrogenase, mitochondrial | Q9H9P8 | 51068.2 | 8.57 | 40 | 0 |
| 1696 | S-arrestin | P10523 | 45262.5 | 6.14 | 33 | 0 |
| 1697 | Septin-2 | Q15019 | 41689.3 | 6.15 | 138 | 100 |
| 1700 | S-arrestin | P10523 | 45262.5 | 6.14 | 38 | 0 |
| 1735 | Heat shock transcription factor, Y-linked | Q96LI6 | 45249.7 | 6.68 | 43 | 7.051 |
| 1773 | Heterogeneous nuclear ribonucleoprotein M | P52272 | 77749.4 | 8.84 | 29 | 0 |
| 1836 | Keratin, type II cytoskeletal 1 | P04264 | 66170.1 | 8.15 | 43 | 0 |
| 1843 | Keratin, type I cytoskeletal 9 | P35527 | 62254.9 | 5.14 | 72 | 99.859 |
| 1869 | E3 ubiquitin-protein ligase | Q969Q1 | 41134 | 4.85 | 34 | 0 |
| 1870 | Keratin, type I cytoskeletal 16 | P08779 | 51578.3 | 4.99 | 94 | 100 |
| 1874 | Heat shock transcription factor, Y-linked | Q96LI6 | 45249.7 | 6.68 | 33 | 0 |
| 1881 | F-actin-capping protein subunit alpha-1 | P52907 | 33073.4 | 5.45 | 140 | 100 |
| *1883 | Annexin A1 | P04083 | 38918.1 | 6.57 | 158 | 100 |
| Φ1914 | Ubiquinone biosynthesis methyltransferase COQ5 | Q5HYK3 | 37401.9 | 6.47 | 33 | 0 |
| *1920 | Annexin A1 | P04083 | 38918.1 | 6.57 | 138 | 100 |
| 1936 | Heterogeneous nuclear ribonucleoprotein H3 | P31942 | 36960.1 | 6.37 | 83 | 99.991 |
| 1977 | Protein phosphatase 1K, mitochondrial | Q8N3J5 | 41484.8 | 6.27 | 25 | 0 |
| 1983 | Inorganic pyrophosphatase 2, mitochondrial | Q9H2U2 | 38409.2 | 7.07 | 43 | 4.886 |
| 2042 | Zinc finger protein 550 | Q7Z398 | 49490.5 | 8.95 | 33 | 0 |
| 2073 | Keratin, type II cytoskeletal 1 | P04264 | 66170.1 | 8.15 | 116 | 100 |
| &2116 | Glutathione S-transferase Mu 5 | P46439 | 25829.3 | 6.9 | 35 | 0 |
| 2130 | Keratin, type I cytoskeletal 9 | P35527 | 62254.9 | 5.14 | 48 | 67.771 |
| *2143 | Annexin A5 | P08758 | 35971.4 | 4.94 | 255 | 100 |
| 2164 | Keratin, type I cytoskeletal 10 | P13645 | 59045.8 | 5.09 | 108 | 100 |
| 2242 | Septin-14 | Q6ZU15 | 50449.4 | 5.87 | 31 | 0 |
| &2302 | Peroxiredoxin-4 | Q13162 | 30748.9 | 5.86 | 321 | 100 |
| *2319 | Calpain small subunit 1 | P04632 | 28468.8 | 5.05 | 55 | 93.999 |
| 2320 | Diphthine--ammonia ligase | Q7L8W6 | 30688.5 | 5.34 | 25 | 0 |
| Φ2333 | NADH-ubiquinone oxidoreductase 30 kDa subunit | O75489 | 30336.8 | 6.99 | 105 | 100 |
| 2337 | Sulfotransferase 1C4 | O75897 | 35667.9 | 8.22 | 50 | 81.022 |
| &2357 | Glutathione S-transferase Mu 5 | P46439 | 25829.3 | 6.9 | 30 | 0 |
| 2420 | Sulfotransferase 1C4 | O75897 | 35667.9 | 8.22 | 31 | 0 |
| &2449 | Peroxiredoxin-2 | P32119 | 22049.3 | 5.66 | 329 | 100 |
| Φ2461 | NADH-ubiquinone oxidoreductase 23 kDa subunit | O00217 | 24202.8 | 6.00 | 90 | 99.998 |
| *2565 | Translocon-associated protein subunit delta | P51571 | 19157.7 | 5.76 | 109 | 100 |
This table is based on Figure 1, different protein spots are separated by 2-D electrophoresis in the combination of curcumin and irinotecan in different manners on the effects of LOVO cells, and then use MALDI-TOF/TOF to identify various spots.
refers to proteins associated with calcium ion channel pathway;
refers to proteins associated with the cellular respiratory chain pathway;
refers to proteins associated cellular redox reaction pathway.
Spots marked Red are interesting protein spots selected in our study, intends to further explore the function that these five spots play in synergy mechanism curcumin combined with irinotecan.
Figure 2.
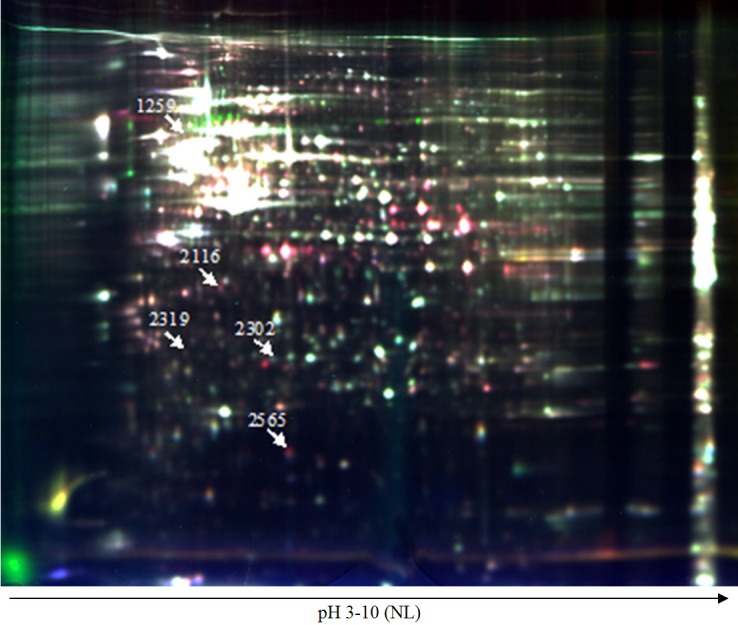
The position of five interesting protein spots in 2-DIGE image. A representative 2-D DIGE image of samples from the five groups (24 cm, pH 3-10, NL). Cy2 (blue) image of proteins from an internal standard is the pool of all the samples, Cy3 (green) image of proteins from NC group, CPT-11 monotherapy group, sequential treatment group, and Cy5 (red) image of proteins from curcumin monotherapy group, mixed-administered group. Spot numbers correspond to those in Table 1.
For the changing curve of Glutathione S-transferase Mu 5 expression between different groups (Figure 3), we found that, compared with the negative control group, after curcumin treatment, GSTM5 expression was downregulated 1.03-fold, however, after be treated by irinotecan, GSTM5 expression was upregulated 3.10-fold, compared with irinotecan monotherapy group, GSTM5 expression in the mixed-administered group and sequential group was gradually decreased by 1.21-fold, 1.58-fold respectively.
Figure 3.
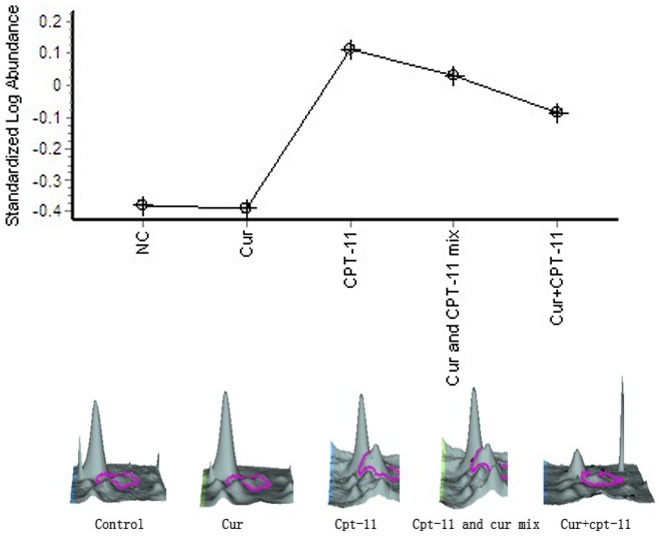
Glutathione S-transferase Mu 5.
According to Peroxiredoxin-4 (PRDX4) variation curve (Figure 4), we found that, compared with the negative control group, PRDX4 expression was upregulated in curcumin monotherapy group, irinotecan monotherapy group, mixed-administered group and sequential administration group, of which the most significant effect was in the curcumin monotherapy group and mixed-administered group, the ratio of PRDX4 expression in the curcumin, irinotecan monotherapy groups compared with the negative control group were upregulated by 1.55-fold, 1.09-fold respectively, compared to irinotecan monotherapy group, PRDX4 expression in the mixed-administered group increased in multiples of 1.38, but which in the sequential administration group decreased by 1.0-fold.
Figure 4.
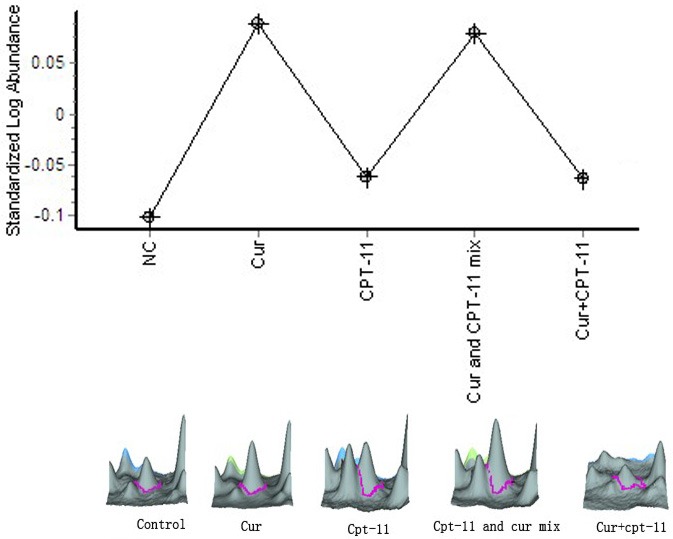
Peroxiredoxin-4.
Base on Calpain small subunit 1 (CAPNS1) variation curve (Figure 5), we found that, compared with the negative control group, CAPNS1 expression in each treatment group was downregulated, of which in the curcumin, irinotecan monotherapy group were reduced by 1.89-fold, 1.37-fold respectively, while in the mixed-administered and sequential group, CAPNS1 expression was gently increased by 1.38-fold, 1.04-fold respectively in contrast to irinotecan monotherapy group, shows that the effect of curcumin for inhibiting CAPNS1 expression was the most significant among all groups, however, when we used the combination of curcumin with irinotecan to treat LOVO cell in vitro, the result showed that not only they did not show their synergistic effect in suppressing CAPNS1 expression, but also display their antagonism, this implies the mechanism the two drugs play in inhibiting the expression of CAPNS1 may be not the same.
Figure 5.
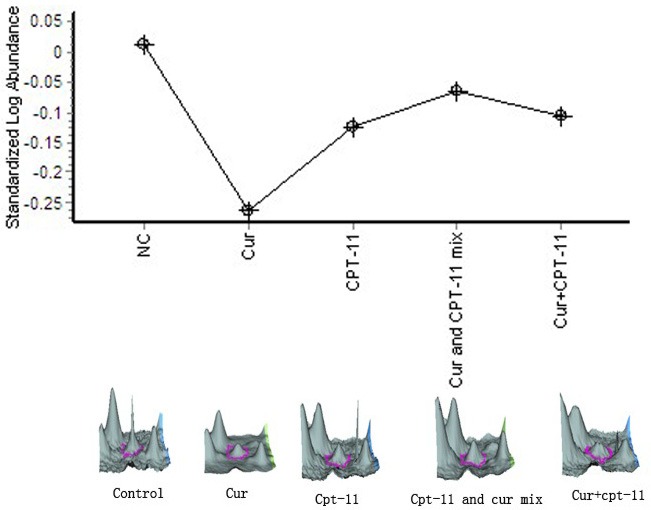
Calpain small subunit 1.
On the basis of Translocon-associated protein subunit delta (SSR4) variation curve (Figure 6), we found that, the changing curve was similar to that of GSTM5, compared with the negative control group, SSR4 expression in the curcumin monotherapy group was decreased by 1.67-fold, while in the irinotecan monotherapy group was increased by 2.44-fold. When using combination of two drugs in mixed-administered and sequential administration ways, we found the SSR4 expression was gradually inhibited in this two groups, reduction at 1.57-fold, 2.08-fold, which was compared to irinotecan monotherapy group.
Figure 6.
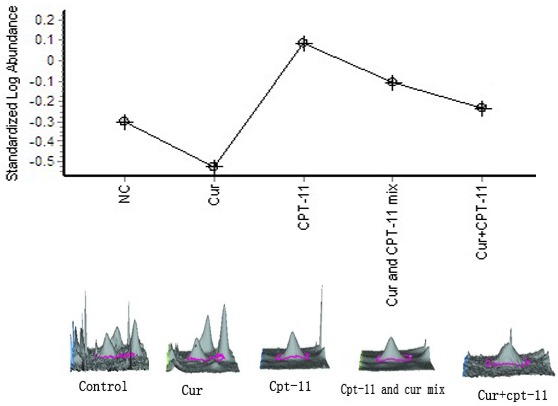
Translocon-associated protein subunit delta.
In the light of protein disulfide-isomerase (P4HB) changing curve (Figure 7), we found that, compared with the negative control group, P4HB expression between curcumin, irinotecan monotherapy group was no significant, and curcumin, irinotecan monotherapy gently raised P4HB expression at 1.06-fold, 1.05-fold respectively, compared to irinotecan monotherapy group, P4HB expression in mixed-administered group was increased by 1.22-fold, while in the sequential group was slightly reduced at 1.07-fold.
Figure 7.
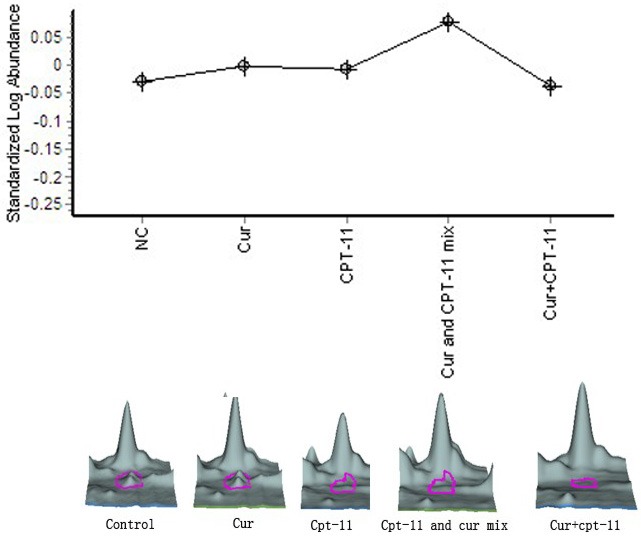
Protein disulfide-isomerase. These five Pictures above are changing curves and three-dimensional maps of five interesting spots selected in this study in different groups, diagram showing the various trends under different treatments, in which the three-dimensional map marked by red arrows and pink circles are interesting proteins.
Based on database STRING, we use STRING9.05 software to search for interactions between the 43 protein spots, which were shown in Figure 8, the score of the required confidence according to STRING9.05 software searching for protein-protein interactions: Low confidence: 0.150; medium confidence: 0.400; high confidence: 0.700; highest confidence: 0.900. In this study, we found that, the score of interaction between P4HB with PRDX4 is 0.682, between SSR4 with PRDX4 the score is 0.602, the score for interaction between P4HB with CAPNS1 is 0.418, in accordance with its standard division, the score for interaction between these four proteins is between medium confidence and high confidence, which illustrates that these four protein spots have a close relationship, and involving intracellular signaling pathways is still worth our further exploration.
Figure 8.
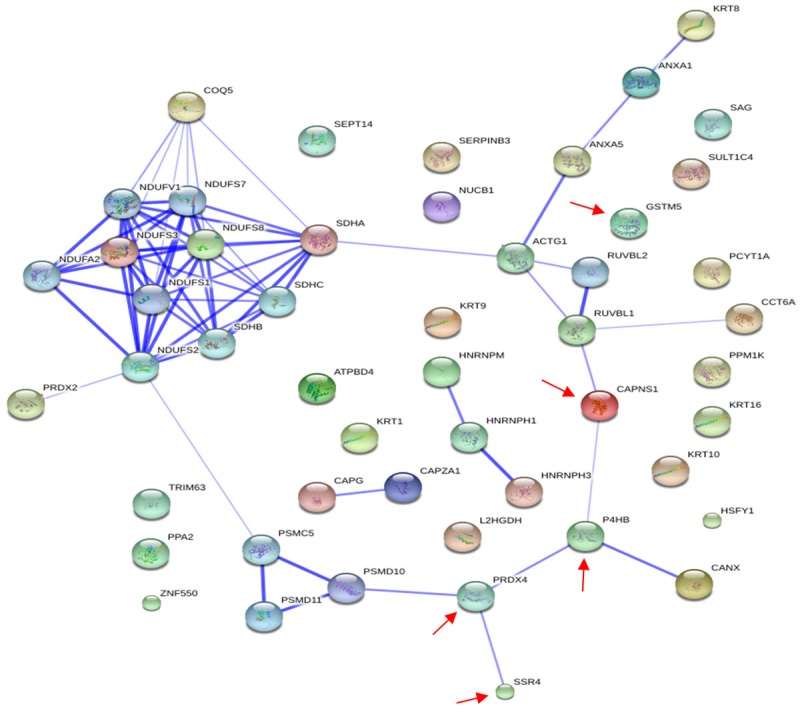
Interaction graph of 43 protein spots. This figure is based on the database STRING, by using software STRING software9.05 to generate automatically interaction graph of 43 protein spots, in which marked by the red arrow are interesting spots in our study.
Discussion
The purpose of this study is to find out targets of curcumin and irinotecan acting on LOVO cells, and further to pick protein spots abnormally expressed in LOVO cells treated with irinotecan and curcumin in different combinational manners. Thus we can figure out the synergy mechanism of these two drugs to LOVO cells. By 2D electrophoresis, we found that, compared with negative control group, curcumin monotherapy group, irinotecan monotherapy group, mixed-administered group and sequential treatment group could change the expression of protein points in LOVO cells. We screened out a total of 54 differential expressed protein points by mass spectrum identification, 11 of which were repeated ones. Through the retrieval of Uniprot database, we preliminarily made clear the function of each protein point According to the function of various protein points, combining with their varying curves in each treatment group, we selected five interesting protein spots, including Peroxiredoxin-4, Glutathione S-transferase Mu 5, Translocon-associated protein subunit delta, Calpain small subunit 1 and Protein disulfide-isomerase. Furthermore, by using STRING database to retrieve the relationship among these proteins above, we found that there exists protein-protein interactions among Peroxiredoxin-4, Translocon-associated protein subunit delta, Calpain small subunit 1 and Protein disulfide-isomerase. Peroxiredoxin-4, Protein disulfide-isomerase and glutathione are involved in the oxidation-reduction reaction pathway in the endoplasmic reticulum. Peroxiredoxin-4, through the consumption of its mercapto group, generates disulfide bonds and catalyzes hydrogen peroxide into water in the endoplasmic reticulum, to maintain the steady-state of endoplasmic reticulum in cells. Protein disulfide-isomerase (PDI) family play an important role in the formation and reduction of disulfides, the native disulfides formation is very important for the protein stability [18-20]. About the five interesting protein spots selected above, we further explore the synergistic mechanisms between the two drugs to LOVO cells. The pathways PDI family members involve in the formation and reduction of disulfides was thoroughly discussed in the review “Multiple ways to make disulfides” by Bulleid NJ, Ellgaard L [18]. From this review, we got the information about the formation and reduction of disulfides in intracellular endoplasmic reticulum (ER), and also understood that Protein disulfide-isomerase (PDI) family plays an important role in the formation and reduction of disulfides, the native disulfides formation is very important for the protein stability. What interested us is that PRDX4, PDI, GSH and FADH2 which are closed to our research result all participate in the formation of disulfides. Base on this review, we believe the interaction between PDI and PRDX4 is that they all involve in the formation of disulfides, and according to our present research result, maybe the combination of curcumin and irinotecan via the affection of PDI and PRDX4 to disturb the formation of disulfides, and results in inducing apoptosis of LOVO cell, which remains to be further explored.
Glutathione S-transferase Mu 5
Glutathione S-transferase family, chiefly located in the cytoplasm, mainly includes Alpha, Mu, Pi and Theta subtribe. Among them, Mu subtribe can also be divided into M1, M2, M3, M4, M5 and M6 [21]. Glutathione transferase in cells has two primary functions: 1, removing the reactive oxygen species (ROS) in the body; 2, detoxification. In Oxidative stress environment, the expression of GST is up-regulated under regulation of nuclear factor-related factor 2 (Nrf2). GST clears ROS to protect the cell by catalyzing the combination of GSH (glutathione) and ROS. The present studies largely focus on the role of GST in multidrug resistance of cancer cells to chemotherapeutic drugs, namely, the detoxification of GST. GST can reduce the killing effect of chemotherapeutics to tumor cell by combining with chemotherapeutic drugs and forming GSH-chemotherapeutic drugs compound, which increases the water solubility of chemotherapeutics and promotes their excretion from cells. Besides, there exists a coordinated expression between GST and ABC Family in tumor cells. When tumor cells are resistant to chemotherapeutics, the expression of these two proteins is both increased. ABC family can also reduce the concentration of chemotherapeutic drugs in tumor cells and decrease their efficacy by transporting GSH-chemotherapy drugs compound out from cancer cells. Besides, due to the effect of adjustment of the tumor cells to protect themselves, glutathione S-transferase within tumor cells can highly express in secondary, which is also an important cause of the secondary resistance of tumor cells to chemotherapeutic drugs [22,23]. Glutathione S-transferase Mu 5, belonging to Mu subtribe, mainly plays a role of detoxification against foreign and endogenous substances in the body [24,25]. Studies have found that the expression of Glutathione S-transferase Mu 5 in liver of cynomolgus macaque could be up-regulated after the injection of estrogen [26]. In this study, we discovered that the expression of Glutathione S-transferase Mu 5 in LOVO cells was up-regulated after treated with irinotecan. Accordingly, we consider that, after treated with irinotecan, LOVO cells start their own protection mechanisms and raise the expression of intracellular Glutathione S-transferase Mu 5, which increases the water-soluble ability of intracellular irinotecan and promote its efflux. With the concentration of irinotecan decreasing, its ability of LOVO cell killing descends. This principle is inferred on the basis of previous studies and this study. The verification of gene knockout and overexpression of Glutathione S-transferase Mu 5 in LOVO cells conduct remain undone in this research, which is the inadequacy of this research. Interestingly, we found that curcumin could reduce the expression of Glutathione S-transferase Mu 5 as well as the combined application of curcumin and irinotecan. Previous studies have found that curcumin could combine with glutathione to generate a conjugated compound, which may restrain the activity of MRP / GSH-X pump and then reduce intracellular chemotherapeutics efflux mediated by this pump, to increase drug concentration in the cell. In addition, curcumin, through Michael addition reaction, consumes glutathione transferase in tumor cells to reduce the amount of GST [27,28]. In the present study, we have not yet ensured that if curcumin suppressed the expression of Glutathione S-transferase Mu 5 via the same mechanism above. However, on the basis of our study and combined with our previous findings, we propose that curcumin may enhance the lethal effect of irinotecan on LOVO cells through lowering the expression of Glutathione S-transferase Mu 5.
Peroxiredoxin-4
Peroxiredoxin widely exists in various organs in the body. PRDX family has six members, including Peroxiredoxin-1, Peroxiredoxi-2, Peroxiredoxi-3, Peroxiredoxin-4, Peroxiredoxin-5 and Peroxiredoxin-6 [29]. In cells, the principal function of Peroxiredoxin is to remove reactive oxygen species (ROS), which plays the role in protecting cells. The study has found that PRDX was closely related to the occurrence and development of tumor. Because of high metabolic characteristics of tumor cells, there generally exist hypoxic microenvironments in most tumor cells. In oxidative stress environment, tumor cells produce reactive oxygen species (ROS) in their own, which is toxic to cells and can induce tumor cells apoptosis. By catalyzing the decomposition of ROS and clearing it in cells, peroxidase plays a protective role in tumor cells. Besides, PRDX has an important effect in the occurrence of tumor cell as well. One of the significant characteristics of cancel cells is their endless division and proliferation. The reason is that in the cell cycle cancer cells can escape cell cycle monitoring and do not enter normal cell apoptosis cycle. In general, through the normal division and proliferation, normal cells become senescent cells, and thus go into senescence and apoptosis pathway under relevant factors’ regulation. If senescent cells fail to enter the normal way of decline and fall due to genetic mutation, then they will transform into tumor cells. In this cancerous transformation, ROS also play an important part because reactive oxygen species can spur senescent cells transform into tumor cells. While PRDX family can remove the senescent cells in body by their anti-oxidant ability, and inhibit the transformation. And in hypoxic microenvironment of tumor cells, through oxidative stress effect, nuclear factor-related factor 2 (Nrf2) can bind with PRDX promoter, trans-activating the promoter domain and prompting transcription of PRDX, results in increasing PRDX expression. Therefore, as the oxidative stress monitor inside the cell, PRDX is very important for protecting both normal cells and tumor cells, and it may be one target for anticancer drugs in the future [30-32]. In this study, we found that the expression of Peroxiredoxin-4 in LOVO cells was both increased after disposed by curcumin and irinotecan, and, compared with curcumin, irinotecan more significantly raised expression of Peroxiredoxin-4. According to previous research, Peroxiredoxin-4 was in a state of high expression in colon cancer tissues, and the downward expression of Peroxiredoxin-4 can induce apoptosis of colon cancer HCT-116 cells [33], which shows that Peroxiredoxin-4 can maintain the growth of tumor cell and anti-tumor effect can be achieved with the inhibition of its expression. In this study, based on the anti-tumor effect of curcumin and irinotecan, we presumed that curcumin and irinotecan may suppress the expression of Peroxiredoxin-4 to promote LOVO cell apoptosis. But we are confused with the experimental result which displayed that the expression of Peroxiredoxin-4 was up-regulated after treated by curcumin and irinotecan in LOVO cells. Respecting that LOVO curcumin could restrain the intracellular respiratory chain pathway, we assume that curcumin may via inhibiting the aerobic respiration of LOVO cells to raise Peroxiredoxin-4 expression, and it also suggests that Peroxiredoxin-4 may not be the direct target of irinotecan and curcumin, but as a powerful weapon of LOVO cells to resist the killing effect of irinotecan and curcumin, and lethal effect of curcumin and irinotecan to LOVO cells may be enhanced when Peroxiredoxin-4 expression was suppressed.
Calpain small subunit 1
Calpain family, a kind of calcium dependent protease in cytoplasm and activated by combined with the calcium ions, regulates the stretching, migration, death, transcription, and signal transduction in cells. In mammalian cells, calpain 1 and calpain 2 are the major species of the calpain family [34]. They differ mainly in their sensitivity to calcium ion in vitro. Calpain 1 is activated by low micromolar free calcium ions. Studies have found that it has a close relationship with tumor’s occurrence and development, and mediates the invasion and metastasis of tumor cells. In lung cancer cell lines, it is found that the inhibition of the activity of calpain 1 could restrain the cell invasion and metastasis [35]. In breast cancer cells (SKBr3, BT474), prostate cancer cells (PC3) and colon cancer cells (HCT116), it is found that Capns1 had interaction relationships with RasGAP and participated in regulating the cell invasion and survival. What’s more, Capns1 gene promoter is regulated by the Nuclear respiratory factor 1 (NRF-1) and Activator Protein 1 (AP-1), so the inhibition of NRF-1 and AP-1 can down-regulate the expression of Capns1 [36,37]. In the present study, we found that curcumin and irinotecan both efficiently suppressed the expression of Capns1 in LOVO cells, especially the former. In the mixed-administered and sequential group, the expression of Capns1 is inhibited, which can be explained that irinotecan and curcumin can restrain the expression of Capns1 together. However, it was not clear that whether Capns1 played a same role in inhibiting apoptosis and promoting the invasion and metastasis of LOVO cells, just like in non-small cell lung cancer, breast cancer, and prostate cancer cells. In addition, whether curcumin and irinotecan reduce the expression of Capns1 by inhibiting NRF-1 and AP-1 or not remains our further study.
Translocon-associated protein subunit delta
Translocon-associated protein subunit delta, together with TRAP-alpha, TRAP-beta, TRAP-gamma, forms the translocon-related protein. Among them, translocon-related protein, a conventional protein located in the membrane of endoplasmic reticulum, mainly helps calcium ions get into the endoplasmic reticulum by coupled with them [38]. Currently only a few studies focus on TRAP-delta, but it has been affirmed that human TRAP-delta located at the q28 region of X chromosome, an area also encoding isocitrate dehydrogenase 3γ subunit (IDH3G), and both of them are subject to the regulation of a 133 bp, CpG-embedded bidirectional promoter, indicating that there may be exist some similarities between these two ones. IDH3G acting as the rate-limiting enzyme in the tricarboxylic acid cycle, has been proven that it can regulate the metabolism of cancer cells, particularly hypoxia of tumor cells [39]. But whether TRAP-delta own a similar function or not has not been confirmed yet. In the UV -induced melanoma tumors, it is found that the expression of TRAP-delta is associated with tumor staging. At the advanced tumors stage, the expression of TRAP-delta is higher [40]. And in melanoma cell lines TD6b and TD15L2, TRAP-delta is also found at high expression levels. Thus it suggests that TRAP-delta may promote the occurrence and metastasis of melanoma induced by ultraviolet rays, and it also can be explained that TRAP-delta may play a protective role in tumor cells and spur the growth and metastasis of tumor [41]. In our research, disposing LOVO cells with curcumin and irinotecan, we found that curcumin could suppress the expression of TRAP-delta while irinotecan raised it. In each treatment group, the various trend of TRAP-delta expression resembles Glutathione S-transferase Mu 5. So we speculate that after treated with irinotecan LOVO cells may launch their own protection mechanisms, increasing the expression of intracellular TRAP-delta and thereby decreasing injuries of irinotecan to their own. While by inhibiting the expression of TRAP-delta, curcumin not only achieves its own anti-tumor effect but also reduces the resistance of tumor cell to irinotecan and enhances the ability of irinotecan to kill LOVO cells, which may be another synergistic effect mechanism of these two drugs.
Protein disulfide-isomerase
Protein disulfide-isomerase, localized in endoplasmic reticulum as a chaperonin and with a molecular weight of 57 KDa, primarily plays the role of thiol peroxidase which catalyzes the formation, breakage and rearrangement of disulfide bond in cells [42]. It has been reported that the expression of protein disulfide-isomerase is activated in the endoplasmic reticulum stress and the unfolded protein response, the induction of which is also an anti-tumor strategy currently. In human ovarian cancer, prostate cancer, lung cancer, melanoma, glioma and acute lymphoma, Protein disulfide-isomerase is found at high expression levels, and inhibiting the activity of Protein disulfide-isomerase can promote apoptosis of tumor cells, suggesting that Protein disulfide-isomerase may be a choice target of anti-tumor drugs [43]. Studies have found that propynoic acid carbamoyl methyl amides (PACMAs) can effectively suppress the activity of Protein disulfide-isomerase and lead to the accumulation of unfolded protein and misfolding protein, to cause endoplasmic reticulum stress and the unfolded protein response, which can promote the death of prostate cancer cells and also reverse the resistance of the prostate cancer cells to first-line chemotherapeutic drugs [44]. It suggests as well that a safe and effective anti-PDI inhibitor can serve as chemotherapy drug of prostate cancer and also may be used as second-line chemotherapy drug after the failure of first-line chemotherapy for prostate cancer. But whether it has a similar effect in colon cancer requests our continuing research. In addition, glutathioned protein disulfide-isomerase can cause unfolded protein response and interfere with the stability and function of ERα to promote the apoptosis of breast cancer cells [45]. But in hepatoma Hep3B cells, it has discovered that nelumbine could effectively induce apoptosis of Hep3B cells, and the action mechanism was also through the induction of endoplasmic reticulum stress in Hep3B cells. However, PDI expression in Hep3B cells is up-regulated after disposed by nelumbine, which indicates that while endoplasmic reticulum stress can be achieved by inhibiting the expression of PDI, this stress in cells occurs in multi-channel and one-sided up-regulation of the expression of PDI is not necessarily to inhibit endoplasmic reticulum stress [46]. In this study, we find that, compared with the negative control group, all the single-agent curcumin or irinotecan and mixed-administered group have no significant effect on the expression of PDI in LOVO cells while mixed-administered group slightly increases it. But in the sequential group, they can effectively suppress the expression of PDI. It is can be explained that the Sequential administration of curcumin and irinotecan may achieves to promote the apoptosis of LOVO cells, by inhibiting the expression of PDI in these cells and triggering endoplasmic reticulum stress and unfolded protein response, PDI may be a target of the sequence combination effect of these two drugs.
In short, based on our study results, we found that the combination of curcumin and irinotecan can indeed enhance the killing effect of irinotecan to LOVO cells. Relevant mechanism has been analyzed above, but there remain lots of deficiencies in this study. Up to date, we have just proved this effect phenomenon and relevant mechanism in LOVO cell, and our research work has not been carried out in other colon cancer cell lines and animal experiments. In the further study, we will continue to improve our research work in other colon cancer cell lines and animal experiments to provide more adequate experimental basis for the two drugs in joint application.
Acknowledgements
Traditional Chinese medicine bureau of GuangDong Province (No. 2010013) and Science and Technology bureau of FoShan City (No. 201308231) provided funding for this analysis.
Disclosure of conflict of interest
None.
References
- 1.Jin P, Wu ZT, Li SR, Li SJ, Wang JH, Wang ZH, Lu JG, Cui XJ, Han Y, Rao J, Sheng JQ. Colorectal cancer screening with fecal occult blood test: A 22-year cohort study. Oncol Lett. 2013;6:576–582. doi: 10.3892/ol.2013.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan DS. Epidemiological trend and control strategy of colorectal cancer in China. Zhonghua Zhong Liu Za Zhi. 2011;33:481–483. [PubMed] [Google Scholar]
- 3.Petrelli N, Douglass HO Jr, Herrera L, Russell D, Stablein DM, Bruckner HW, Mayer RJ, Schinella R, Green MD, Muggia FM, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: A prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J. Clin. Oncol. 1989;7:1419–1426. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 4.Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: Evidence in terms of response rate: Advanced Colorectal Cancer Meta-Analysis Project. J. Clin. Oncol. 1992;10:896–903. doi: 10.1200/JCO.1992.10.6.896. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell MJ. A phase III trial of 5-fluorouracil and leucovorin in the treatment of advanced colorectal cancer. A Mayo Clinic/North Central Cancer Treatment Group study. Cancer. 1989;63:1026–1030. doi: 10.1002/1097-0142(19890315)63:6+<1026::aid-cncr2820631307>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Bleiberg H. Role of chemotherapy for advanced colorectal cancer: new opportunities. Semin Oncol. 1996;3:42–50. [PubMed] [Google Scholar]
- 7.Ragnhammar P, Hafstrom L, Nygren P, Glimelius B. Asystematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308. doi: 10.1080/02841860151116367. [DOI] [PubMed] [Google Scholar]
- 8.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 9.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 10.De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 11.Arun B, Frenkel EP. Topoisomerase I inhibition with topotecan: pharmacologic and clinical issues. Expert Opin Pharmacother. 2001;2:491–505. doi: 10.1517/14656566.2.3.491. [DOI] [PubMed] [Google Scholar]
- 12.Lucas AS, O’Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10:238–244. doi: 10.1016/j.clcc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Schaffer M, Schaffer PM, Zidan J, Bar SG. Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metab Care. 2011;14:588–597. doi: 10.1097/MCO.0b013e32834bfe94. [DOI] [PubMed] [Google Scholar]
- 14.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S, Kurzrock R. Development of curcumin as an epigenetic agent. Cancer. 2010;116:4670–4676. doi: 10.1002/cncr.25414. [DOI] [PubMed] [Google Scholar]
- 16.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 17.Wang JZ, Chen XW, Zhu DJ, Ju YL. The influence of curcumin combined with irinotecan to the growth of colorectal cancer LOVO cells in vitro. Zhonghua Shi Yan Wai Ke Za Zhi. 2013;30:468–470. [Google Scholar]
- 18.Bulleid NJ, Ellgaard L. Multiple ways to make disulfides. Trends Biochem Sci. 2011;36:485–492. doi: 10.1016/j.tibs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Kakihana T, Nagata K, Sitia R. Peroxides and peroxidases in the endoplasmic reticulum: integrating redox homeostasis and oxidative folding. Antioxid Redox Signal. 2012;16:763–771. doi: 10.1089/ars.2011.4238. [DOI] [PubMed] [Google Scholar]
- 20.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whalen R, Boyer TD. Human glutathione S-transferases. Semin Liver Dis. 1998;18:345–358. doi: 10.1055/s-2007-1007169. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;15:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Leung FC. Acquisition of inverted GSTM exons by an intron of primate GSTM5 gene. J Hum Genet. 2009;54:271–276. doi: 10.1038/jhg.2009.23. [DOI] [PubMed] [Google Scholar]
- 24.Rowe JD, Patskovsky YV, Patskovska LN, Novikova E, Listowsky I. Rationale for reclassification of a distinctive subdivision of mammalian class Mu glutathione S-transferases that are primarily expressed in testis. J Biol Chem. 1998;273:9593–9601. doi: 10.1074/jbc.273.16.9593. [DOI] [PubMed] [Google Scholar]
- 25.Ise R, Kito G, Uno Y. Expression profile of early estradiol-responsive genes in cynomolgus macaque liver: implications for drug-metabolizing enzymes. Drug Metab Pharmacokinet. 2012;27:451–455. doi: 10.2133/dmpk.dmpk-11-nt-147. [DOI] [PubMed] [Google Scholar]
- 26.Sau A, Pellizzari Tregno F, Valentino F, Federici G, Caccuri AM. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 2010;500:116–122. doi: 10.1016/j.abb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Tew KD, Townsend DM. Glutathione-s-transferases as determinants of cell survival and death. Antioxid Redox Signal. 2012;17:1728–1737. doi: 10.1089/ars.2012.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oetari S, Sudibyo M, Commandeur JN, Samhoedi R, Vermeulen NP. Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem Pharmacol. 1996;51:39–45. doi: 10.1016/0006-2952(95)02113-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Wang Y, Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009;286:154–160. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Neumann CA, Fang Q. Are peroxiredoxins tumor suppressors? Curr Opin Pharmacol. 2007;7:375–380. doi: 10.1016/j.coph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Butterfield LH, Merino A, Golub SH, Shau H. From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxid Redox Signal. 1999;1:385–402. doi: 10.1089/ars.1999.1.4-385. [DOI] [PubMed] [Google Scholar]
- 32.Nyström T, Yang J, Molin M. Peroxiredoxins, gerontogenes linking aging to genome instability and cancer. Genes Dev. 2012;26:2001–2008. doi: 10.1101/gad.200006.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leydold SM, Seewald M, Stratowa C, Kaserer K, Sommergruber W, Kraut N, Schweifer N. Peroxireduxin-4 is Over-Expressed in Colon Cancer and its Down-Regulation Leads to Apoptosis. Cancer Growth and Metastasis. 2011;4:7–23. [Google Scholar]
- 34.Zhang B, Wang Y, Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009;286:154–160. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Deng X. Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of mu- and m-calpains. J Biol Chem. 2006;281:35567–35575. doi: 10.1074/jbc.M607702200. [DOI] [PubMed] [Google Scholar]
- 37.Pamonsinlapatham P, Gril B, Dufour S, Hadj-Slimane R, Gigoux V, Pethe S, L’hoste S, Camonis J, Garbay C, Raynaud F, Vidal M. Capns1, a new binding partner of RasGAP-SH3 domain in K-Ras(V12) oncogenic cells: modulation of cell survival and migration. Cell Signal. 2008;20:2119–2126. doi: 10.1016/j.cellsig.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Asangani IA, Rasheed SA, Leupold JH, Post S, Allgayer H. NRF-1, and AP-1 regulate the promoter of the human calpain small subunit 1(CAPNS1) gene. Gene. 2008;410:197–206. doi: 10.1016/j.gene.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann E, Görlich D, Kostka S, Otto A, Kraft R, Knespel S, Bürger E, Rapoport TA, Prehn S. A tetrameric complex of membrane proteins in the endoplasmic reticulum. Eur J Biochem. 1993;214:375–381. doi: 10.1111/j.1432-1033.1993.tb17933.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, VandeBerg JL. Cloning and molecular characterization of a human ortholog of Monodelphis TRAPD in ultraviolet B-induced melanoma. Melanoma Res. 2004;14:107–114. doi: 10.1097/00008390-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Holthuis JC, van Riel MC, Martens GJ. Translocon-associated protein TRAP delta and a novel TRAP-like protein are coordinately expressed with pro-opiomelanocortin in Xenopus intermediate pituitary. Biochem J. 1995;312:205–213. doi: 10.1042/bj3120205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenner V, Nyakatura G, Rosenthal A, Platzer M. Genomic organization of two novel genes on human Xq28: compact head to head arrangement of IDH gamma and TRAP delta is conserved in rat and mouse. Genomics. 1997;44:8–14. doi: 10.1006/geno.1997.4822. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, Zandi E, Petasis NA, Neamati N. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci U S A. 2012;109:16348–16353. doi: 10.1073/pnas.1205226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong Y, Manevich Y, Tew KD, Townsend DM. S-Glutathionylation of Protein Disulfide Isomerase Regulates Estrogen Receptor α Stability and Function. Int J Cell Biol. 2012;2012:273549. doi: 10.1155/2012/273549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon JS, Kim HM, Yadunandam AK, Kim NH, Jung HA, Choi JS, Kim CY, Kim GD. Neferine isolated from Nelumbo nucifera enhances anti-cancer activities in Hep3B cells: Molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine. 2013;20:1013–1022. doi: 10.1016/j.phymed.2013.03.024. [DOI] [PubMed] [Google Scholar]