Abstract
This study is to detect the co-expression of embryonic stem cell-related markers (Oct4 and Sox2) in the carcinogenesis of oral mucosa. The expression profile of these markers was studied by immunohistochemistry assay in rat and human samples. The normal oral mucosa (20 cases) and the transforming oral mucosa (20 cases) were performed in rat samples. The precancerous lesions (20 cases), OSCCs in primary site (116 cases), corresponding epithelial non-cancer tissues adjacent to the OSCC (20 cases) and 46 paired metastatic OSCCs in lymph nodes were performed in human samples. The co-expression of the two markers was defined as both of them are positively detected in the same site of one case under one selected field of microscope. The results indicated that Oct4 and Sox2 were individually detected in normal oral mucosa, but they cannot be co-expressed in the same site of one case. The co-expression of Oct4 and Sox2 (Oct4+Sox2+) was frequently detected in the transforming oral mucosa of rat (16/20), precancerous lesions of human (12/20) and epithelial non-cancer tissues adjacent to the OSCC (18/20). Also, Oct4+Sox2+ profile was remarkable noted in the primary sites of OSCCs (38/116). In the 46 paired OSCCs (primary sites with lymph node metastasis), Oct4+Sox2+ profile (8/46) was less frequently detected than Oct4low/-Sox2low/- (14/46) profile in the metastatic sites. To conclude, this study suggests Oct4 and Sox2 are expressed in normal oral mucosa, premalignant diseases, primary sites of OSCCs and metastasis sites of OSCCs. Oct4+Sox2+ profile may contribute to the malignant transformation of oral mucosa.
Keywords: Carcinogenesis, Oct4, Sox2
Introduction
Oral squamous cell carcinoma (OSCC) occurs in the lining of upper digestive tract, has a remarkable incidence worldwide [1]. OSCC often occurs with metastasis to adjacent and distant organs, and patients suffer from pain, facial nerve paralysis, pathological fractures, recurrence and frequently die under very distressing circumstances [2]. The majority of OSCC patients often have cervical lymph nodal metastasis when diagnosed even at early stage. Hence, it is critical to understand key molecular mechanisms in the carcinogenesis and spread of OSCC, with a view to design individualized- targeted therapies.
Cancer stem cells (CSCs) have been defined as a unique subpopulation in cancers that posse the ability to initiate neoplasm and sustain tumour self-renewal [3]. Also, self-renewal is the property of embryonic stem cells (ESCs), thus suggesting common molecules might exist between CSCs and ESCs [4]. Embryonic stem cell markers (Oct4 and Sox2) can be co-expressed in ESCs, are essential for the maintenance of ESCs pluripotency [5]. In addition, somatic cells can be reprogrammed into induced pluripotent stem cells (iPSC) by Oct4, Sox2 in combination with Klf4 (Kruppel-like factor 4) and c-Myc [6]. Seeding iPS cells in immune-deficient mice can form teratomas [7]. Therefore, the process of reprogramming of iPSC is similar to the generation of cancer stem cells.
Oct4 and Sox2 are expressed in many types of cancer [8,9]. Knocking down these genes could decrease tumour-sphere formation in vitro and inhibit tumour formation in xenograft mouse models [8]. The individual expression of Oct4 and Sox2 are noted in lung adenocarcinoma [10], gliomas [11], gastric carcinoma [12] and OSCCs [9]. Oct4 has two isoforms in human tissues, Oct-4A and Oct-4B [13]. Oct-4A protein, localized to the nuclei of ESCs, is functional for maintaining the pluripotency of ESCs and promoting tumorigenesis. Oct-4B protein, localized to the cytoplasm of ESCs, is not sufficient to maintain undifferentiated state and activate transcription of Oct4 gene [14]. The two variants of Oct4 often lead to misunderstandings in stem cell research and current knowledge of cancer progression.
At present, the correlations of Oct4 and Sox2 with metastasis features of OSCC patient remains poorly understood. In consideration that cancer stem cells might be responsible for metastasis of cancer, we hypothesized that these molecules (Oct4 and Sox2) should represent cancer stem cells and contribute to the carcinogenesis of oral mucosa. In this study, we aimed to investigate the differential expression profile of Oct4 and Sox2 in our OSCC cohort as tumour progress in different grades. In addition, the expression patterns of these ESCs proteins in normal oral mucosa, transforming oral mucosa and precancerous lesions of oral mucosa also were examined.
Materials and methods
Patients and specimens
All biopsies of 116 OSCC patients, epithelial non-cancer tissues adjacent to the OSCC (20 cases) and 20 precancerous lesions were collected from the Department of Pathology, The First Affiliated Hospital of Zhengzhou University, China, between 2011 and 2012. All patients of OSCC did not undergo preoperative radiotherapy and chemotherapy. The clinical data of patients was reviewed according to the International Union against Cancer rules for Head/Neck Cancer reporting [15]. Written informed consent was obtained from all of patients in this analysis and this study was approved by Zhengzhou University Ethics Committee.
Forty adult BALB/c rats (16-18 g, all males) were obtained from the Laboratory Animal Center of Zhengzhou University (Zhengzhou, China). The Ethical Committee of Experimental Animals of Zhengzhou University approved all procedures. Forty rats were randomly equally divided into experimental group (20 cases) and control group (20 cases). The 0.5% 4-nitroquinoline 1-oxide (4-NQO) in propylene glycol was painted on dorsal of tongue three times per week in experimental group rats. After 6 weeks, all forty rats were sacrificed. The tongues were cut off and fixed in 10% neutral formalin for immunohistochemistry assay.
Immunohistochemistry assay
All formalin-fixed, paraffin embedded specimens (human and rat) were used in this study. 5 μm serial paraffin sections were cut off and de-waxed using xylene and re-hydrated through graded alcohols and water. Tissue endogenous peroxidase activity was blocked by incubating sections with 3% H2O2. Antigen retrieval was performed by incubation in 10 mM citrate buffer (pH 6.0) in a pressure vessel for 5 min at 120°C. After the slides were cooled, they were rinsed with distilled water and then covered with 0.1% trypsin (Invitrogen, Grand Land, NY, USA) (pH=7.8) in room temperature for 5 min. The slides were then treated with 5% bovine serum albumin (Invitrogen) to block non-specific binding. The staining of immunohistochemistry assay was done separately for Oct4 and Sox2. The concentration of each primary antibody was Oct4 (Oct-4A, Cell Signaling Technology, Danvers, MA, USA, 1:50) and Sox2 (Stemgent, Cambridge, MA, USA, 1:30). In each reaction, the primary antibody was incubated at 4°C overnight. Then, secondary antibody (horseradish peroxidase labelled goat-anti mouse or rabbit; Invitrogen) was added at room temperature for 30 min, washed three times with PBS. The sections were then stained with diaminobenzidine (Invitrogen) for 3 min. Positive control was an embryonal carcinoma from ovary. Replacement of the antibodies by normal serum was used as negative control. The immunoreactivity of the two proteins was evaluated by two independent pathologists who had no knowledge of the clinicopathologic factors of the patients. The co-expression of these ESC markers (Oct4 and Sox2) is defined as both of them are positively detected in the same site of one case under one selected field of microscope.
Statistical analysis
Comparisons between groups were performed using the paired Student’s t-test. The significance level was taken at P<0.05. The statistical tests were performed with the program, Statistical Package for Social Sciences (SPSS version 14.0, Chicago, IL, USA).
Results
Expression profile and localization of Oct4 and Sox2 in embryonal carcinoma
Three ESC-related markers (Oct4 and Sox2) were nuclear co-expressed in embryonal carcinoma (Figure 1). The expression profile and localization of these markers in embryonal carcinoma was set as a standard in the following immunohistochemical study.
Figure 1.
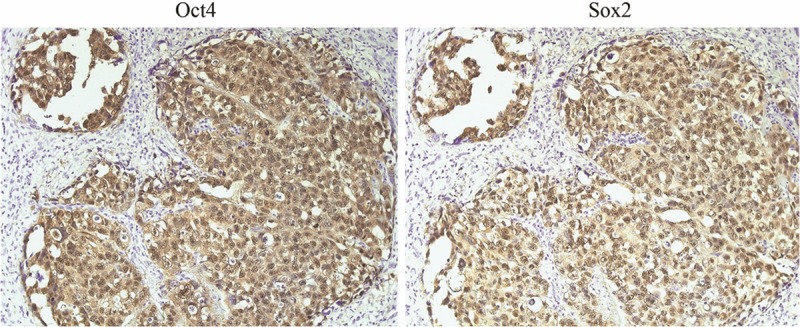
The expression profile of Oct4 and Sox2 in an embryonal carcinoma. Under one selected field of microscope (×200), Oct4 and Sox2 are co-expressed in cell nuclei (Oct4+Sox2+).
Expression profile of Oct4 and Sox2 in rat samples (normal oral mucosa and transforming oral mucosa)
The immunohistochemical results showed that Oct4 and Sox2 could be detected in the basal layer of normal oral mucosa. Sox2 (Figure 2A) and Oct4 (Figure 2B) exhibited nuclear staining, respectively. Of note, they cannot be co-expressed in the same site of one case. In transforming oral mucosa, simple hyperplasia was exhibited. Sox2 nuclear staining was much more frequently detected than that in normal oral mucosa (Table 1). Of note, the nuclear co-expression of Oct4 and Sox2 was observed in these transforming samples.
Figure 2.
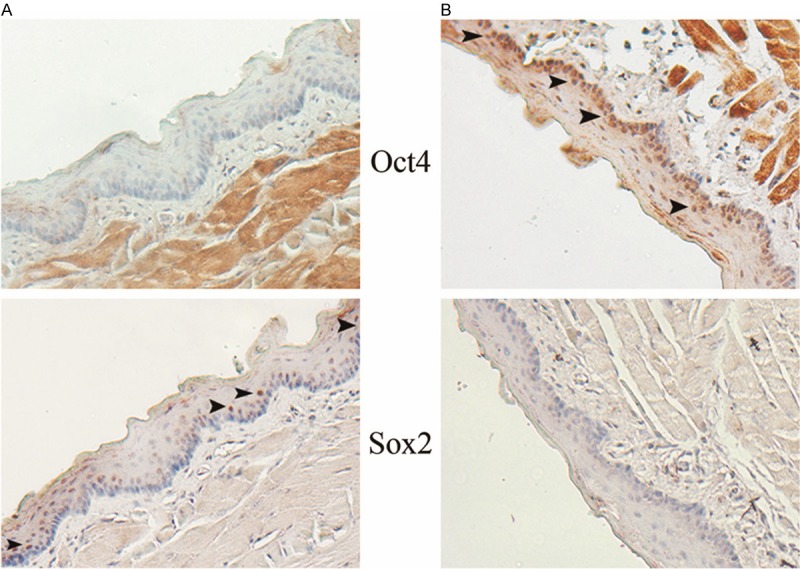
The expression profile of Oct4 and Sox2 in normal oral mucosa of rat samples. Under one selected field of microscope (×160), Oct4 and Sox2 nuclear positive cells are individually detected in different cases, respectively. The arrow heads show the locations of these positive cells are basal layers.
Table 1.
Association of expression profile of ESC-related markers in rat samples
| Oct4 positive (%) | Sox2 positive (%) | Both high expression (%) | Both low expression or negative (%) | |
|---|---|---|---|---|
| Normal (20 cases) | 16 (80) | 5 (25)* | 0 (0)# | 1 (5) |
| Experimental (20 cases) | 17(85) | 16 (80)** | 14 (70)## | 2 (10) |
p<0.05;
p<0.05;
p<0.01;
p<0.01.
Expression profile of Oct4 and Sox2 in human samples (precancerous lesions, epithelial non-cancer tissues adjacent to the OSCC and OSCCs)
There were 12 oral leukoplakia (OLK) and 8 oral lichen planus (OLP) involved in this group of precancerous lesions. Oct4 (14/20) and Sox2 (18/20) were positively detected, respectively. In the co-expression study, the Oct4+Sox2+ profile (12 cases) was predominantly found (Figure 3B).
Figure 3.
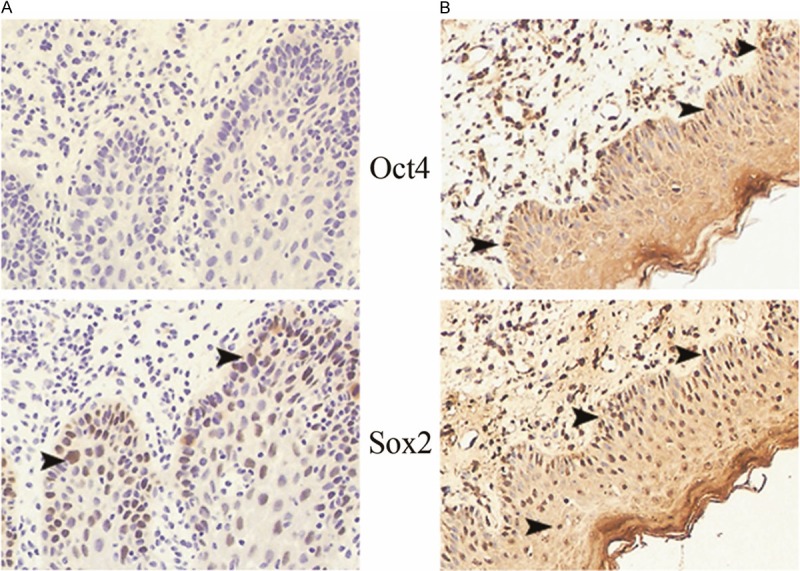
The expression profile of Oct4 and Sox2 in precancerous lesions of human samples (×160). A: A representative sample of the expression profile of Oct4 and Sox2 in OLK. Under one selected field of microscope, nuclear positive cells of Sox2 are demonstrated (arrow heads). The expression of Oct4 is negative. B: A representative sample of the expression profile of Oct4 and Sox2 in OLP. Under one selected field of microscope, Oct4 and Sox2 double positive cells (Oct4+Sox2+) are noted (arrow heads).
Similar results for ESC-related markers were demonstrated in the epithelial non-cancer tissues adjacent to the OSCCs. Oct4 (nuclear) and Sox2 (nuclear) were positively detected, respectively. There were 18 cases showing Oct4+Sox2+ profile in the co-expression study (Figure 4A).
Figure 4.
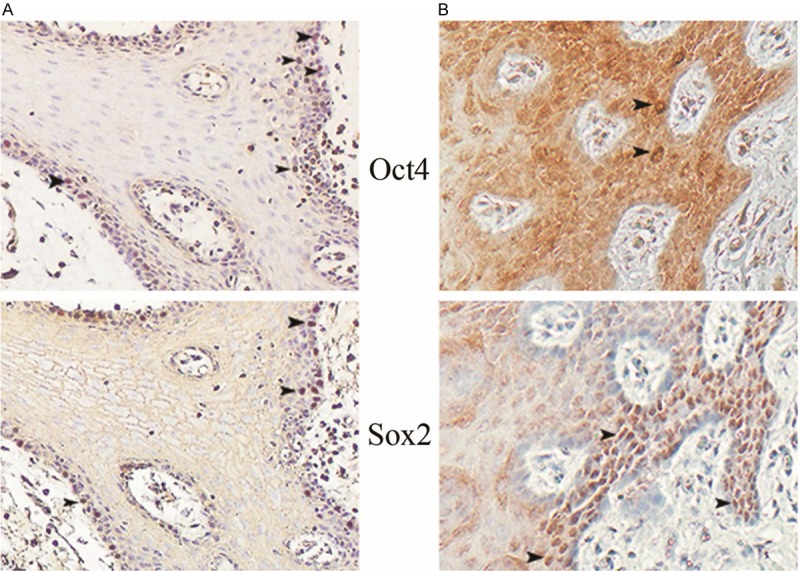
The expression profile of Oct4 and Sox2 in the adjacent non-cancer tissues of human samples and in the transforming tissues of rat samples. The adjacent non-cancer tissues of human samples (A) and the transforming tissues of rat samples (B) show simple hyperplasia in histology. In these two models, Oct4 and Sox2 double nuclear positive cells (Oct4+Sox2+) are frequently observed under one selected field of microscope (arrow heads ×160).
In the OSCC group, there were 116 patients with (46 cases) or without lymph node metastasis (70 cases) involved in this study. According to the TNM classification, 70 cases without lymph node metastasis were in grade I-II (T1-2N0M0), 46 cases with lymph node metastasis were in grade III-IV (T1-4N1-3M0). The clinicopathological details of these OSCC patients were summarized in Table 2. The differential expression profile of Oct4 and Sox2 were summarized in Table 3. In the primary site of 116 cases, the individual expression of Oct4 (70/116) and Sox2 (74/116) were detected in differential clinical grade of OSCCs. The Oct4+Sox2+ profile (38/116) was more frequently demonstrated than Oct4low/-Sox2low/- (11/116) profile. In the 46 cases of lymph nodes, the individual expression of Oct4 (22/46) and Sox2 (30/46) also were detected. However, the Oct4low/-Sox2low/- profile (14/46) was more frequently observed than Oct4+Sox2+ profile (8/46) that contrary to the expression pattern in the primary sites. Within the 46 paired OSCCs (primary sites with lymph node metastasis, grade III-IV), Sox2 profile (single expression or co-expression with Oct4) was detected in all the primary sites (46/46) (Figures 5, 6 and 7). However, the expression profile of Sox2 in the lymph nodes was not always in line with their corresponding primary sites. e.g. Oct4+Sox2+ double positive profile was in the primary site but Oct4low/-Sox2low/- double negative profile was in the metastasis site (Figure 7).
Table 2.
Clinicopathological factors in 116 patients with OSCCs
| No. of cases | |
|---|---|
| OSCCs in primary site (116 cases) | |
| Tongue | 50 |
| Gingiva | 20 |
| Cheek | 40 |
| Hard palate | 6 |
| Gender | |
| Male | 80 |
| Female | 36 |
| Age | |
| <50 years | 46 |
| >50 years | 70 |
| Pathological grade | |
| Well | 54 |
| Mod | 46 |
| Poor | 16 |
| Clinical grade | |
| I-II | 70 |
| III-IV | 46 |
| OSCCs in lymph nodes (46 cases) | |
| N1 | 20 |
| N2 | 22 |
| N3 | 4 |
Table 3.
Association of expression profile of ESC-related markers in precancerous lesions and OSCCs
| Oct4 positive (%) | Sox2 positive (%) | Both high expression (%) | Both low expression or negative (%) | |
|---|---|---|---|---|
| Precancerous lesions (20 cases) | 14 (70) | 18 (90) | 12 (60) | 1 (5) |
| Primary sites of OSCCs (116 cases) | 70 (60) | 74 (64) | 38 (33)a | 11 (9)aa |
| Paired OSCCs (46 cases) | ||||
| Primary sites | 26 (57)* | 46 (100)# | 19 (41) | 0 (0) |
| Metastatic sites | 22 (48) | 30 (65) | 8 (17)b | 14 (30)bb |
| Nodal-negative OSCCs (70 cases) | 44 (63)** | 28 (40)## | 19 (27) | 11 (16) |
p>0.05;
p>0.05;
p<0.05;
p<0.05;
p<0.05;
p<0.05;
p<0.05.
p<0.05.
Figure 5.
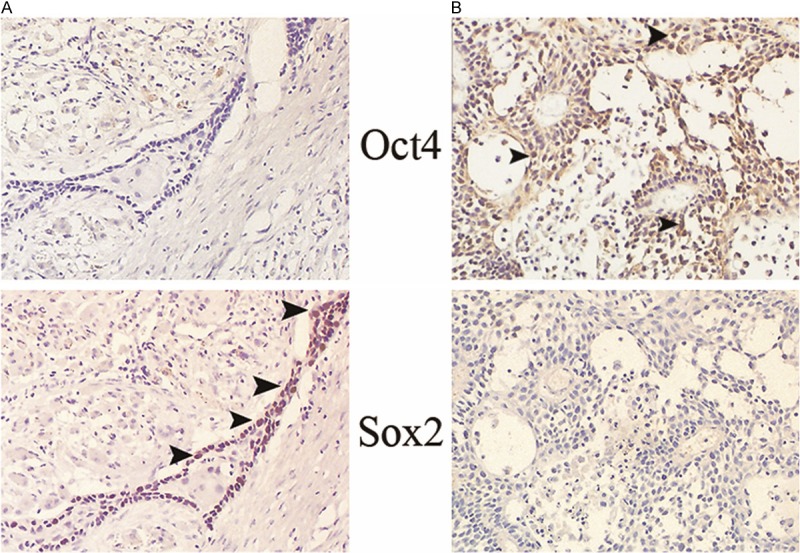
The expression profile of Oct4 and Sox2 in a paired OSCC (well differentiation). Sox2 single positive pattern in the primary site (A), Oct4 single positive pattern in the metastatic site (B) are noted. All the positive cells are demonstrated by arrow heads in the magnification of ×160.
Figure 6.
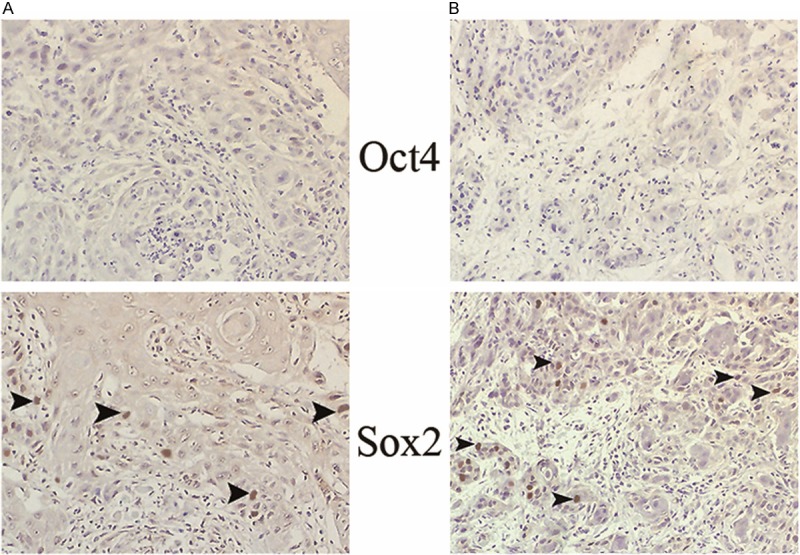
The expression profile of Oct4 and Sox2 in a paired OSCC (moderate differentiation). Sox2 single positive pattern in the primary site (A) and in the metastatic site (B) are noted, respectively. All the positive cells are demonstrated by arrow heads in the magnification of ×160.
Figure 7.
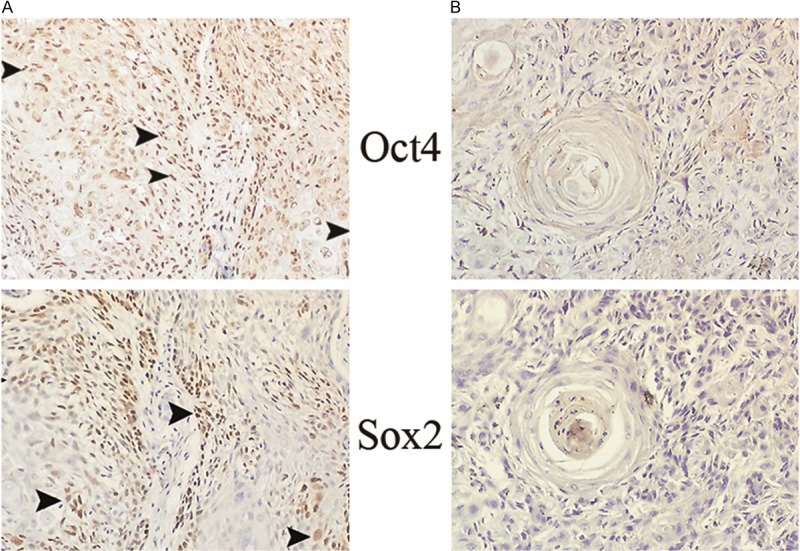
The expression profile of Oct4 and Sox2 in a paired OSCC (poor differentiation). Oct4 and Sox2 double positive pattern in the primary site (A), Oct4 and Sox2 double negative pattern in the metastatic site (B) are noted. All the positive cells are demonstrated by arrow heads in the magnification of ×160.
Discussion
As transcriptional factors, Oct4 and Sox2 can be co-expressed in ESCs, described as a nuclear protein [16]. Similarly, in embryonal carcinoma (positive control), this double positive co-expression was noted in this study. Epidermal stem cells are adult stem cells without pluripotency, locating in basal layers of oral mucosa [17]. In our study, the individual expression of Oct4 and Sox2 was shown in the epithelial basal layers. Of note, the double positive co-expression profile of these markers cannot be demonstrated in normal oral mucosa. In contrast, the co-expression of Oct4 and Sox2 (Oct4+Sox2+ profile) was frequently detected in transforming oral mucosa of rat, precancerous lesions of human, epithelial non-cancer tissues adjacent to the OSCC and primary sites of OSCCs.
The importance of the hyperplasia - dysplasia - neoplasia continuum in many epithelial tissues is now well understood [18]. In this study, the clinical cases (precancerous lesions of human, epithelial non-cancer tissues adjacent to the OSCC) and the samples from animal model (transforming oral mucosa of rat) displayed simple hyperplasia. They were in the early stage of carcinogenesis of oral mucosa. The information from them suggested Oct4+Sox2+ profile should reflect a situation in which carcinomatous changes of epithelial cells were in process. Together with the data from the primary sites of OSCCs, we proposed that these double positive cells (Oct4+Sox2+) might be cancer stem cells. Of note, although Oct4+Sox2+ profile was detected in the metastatic OSCCs as well, Oct4low/-Sox2low/- profile was the more frequently expression pattern in the metastatic sites. Hence, metastatic cancer cells may own the independent evolution profile once the cells fight for survival in a strange environment, while cancer cells in lymph nodes are disseminated from the primary sites of OSCCs.
The correlation of Oct4 expression with cisplatin resistance in OSCC was well studied [19]. While, the relationship between Sox2 expression and tumour behavior of OSCC is under debate. Du et al. [20] showed a significant association of high Sox2 nuclear expression in nodal-negative (pN0) oral tongue squamous cell carcinoma with poor overall and disease-free survival. Michifuri et al. [21] further indicated the poor prognosis accompanying with lymph node metastasis was significant correlated with the high expression of Sox2 in the primary sites of OSCCs. However, by using of tissue microarray technique and immunohistochemistry assay, Züllig et al. [22] showed high expression levels of Sox2 significantly correlated with negative lymph node status implying good prognosis in OSCC patients. Züllig and the colleagues indicated that the heterogeneity of primary tumors might be one of the reasons for such controversial results [22]. In our study, the Oct4 expression was not correlated with lymph node metastasis (nodal-positive VS nodal-negative, P>0.05). In contrast, 100% of Sox2 expression was detected in the primary sites of 46 nodal-positive OSCCs, while only 40% of Sox2 expression was detected in the primary sites of 70 nodal-negative OSCCs. The Sox2 expression was significantly correlated with lymph node metastasis (nodal-positive VS nodal-negative, P<0.05). The results of us were in line with the findings of Michifuri’s group.
In summary, this study suggests that Oct4+Sox2+ profile should be the biomarker of stem cells which drive epithelial cells to OSCCs. Contrast to traditional studies, this co-expression profile could narrow the misunderstandings caused by tumour heterogeneity in searching cancer stem cells.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81200796), the Henan Province Department for Science and Technology (Grant No. 122300410159) and funding from Youth Foundation of The First Affiliated Hospital of Zhengzhou University.
Disclosure of conflict of interest
None.
References
- 1.McDowell JD. An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol Clin North Am. 2006;39:277–94. doi: 10.1016/j.otc.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Qiao B, Johnson NW, Gao J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int J Oncol. 2010;37:663–8. doi: 10.3892/ijo_00000715. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Levasseur D. Transcriptional regulatory mechanisms that govern embryonic stem cell fate. Methods Mol Biol. 2013;1029:191–203. doi: 10.1007/978-1-62703-478-4_13. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19:469–80. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–95. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 10.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–44. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, Zhang Y, Ling EA, Gao J, Hao A. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–75. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, Sawada T, Ohira M, Hirakawa K. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012;174:130–5. doi: 10.1016/j.jss.2010.11.903. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Wei X, Ling J, Wu L, Xiao Y. Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture of human dental pulp derived cells. J Endod. 2011;37:466–72. doi: 10.1016/j.joen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281:33554–65. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003;21:30–42. doi: 10.1002/ssu.10019. [DOI] [PubMed] [Google Scholar]
- 16.Chan YS, Yang L, Ng HH. Transcriptional regulatory networks in embryonic stem cells. Prog Drug Res. 2011;67:239–52. doi: 10.1007/978-3-7643-8989-5_12. [DOI] [PubMed] [Google Scholar]
- 17.Tao Q, Qiao B, Lv B, Zheng C, Chen Z, Huang H. p63 and its isoforms as markers of rat oral mucosa epidermal stem cells in vitro. Cell Biochem Funct. 2009;27:535–41. doi: 10.1002/cbf.1612. [DOI] [PubMed] [Google Scholar]
- 18.Delahunt B, Scolyer RA, Srigley JR. Premalignancy: the lull before the storm. Pathology. 2013;45:207–8. doi: 10.1097/PAT.0b013e32835f618e. [DOI] [PubMed] [Google Scholar]
- 19.Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:621–8. doi: 10.1111/j.1600-0714.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 20.Du L, Yang Y, Xiao X, Wang C, Zhang X, Wang L, Zhang X, Li W, Zheng G, Wang S, Dong Z. Sox2 nuclear expression is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral Oncol. 2011;47:709–13. doi: 10.1016/j.oraloncology.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Michifuri Y, Hirohashi Y, Torigoe T, Miyazaki A, Kobayashi J, Sasaki T, Fujino J, Asanuma H, Tamura Y, Nakamori K, Hasegawa T, Hiratsuka H, Sato N. High expression of ALDH1 and SOX2 diffuse staining pattern of oral squamous cell carcinoma correlates to lymph node metastasis. Pathol Int. 2012;62:684–9. doi: 10.1111/j.1440-1827.2012.02851.x. [DOI] [PubMed] [Google Scholar]
- 22.Züllig L, Roessle M, Weber C, Graf N, Haerle SK, Jochum W, Stoeckli SJ, Moch H, Huber GF. High sex determining region Y-box2 expression is a negative predictor of occult lymph node metastasis in early squamous cell carcinoma of the oral cavity. Eur J Cancer. 2013;49:1915–22. doi: 10.1016/j.ejca.2013.01.005. [DOI] [PubMed] [Google Scholar]


