Abstract
Regulatory T cells (Treg) are important for maintaining immune homeostasis. Adoptive transfer of Tregs is protective in renal disease models in both immunocompetent and immunodeficient mice. However the involvement of TCR recognition of renal antigens remains to be clarified. To address this question, we made use of Tregs from the DO11.10 mouse (a TCR transgenic (Tg) mouse), that recognise the non-murine antigen Ovalbumin (OVA) and therefore are not activated by renal antigens. DO11.10 Tregs were assessed functionally in vitro and demonstrated equivalent suppression to WT BALB/c Tregs. Adriamycin Nephropathy (AN) was induced in mice which had been transfused with CD4+CD25+Tregs isolated from DO11.10 or BALB/c mice. To eliminate the memory/activation state as a cause of differences in activity, the protective capacity of DO11.10 Tregs pre-activated with OVA in vivo was assessed. Transfer of WT BALB/c Tregs significantly attenuated the development of AN with less glomerulosclerosis, tubular atrophy and macrophage infiltration as compared to AN mice without Treg transfer. However, mice receiving either naïve or pre-activated DO11.10 Tregs were not protected from AN. The lack of protection by DO11.10 Tregs was not due to failure to traffic to the affected kidney. These results suggest that antigen recognition in the kidney is important for Treg protection against injury.
Keywords: DO11.10 Tregs, antigen specificity, Adriamycin Nephropathy
Introduction
Tregs play an important role in maintaining immune homeostasis and defects in Treg cell number or function are associated with various autoimmune diseases, including myasthenia gravis, multiple sclerosis, and polyglandular syndrome [1-3]. Different subsets of Tregs have been identified, among which CD4+CD25+Tregs are the best characterised [4,5]. The suppressive functions of CD4+CD25+Tregs are either cell contact-dependent [6] or mediated by secretion of immunosuppressive cytokines such as IL-10 and TGF-β [7,8]. Other mechanisms involve expression of costimulatory molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) [9], and enzymes like CD39 [10,11]. They appear to act on a wide variety of cells in both the cognate and innate immune system. Natural CD4+CD25+Tregs are generated in the thymus via recognition of self-antigens [12] and expression of various homing receptors such as CCR4, CCR5, CCR7, CD62L and CD103 allow Tregs to traffic to the sites of inflammation and exert their suppressive effects [13,14]. Furthermore, it has been shown that peripheral shaping of TCR repertoire plays an important role in Treg function [15-18].
Antigen-specific Tregs appear to be more potent than polyclonal Tregs in suppressing autoimmune diabetes, graft-versus-host disease (GVHD) and inducing transplantation tolerance [19-24], however the role of antigen recognition in the action of antigen-specific Tregs is not fully defined in nephritis. In vitro studies have shown that Treg activation requires T cell receptor (TCR) engagement with specific antigen, but activated Tregs function in a bystander mode [25]. We therefore compared the efficacy of antigen-specific Tregs with specificity to an exogenous antigen to that of natural Tregs in AN, an experimental model of chronic kidney disease (CKD). For this purpose, we used Tregs from the DO11.10 mouse, a TCR transgenic (Tg) mouse [26,27]. DO11.10 Tregs recognise the non-murine antigen OVA and were used as a source of Tregs that would not be activated by renal antigens. We further used DO11.10 Tregs that had been pre-activated with OVA to assess the effect of naïvety versus activation/memory as a cause of limited activity. Previous studies by us and others have shown that adoptive transfer of natural CD4+CD25+Tregs has protective effects in several CKD models [28-30]. Natural Tregs from normal BALB/c mice therefore served as a good control in this study.
AN is a well-established experimental model of CKD and end-stage renal disease (ESRD) [31]. It is characterised by glomerulosclerosis and tubulointerstitial inflammation. Both T and B lymphocytes as well as macrophages are important for disease progression of AN [32].
Materials and methods
Mice
Breeding pairs of DO11.10 mice were kindly provided by Prof Stephen R Holdsworth from Monash University. 6-8 week old male BALB/c mice were purchased from the Animal Resources Centre in Perth, Australia. All animals were maintained under standard sterile conditions in the Department of Animal Care at Westmead Hospital. Experiments were carried out in accordance with protocols approved by the Animal Ethics Committee of Sydney West Area Health Service.
Antibodies
Antibodies used for immunohistochemistry included: rat anti-mouse F4/80 (Serotec, Oxford, UK), rat anti-mouse/rat Foxp3 (eBioscience, CA) and biotinylated goat anti-rat immunoglobulin (Zymed Laboratories, San Francisco, CA). APC-rat anti-mouse CD4 (BD PharmingenTM, CA), FITC-rat anti-mouse KJ1-26 (Biolegend, CA), PE-Cy5-rat anti-mouse/rat Foxp3 (eBioscience, CA) and PE-rat anti-mouse CD25 (BD PharmingenTM, CA) were used for flow cytometry stainings. Anti-mouse CD3 antibody used for in vitro suppression assay was from BD PharmingenTM, CA.
Isolation of CD4+CD25+Tregs
Mouse spleen was harvested from BALB/c or DO11.10 mice and passed through a 40 μm cell strainer to obtain a single-cell suspension, which was followed by red blood cell lysis. CD4+CD25+Tregs were isolated using a mouse CD4+CD25+ regulatory T cell isolation kit according to manufacturer’s manual (Miltenyi Biotec, GmbH, Germany). Briefly, non-CD4+ T cells were depleted using biotinylated antibody cocktail and anti-biotin microbeads. CD4+CD25+ T cells were separated from CD4+CD25- T cells by positive selection using PE anti-CD25 mAb and anti-PE microbeads.
Adoptive transfer of CD4+CD25+Tregs and induction of AN
After cell isolation, ~1.5 million CD4+CD25+Tregs were injected into the tail vein of mice. Eight days after the adoptive transfer or OVA injection, AN was induced by tail vein injection of Adriamycin (9.8 mg/kg body weight). Mice were divided into five groups: (1) BALB/c Treg-AN group (n=6); (2) naïve DO11.10 Treg-AN group (n=7); (3) activated DO11.10 Treg-AN group (mice injected with 5 μg of OVA one day after adoptive transfer and 8 days prior AN induction) (n=7); (4) normal group (n=4); (5) AN group (n=5).
Renal function
Blood and urine samples were collected every two weeks after the induction of AN. Urine protein concentration was measured using colorimetric assay (Bio-Rad, Hercules, CA) based on the method of Bradford. Urine creatinine and serum creatinine were analysed using an automated chemistry analyser VITROS (Ortho Clinical Diagnostics, Johnson & Johnson) by staff at the Institute of Clinical Pathology and Medical Research at Westmead Hospital.
Renal histology and immunohistochemistry
Coronal sections of the kidney were fixed in 10% neutral-buffered formalin and embedded in paraffin. 4 μm sections of paraffin block were stained with periodic acid-Schiff’s (PAS) reagent and counterstained with haematoxylin. Renal histopathology was graded as previously described [29]. Immunohistochemical staining was performed to determine macrophage infiltration in the kidney. Sections were incubated with primary antibody (16 h, 4°C) followed by secondary antibody incubation (30 min, RT) as previously described previously [33,34].
Flow cytometry analysis
Single cell suspensions were directly stained with fluorochrome-conjugated rat anti-mouse antibody to cell surface antigens followed by permeabilization and intracellular staining of Foxp3. All samples were analysed on LSR II flow cytometer (Becton Dickinson, Mountain View, CA). Flowjo software was used for analysis (Tree Star, Inc. Ashland, Oregon).
In vitro proliferation assay
Cells were cultured in triplicate in round-bottomed 96-well plates in RPMI1640 (Lonza, Basel, Switzerland) supplemented with 10% FCS, 10 mM Hepes, 50 u/ml Penicillin/50 μg/ml streptomycin, 50 μM 2-ME and 2 mM L-Glutamine. BALB/c CD4+CD25- indicator T cells were stimulated with plate-bound anti-mouse CD3 antibody (clone number: 145-2C11) (plates pre-coated with 100 μl of 1 μg/ml anti-mouse CD3 antibody, overnight, 4°C) in the presence of irradiated BALB/c antigen-presenting cells (APC) from splenocytes. DO11.10 CD4+CD25- indicator T cells were stimulated with irradiated BALB/c APC pulsed with 10 μg/ml OVA peptide (2 h, 37°C). Coculture consisted of 5x104 indicator T cells, 7.5x104 APC and Tregs at 3 different doses (5x104, 2.5x104 and 1.25x104). After 3 days of culture, proliferation was determined by overnight (18 h) incorporation of [3H] thymidine (1 μCi per well). Cells were harvested onto glass fiber filters (PerkinElmer, Massachusetts) and the radioactivity incorporated was quantified using a Wallac Betaplate liquid scintillation counter (Beckman, Fullerton, CA). Results were expressed as mean counts per minute (CPM) of triplicate cultures ± SD.
Real-time PCR
Total RNA was extracted using RNeasy Mini kit (QIAGEN, Hilden, Germany) and reverse transcription was performed using SuperScriptTM III First-Strand Synthesis system (Invitrogen, Carlsbad, CA). cDNA was subjected to quantitative PCR analysis using Taqman® Gene Expression Assays specific for the genes of interest (Applied Biosystem, Australia). PCR reaction mix was subjected to Rotor-Gene 3000 thermal cycler (Corbett Life Science) for acquisition and analysis.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) for multiple comparisons. For comparison between two groups, student t-test was performed. Results are expressed as the group mean ± SD. Differences were considered significant at P<0.05. Statistical analysis was performed using Prism 5 (GraphPad Software).
Results
In vivo OVA challenge induced the proliferation of a KJ1-26+Foxp3+ Treg population in the spleen
Activation of antigen-specific Tregs requires TCR engagement with specific antigen [25]. Previous studies have shown that single intravenous (i.v.) injection of OVA could induce the proliferation of peripheral OVA specific Tregs [35]. We adoptively transferred 1.5 million Tregs from DO11.10 mice into BALB/c mice, followed by i.v. injection of 5 μg of OVA the following day. Anti-KJ1-26 antibody specifically binds the TCR expressed on DO11.10 mice, which allows differentiation between the adoptively transferred DO11.10 and the endogenous Treg population. Splenic KJ1-26+Foxp3+ Tregs were assessed by flow cytometry at day 3 and day 8 post OVA injection. As shown in Figure 1, KJ1-26+Foxp3+ Tregs proliferated in the presence of OVA, whereas in the absence of OVA, a reduction of this population was observed.
Figure 1.
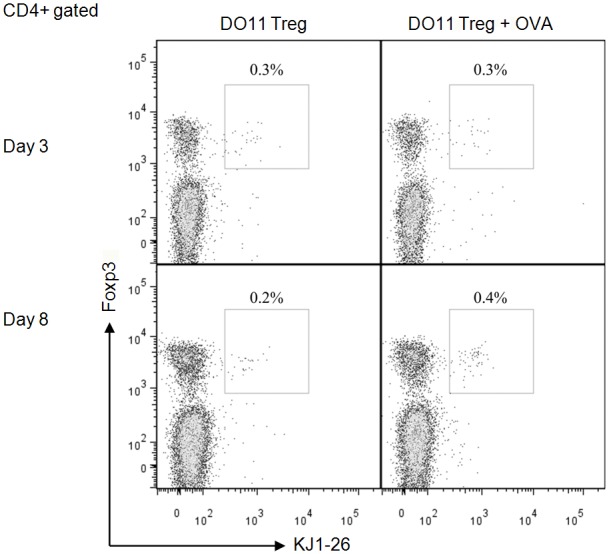
DO11.10 Tregs induced by OVA injection. Flow cytometry analysis of DO11.10 Tregs (KJ1-26+) in the spleen was conducted after single i.v. injection of OVA. BALB/c mice were adoptively transferred with 1.5 million DO11.10 Tregs (day 0), followed by i.v. injection of 5 μg OVA (day 1). Spleens of transferred mice were harvested on day 4 and day 9 and analysed by flow cytometry.
WT Tregs were more potent that activated or naïve DO11 Tregs
Our previous studies have shown that CD4+CD25+Tregs from normal BALB/c mice protect against AN [28,29], however the involvement of TCR recognition of renal antigen in Treg’s protective effects is not known. We addressed this question with the use of DO11.10 mice, TCR transgenic mice whose T cells recognise a non-renal antigen OVA. Tregs have been shown to function in a non-antigen-specific way after the initial activation with specific antigen [25]. We compared the effects of adoptively transferred BALB/c Tregs to DO11.10 Tregs in AN. To activate adoptively transferred DO11.10 Tregs, OVA was injected one day after adoptive transfer (activated DO11.10 Treg-AN) and 9 days prior to ADR administration and for comparison purposes, a group of mice was adoptively transferred with DO11.10 Tregs without OVA injection (naïve DO11.10 Treg-AN). Urine protein/creatinine and serum creatinine were measured 4 weeks after induction of AN. WT Tregs provided greater protection that activated or naïve DO.11 Tregs (Figure 2).
Figure 2.
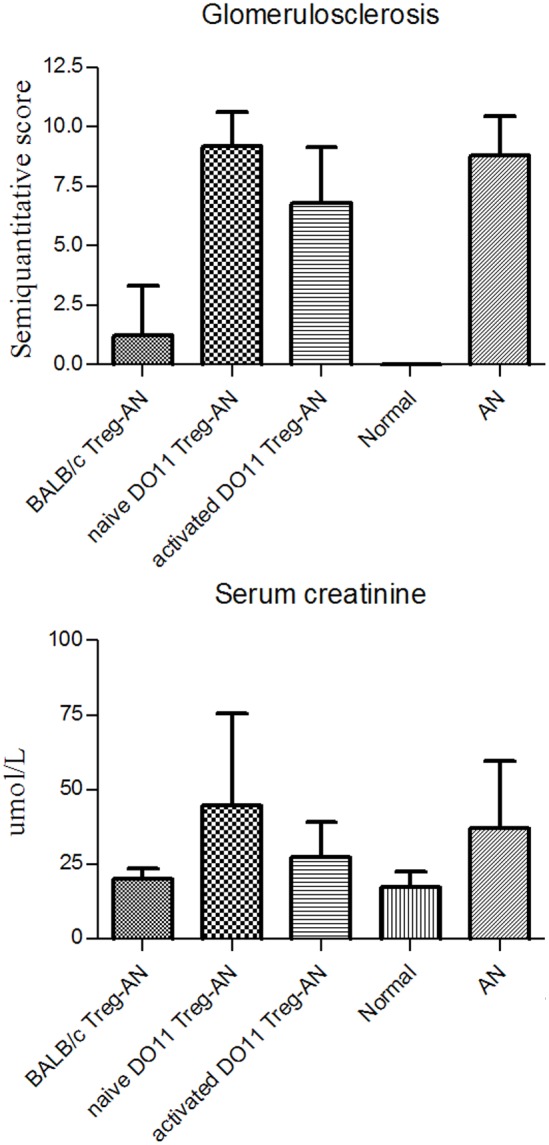
DO11.10 Tregs do not protect renal function. Renal function was assessed 4 weeks after induction of AN. Urine and sera were collected and urine protein/creatinine and serum creatinine were measured. All results are expressed as mean ± SD (*P<0.05).
WT Tregs but not activated DO11.10 Tregs ameliorated renal structural injury
Renal injury was assessed histologically by evaluating glomerulosclerosis and tubular atrophy (Figure 3A). WT BALB/c Tregs significantly reduced renal injury whereas activated and naïve DO11.10 Tregs did not ameliorate either glomerulosclerosis or tubular atrophy, when compared to AN group (Figure 3B).
Figure 3.
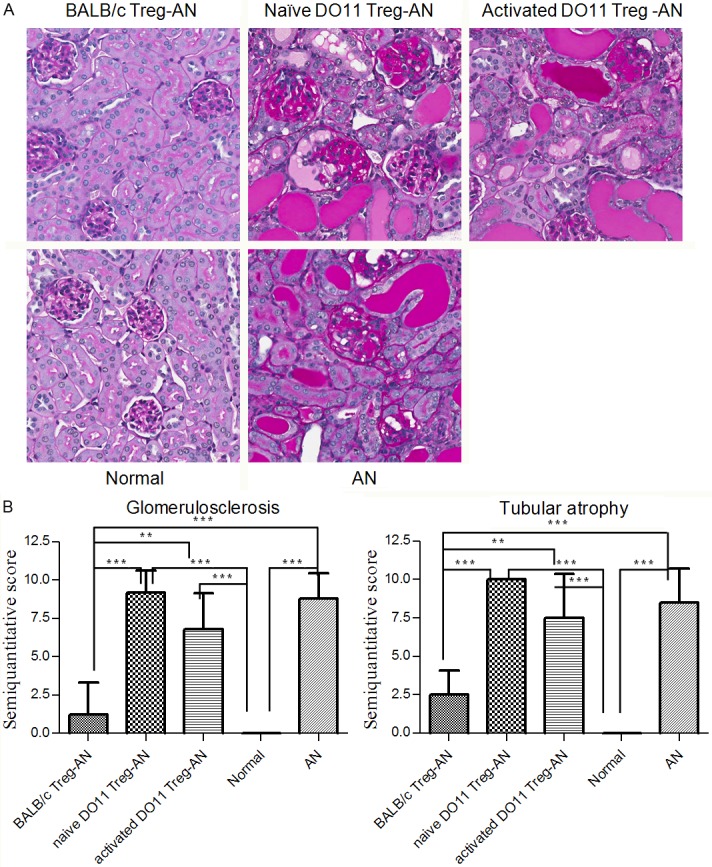
BALB/c Tregs but not DO11.10 Tregs ameliorated renal structural injuries. Kidney tissues harvested at week 4 post AN were analysed with periodic acid-Schiff (PAS) staining. A: Representative pictures from each group were shown (magnification 200x). B: Semiquantitative scoring of glomerulosclerosis and tubular atrophy by a blinded observer. Results are expressed as mean ± SD (**P<0.01; ***P<0.001).
Renal macrophage infiltration correlated with disease severity
Immune cell infiltration was assessed by immunohistochemical staining of kidney sections with antibodies specific for macrophages at week 4 post AN induction. As shown in Figure 4, the BALB/c Treg-AN group had less macrophage cell infiltration than the AN group, whereas naïve or activated DO11.10 Treg-AN groups were equivalent to the AN group.
Figure 4.
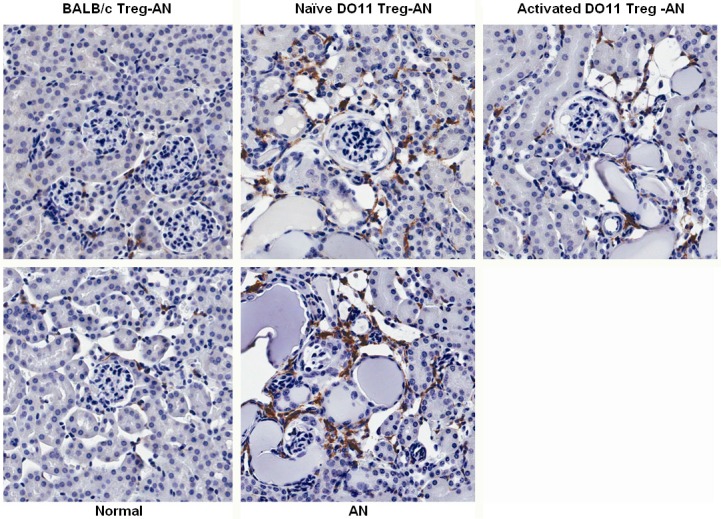
Macrophage infiltrations in renal cortex. Immunohistochemistry staining was performed to assess the number of F4/80+ macrophages in renal cortex at week 4 post AN. Representative pictures from each group were shown (magnification 200x).
Foxp3 positive cells were detected in the spleen and kidneys of both naïve and activated DO11.10 Treg-AN mice
In order to understand why WT Tregs but not naïve or activated DO11.10 Tregs ameliorated AN, Foxp3+ T cells in the spleen and kidney sections were assessed by immunohistochemical staining. Foxp3+ cells were found in spleen sections of all the groups and no significant difference was observed among the groups (Figure 5). KJ126 staining for DO11 T cells was unsuccessful in spleen and so it was not possible to distinguish transferred Tregs from native Tregs.
Figure 5.
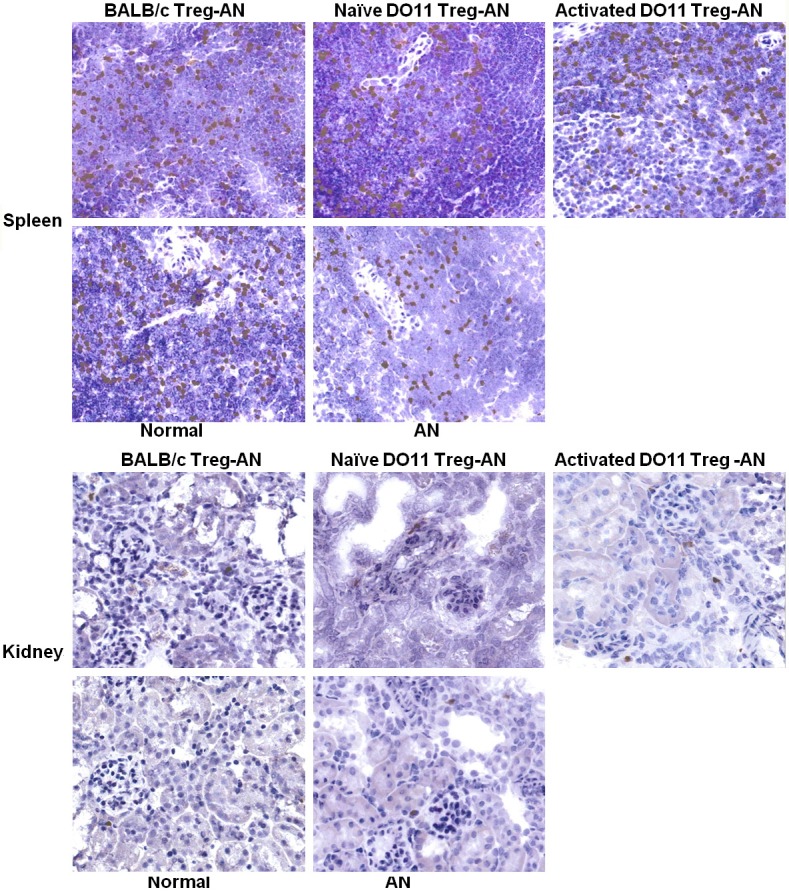
Foxp3+ Tregs in the spleen. Immunohistochemistry staining of Foxp3+ Tregs were performed on spleen sections collected at week 4 post AN. Representative pictures of the staining were shown (magnification 400x).
We further examined expression of Foxp3 in kidney at week 4 post AN by semiquantitative real-time PCR. Foxp3 mRNA level was up-regulated in all diseased groups (Figure 6).
Figure 6.
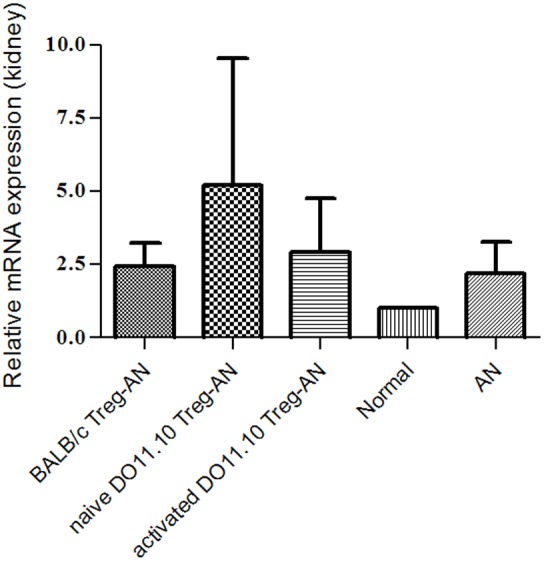
Foxp3 mRNA expression of the kidney. Expression was normalized against T-cell receptor Vβ constant region (TRBC). Results are expressed as mean ± SD.
In vitro assessment of WT and DO.11 Treg suppressor function
Our in vivo data showed that neither activated or naive DO11.10 Tregs protected mice against renal injury in AN, which suggested that recognition of organ-specific antigens was required for in vivo function of Tregs. Functional assessment of the WT and DO.11 Tregs was assessed in vitro using a standard suppression assay. Naïve DO11.10 Tregs suppressed the proliferation of BALB/c responders (Figure 7A), suggesting differing mechanisms to those with in vivo suppression. With prior activation, DO11.10 Tregs suppressed proliferation of responders to a greater degree than WT BALB/c Tregs, consistent with previous observations [25] (Figure 7B).
Figure 7.
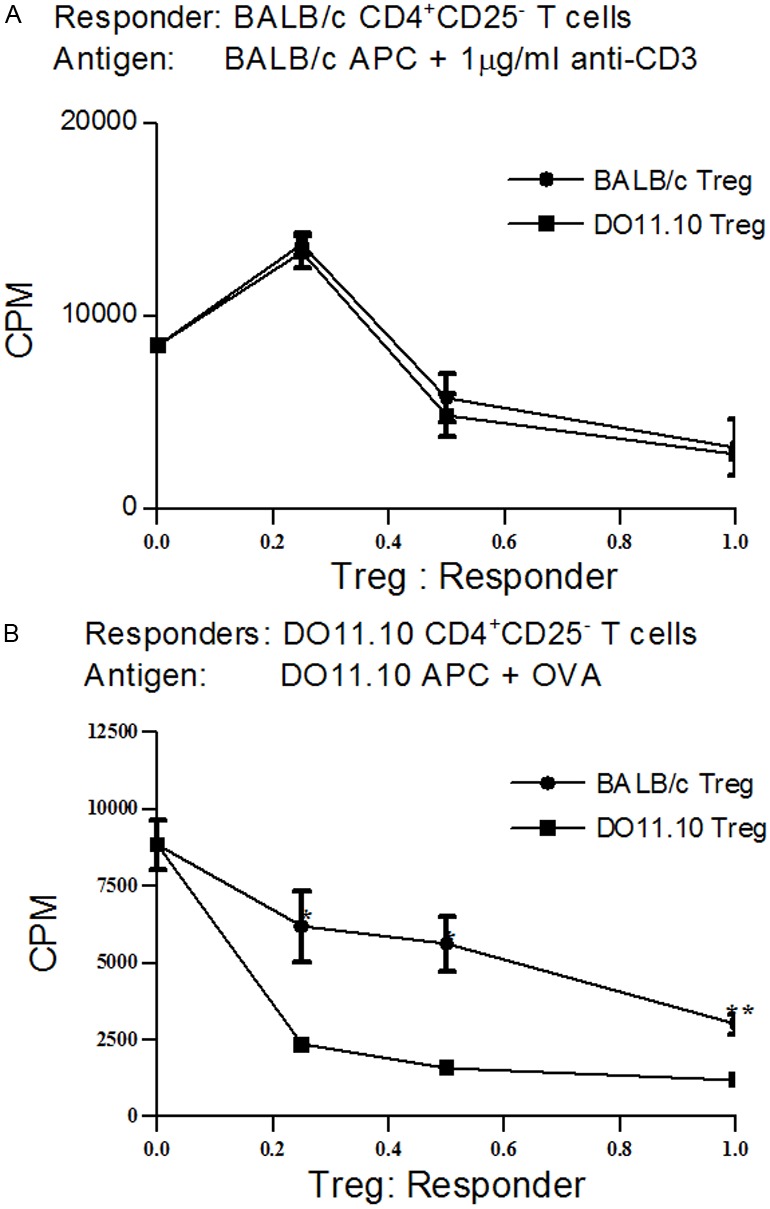
In vitro proliferation assay. 5x104 CD4+CD25- indicator T cells were cocultured with three doses of Tregs (1.25x104, 2.5x104 and 5x104). After 3 days of culture, proliferation was determined by overnight incorporation of [3H] thymidine. A: Tregs from BALB/c and DO11.10 displayed similar suppressive effects in inhibiting proliferation of anti-CD3-induced BALB/c CD4+CD25-; B: DO11.10 Treg was more efficient in suppressing OVA-induced DO11.10 CD4+CD25- effector T cells than BALB/c Tregs.
Discussion
Tregs are a subset of T cells generated in the thymus providing suppression of autoimmune activity in the periphery [4,12]. They have been shown to go to specific organs including bowel and kidney and to demonstrate antigen specific expansion in resident tissues [30,36]. However the role of antigen specificity has not been addressed in Tregs established protection against renal disease.
Treg function in renal and other immune mediated disease has been shown to be affected by depletion of Tregs leading to worsening of disease, while therapeutic delivery of natural or transduced Tregs can protect against renal injury [29]. Tregs can protect against renal injury in the absence of other cognate immune cells in renal disease in SCID mice suggesting the mechanisms of protection involve direct effects of Tregs on the innate immune system and the injured organ itself [28]. In addition to cytokines and costimulatory mechanisms, Tregs utilise a number of other strategies including the removal of dangerous extracellular purines [10,11].
Both antigen specificity and activation status have previously been identified as key factors determining the capacity of non-renal Tregs to protect against disease [15,17]. Tregs have been shown to develop particular effector phenotypes following activation. These have included increased capacity to traffic to inflamed tissues involving a variety of chemokine and chemokine receptors [37]. Recently antigens involved in autoimmune renal disease have been defined including PLA2R and CD10 suggesting that specific antigens may drive autoimmune renal disease and that potentially Treg protection against autoimmune renal disease may involve antigen recognition [38-40]. The DO11 TCR transgenic on the same BALB/c background required for AN and recognising an exogenous antigen allows study of the requirement for renal antigen recognition in the protection against renal disease [26].
Our studies show that while DO.11 Tregs in vitro, particularly after activation, have strong suppressor activity, they do not protect against renal injury induced by Adriamycin to the same extent as WT Tregs. This suggests that renal antigen recognition is required for Treg protection in AN. This supports the use of antigen specific Tregs as cell therapy in the protection against renal disease.
Acknowledgements
This work was supported by research grants from the National Health and Medical Research Council of Australia (NHMRC grant ID632519). We thank the staff of the Westmead Hospital Animal House Facility for care of the animals. We thank Virginia James (Westmead Millennium Institute, Australia) for her technical assistance with histology.
Disclosure of conflict of interest
None.
Abbreviations
- AN
Adriamycin Nephropathy
- APC
Antigen-Presenting Cells
- CTLA-4
cytotoxic T-Lymphocyte Antigen 4
- CKD
Chronic Kidney Disease
- CPM
Counts Per Minute
- ESRD
End-Stage Renal Disease
- GVHD
Graft-Versus-Host Disease
- OVA
Ovalbumin
- PAS
Periodic Acid-Schiff’s
- Treg
Regulatory T cells
- TCR
T Cell Receptor
References
- 1.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–1291. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4(+)CD45RC- cells and CD4(+)CD8(-) thymocytes. J Exp Med. 1999;189:279–288. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 2012;119:5155–63. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salcido-Ochoa F, Tsang J, Tam P, Falk K, Rotzschke O. Regulatory T cells in transplantation: does extracellular adenosine triphosphate metabolism through CD39 play a crucial role? Transplant Rev (Orlando) 2010;24:52–66. doi: 10.1016/j.trre.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 12.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 13.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taguchi O, Kontani K, Ikeda H, Kezuka T, Takeuchi M, Takahashi T. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 1994;82:365–369. [PMC free article] [PubMed] [Google Scholar]
- 17.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenen HJ, Joosten I. Antigen-specific regulatory T-cell subsets in transplantation tolerance regulatory T-cell subset quality reduces the need for quantity. Hum Immunol. 2006;67:665–675. doi: 10.1016/j.humimm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol. 2005;175:3053–3059. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 22.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells--ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 27.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan D, Wang Y, Qin X, Zheng G, Wang YM, Alexander SI, Harris DC. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006;17:2731–2741. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- 29.Wang YM, Zhang GY, Wang Y, Hu M, Wu H, Watson D, Hori S, Alexander IE, Harris DC, Alexander SI. Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J Am Soc Nephrol. 2006;17:697–706. doi: 10.1681/ASN.2005090978. [DOI] [PubMed] [Google Scholar]
- 30.Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, Mayer G, Gunsilius E, Rosenkranz AR. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol. 2005;16:1360–1370. doi: 10.1681/ASN.2004100837. [DOI] [PubMed] [Google Scholar]
- 31.Mason PD, Pusey CD. Glomerulonephritis: diagnosis and treatment. BMJ. 1994;309:1557–1563. doi: 10.1136/bmj.309.6968.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–1804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Wang Y, Tay YC, Zheng G, Zhang C, Alexander SI, Harris DC. DNA vaccination with naked DNA encoding MCP-1 and RANTES protects against renal injury in adriamycin nephropathy. Kidney Int. 2005;67:2178–2186. doi: 10.1111/j.1523-1755.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 34.Zheng G, Wang Y, Xiang SH, Tay YC, Wu H, Watson D, Coombes J, Rangan GK, Alexander SI, Harris DC. DNA vaccination with CCL2 DNA modified by the addition of an adjuvant epitope protects against “nonimmune” toxic renal injury. J Am Soc Nephrol. 2006;17:465–474. doi: 10.1681/ASN.2005020164. [DOI] [PubMed] [Google Scholar]
- 35.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 36.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nortier JL, Debiec H, Tournay Y, Mougenot B, Noel JC, Deschodt-Lanckman MM, Janssen F, Ronco P. Neonatal disease in neutral endopeptidase alloimmunization: lessons for immunological monitoring. Pediatr Nephrol. 2006;21:1399–1405. doi: 10.1007/s00467-006-0203-6. [DOI] [PubMed] [Google Scholar]
- 39.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 40.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]


