Abstract
Background: Oral propranolol (PRN) has recently been shown to be highly effective for infantile hemangiomas (IHs), and is currently recommended as the first-line treatment of complicated IHs. However, the therapeutic mechanism(s) still remain unclear. Methods: In this study, we tested hemangioma-derived stem cells for expression of vascular endothelial growth factor (VEGF) in vitro and studied the inhibition of VEGF expression. We used PCR, Elisa, Western blotting and immunohistochemistry in vivo and in vitro trial. Results: The study demonstrated that application of PRN at a “normal” concentration equivalent to plasma concentration did not inhibit proliferation or promote apoptosis of hemangioma derived stem cells (HemSCs) isolated from IH patients. PRN suppressed expression of vascular endothelial growth factor (VEGF) and basic Fibroblast Growth Factor (bFGF) in HemSCs in vitro. Morphological, histological and immunohistological improvement were observed in vivo using murine IH model in which HemSCs pre-treated with PRN were implanted into BALB/c-nu mice. In the pre-treated HemSC grafts, mean micro-vessel density (MVD) significantly decreased and protein levels of VEGF markedly decreased, while bFGF was still detectable. Conclusions: The results suggested PRN inhibited angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. These findings provide critical insight into the potential mechanisms of PRN action on IH.
Keywords: Hemangioma-stem cell, propranolol, pharmacokinetics, vascular endothelial growth factor (VEGF)
Introduction
Infantile hemangioma (IH) is the most common tumor of childhood. It is estimated to affect up to 10% of Caucasians. Approximately 60% of IH occur in the head and neck [1,2]. Previous studies have suggested that hemangioma-derived stem cells (HemSCs) are the cellular precursors of IH. These cells have been used to develop a hemangioma mouse model [3,4]. An early rapid growth occurs in the first year following birth, followed by a slow spontaneous regression phase occurring within a period of 3 to 7 years. However, many patients still require treatment to avoid followed deformities and functional impairment. Since 2008, Léauté-Labrèze et al [5] discovered the occasionally good effects of the non-selective beta blocker propranolol (PRN) for IH, additional clinical research confirmed its efficacy. To date PRN had been recommended as the first-line medicine in treating IH. But there is currently nothing to known about the mechanism of action of PRN in IH. We investigated the effect of PRN in apoptosis, growth and expression of angiogenic factors of HemSC using both in vitro and in vivo models.
Materials and methods
We obtained surgical specimens of proliferating infantile hemangiomas, human umbilical vein endothelial cells (HUVECs) and normal human dermal fibroblasts (NHDFs) under a human-subject protocol approved by the ethics committee at Ninth People’s Hospital, Shanghai Jiao Tong University. All parents of participants were conformation of written informed consent for the original human work that produced the tissue samples.
Cell culture, cell cycle and apoptosis analysis
HemSCs were isolated from Chinese proliferating hemangioma tissues by CD133 immunomagnetic beads. Human umbilical vein endothelial cells (HUVECs) and normal human dermal fibroblasts (NHDFs) were obtained with institutional research board approval from Ninth People’s Hospital, Shanghai Jiao Tong University. Cells were grown in endothelial basal medium (EBM-2, CC-3162 Lonza USA) supplemented with 20% FBS and SingleQuot (CC-4176, Lonza USA). Three types of single-cell suspensions were each respectively plated onto 96-well plates at a density of 4000 cells/well in 100 μL complete medium and incubated in a 5% carbon dioxide-humidified atmosphere at 37°C for 24 h. Cells were then incubated for 48 hours with the following concentrations of PRN: 0, 1, 10, 50, 100, 200 and 400 μM (Sigma, USA) in EBM-2 medium lacking FBS and supplemented with SingleQuots. Four duplicate wells were used for each experimental group and control group. Cell proliferation was measured at different time points (from 1 to 7 days) using the methyl thiazolyl tetrazolium (MTT) assay (Sangon Biotech Shanghai China). 20 μl solution was added to each well, and the plates incubated for an additional 4 hrs. Then, the supernatant was removed and 150 μl of DMSO was added to each well. The optical density of each well was measured at 490 nm using a microplate reader (Tecan Austria). A dose-effective curve was made in Excel, and IC50 determined using by SPSS (Statistical Product and Service Solutions) software. To assess cellular apoptosis, cells were double stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (Annexin V-FITC apoptosis detection kit I; BD Biosciences, USA).
RNA isolation and reverse-transcriptase-polymerase-chain-reaction (RT-PCR), real-time PCR, enzyme-linked immunosorbent assay (ELISA)
Prior to running RT-PCR, Real-time PCR and ELISA for VEGF, media containing PRN was washed out and cells serum-starved for 24 h before assay. Total RNA was extracted using Trizol according to the manufacturer’s instructions. All primers were designed by Sangon Biotech (Shanghai China). The RT-PCR and real-time PCR were performed with the following primers (VEGF-A 5’ GCG GAT CAA ACC TCA CCA AG 3’ and 5’ GCT TTC GTT TTT GCC CCT TTC 3’, FGF-2 5’ AGT GTG TGC TAA CCG TTA CCT 3’ and 5’ ACT GCC CAG TTC GTT TCA GTG 3’, MMP-1 5’ ACA CAT CTG ACC TAC AGG ATT GA and 5’ GTG TGA CAT TAC TCC AGA GTT GG 3’). Isolated RNA was converted to cDNA using Ex Taq reverse transcriptase enzyme (Reverse transcription reagents; Takara, Japan). Reactions were carried out for 40 cycles at an annealing temperature of 60°C. An enzyme-linked immunosorbent assay (ELISA) was performed with the use of VEGF (R&D USA).
Western blotting
HemScs were digested with 0.25% trypsin, and then suspended in 100 ml of lysis buffer. The lysates were solubilized in Laemmli sample boiled for 5 min, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) Proteins were then transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA) and blocked with 5% non-fat milk in PBS-Tween 20 (0.05%) for 1 h at room temperature. The membranes were incubated with the primary antibody mouse monoclonal anti-human FGF2 antibody (1:500, Peprotech, USA) at 4°C overnight under agitation. Membranes were then washed three times with PBS, followed by 1 hr incubation with secondary antibodies in darkness and subsequently washed three times with PBST. Bands were visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences, USA).
Murine hemangioma model
The murine model of infantile hemangioma (IH) was adapted from Khan et al [3]. HemSCs and human umbilical vein endothelial cells (HUVECs) isolated from human umbilical-cord were mixed at density of 2×107 and 1×107 per mouse respectively, sedimented, resuspended in 200 uL of Matrigel (BD Biosciences, USA) and injected subcutaneously into the backs of BALB/C-nu mice (4 weeks, male). Mice were purchased from Shanghai Laboratory Animal Center. This study was approved by the Ethics Committee of Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
Effect of propranolol and immunohistochemical analyses of VEGF and bFGF
We treated hemangioma-derived stem cells alone with propranolol for 3 days, and then propranolol was washed out. Mixed two kinds of cells in the same quantity and proportion same as the murine IH model were injected into 4-week-old mice (200 uL Matrigel/animal). H&E staining of sections and micro-vessel density (MVD) analysis were followed carried out. Immunohistochemical staining for vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were performed on cryosections as follows: slides were fixed with 4% polyoxymethylene, blocked with 5% serum, and the antigens repaired for 10 min. Sections were then incubated with rabbit anti-human VEGF and bFGF polyclonal antibodies (1:50; Abcam, USA) for 24 h at 4°C, and subsequently incubated with anti-rabbit antibodies (1:1000; Abcam) for 20 min at 37°C.
Results
PRN inhibits HemSC proliferation
As shown in Figure 1, the growth of HemSCs, HUVECs and NHDFs were inhibited by PRN in a dose-dependent manner with a half-maximal inhibition (IC50) of 133 μM, 62.75 μM and 112 μM respectively. HUVECs were the most sensitive to the inhibitory effect of PRN; the tolerance of HemSCs was lower and similar to that of the NHDFs. There was no inhibition of the three cell types when PRN concentration was lower than 20 μM. However, at a higher concentration of 50 μM PRN, the activation ratio (%) between the control and treated groups decreased to 57±5.8, 85±2.3 and 66.7±2.1 for HUVECs, HemSCs and NHDFs respectively. When PRN concentration was 100 μM, the activation ratio (%) of three cells was 18.9±1.5 (HUVECs), 70.3±0.6 (HemSCs) and 72.6±3.9 (NHDFs). Once PRN concentration reached 200 μM, both HemSCs’ and NHDFs’ viabilities were lower than 20%. There was no inhibition about the three cells when PRN concentration was between 0 and 20 μM.
Figure 1.
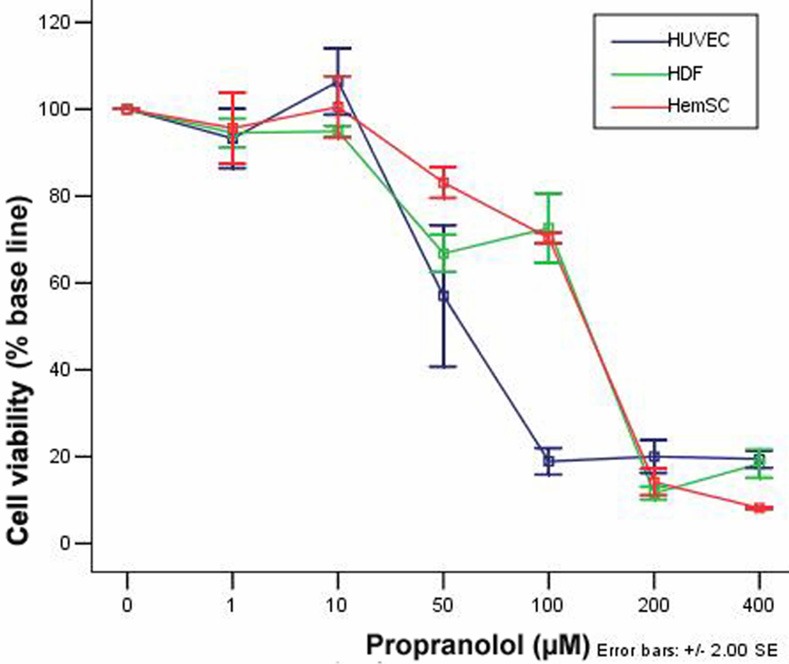
PRN inhibits HemSC proliferation. Graph showing the cell viability of HemSCs, HUVECs and NHDFs with increasing concentration of PRN.
Effect of PRN on HemSC apoptosis
Flow cytometric analysis of HemSCs double-stained with annexin V and PI and incubated with different concentrations of PRN demonstrated that PRN could not induce a significant rate of apoptosis: PRN was not effective at a concentration lower than 50 μM and even at a concentration of 100 μM, the rate of apoptosis was only slightly increased (Figure 2).
Figure 2.
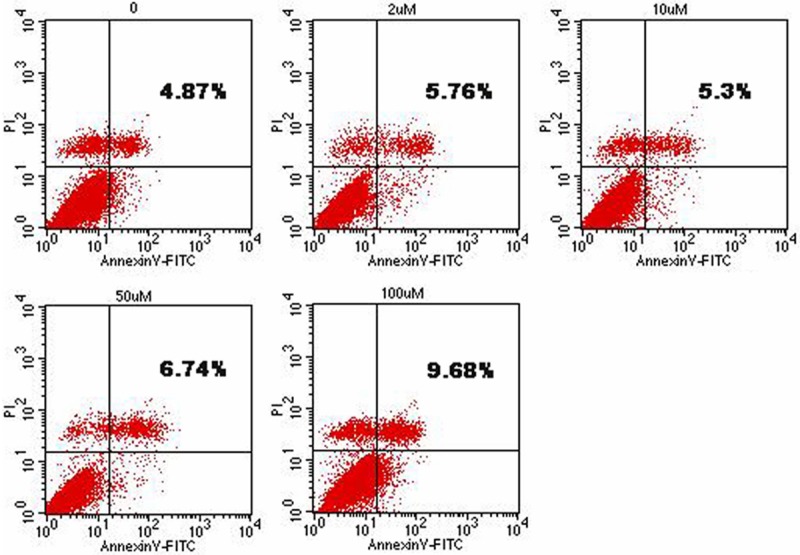
Effect of PRN on HemSC apoptosis. PRN does not affect HemSC apoptosis. Total apoptosis rates of HemSCs treated with different concentrations of PRN.
Effect of PRN on VEGF expression by HemSCs
HemSCs expressed more VEGF mRNA than did NHDFs and HUVECs; this result was consistent with that reported by Shoshana Greenberger, et al [4]. We observed significant down-regulation of VEGF mRNA when the HemSCs were treated with PRN compared with the untreated group (Figure 3A and 3C). Maximum inhibition achieved at 2 μM of PRN. The expression of mRNA was less than 50% (Figure 3A). But there was not remarkable variation between the five groups treated with different propranolol. RT-PCR results were consistent with those from the real-time PCR; gel electrophoresis showed that when the PRN concentration was higher and the band expression of VEGF mRNA is diminished (Figure 3C). There was notable variation in VEGF protein produced by HemSCs in groups treated with PRN compared with the untreated control group (Figure 3B). This effect was significant at the lowest concentration of 0.02 μM. At 2 μM, the expression of VEGF protein was less than 50% of the control untreated group. PRN-mediated inhibition of VEGF protein production was dose-dependent up to, but not beyond 2 μM (Figure 3B).
Figure 3.
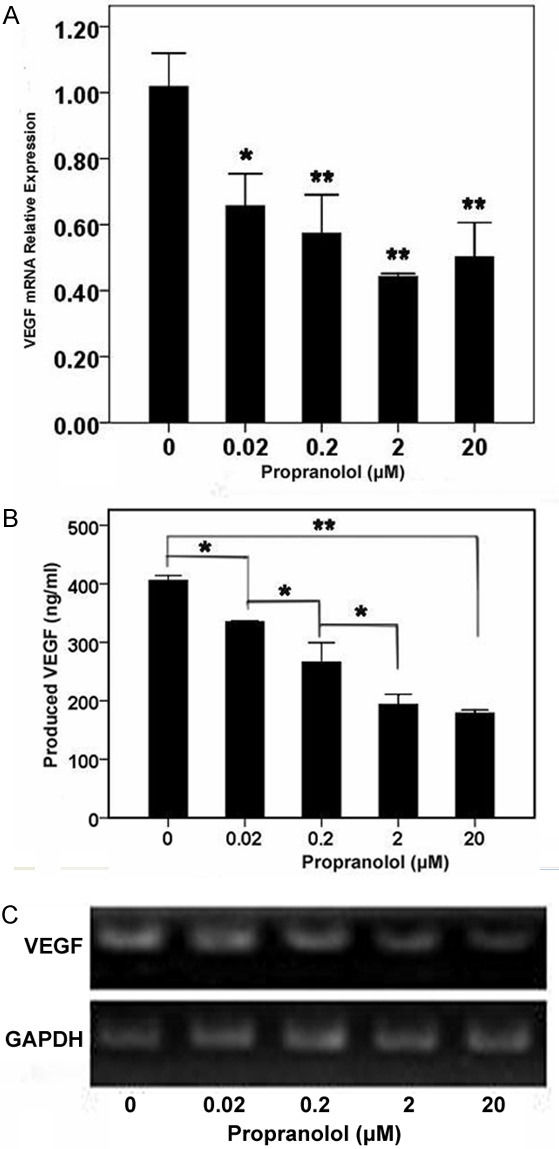
Effect of PRN on VEGF expression by HemSCs. PRN decreases mRNA expression and protein production of VEGF by HemSCs. A. Real-time PCR analysis of VEGF mRNA transcript levels; B. ELISA of VEGF in supernatants of HemSCs treated with different concentrations of PRN. Note the dose-dependent inhibition of VEGF secretion; C. RT-PCR showing decrease in mRNA expression of VEGF with increasing concentration of PRN (*P<0.05, **P<0.01).
Effect of PRN on bFGF expression by HemSCs
RT-PCR results expression of bFGF mRNA is lowest at a concentration of 0.2 μM (Figure 4). The band was not lighter followed by higher concentrated PRN. This suggests that the inhibitory effect of PRN on bFGF expression was not strong as pronounced as that observed for VEGF expression (Figure 4). Western blot analysis shows a similar, marked reduced bFGF protein levels expression at 2 and 20 μM of PRN treatment group, there was not notable variation in 2 μM group and 20 μM group. This decrease in bFGF protein production was consistent with the reduced expression of bFGF mRNA (Figures 4 and 5).
Figure 4.
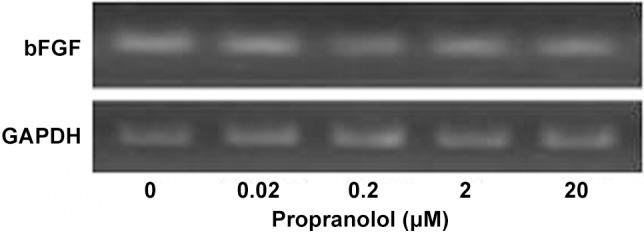
Effect of PRN on bFGF expression by HemSCs. RT-PCR showing bFGF mRNA expression by HemSCs incubated with different concentrations of PRN.
Figure 5.
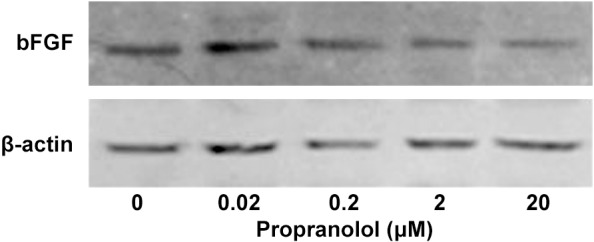
Effect of PRN on bFGF expression by HemSCs. Western blot of bFGF protein in HemSCs treated with different concentrations of PRN.
Effect of PRN and immunohistochemical analysis of VEGF and bFGF in the murine IH model
Pretreatment of HemSCs with PRN led to a significant decrease in H&E staining in the murine tumors of IH mice (Figure 6A). In addition, the MVD reduced from 140±35/mm2 to 67±21/mm2 (P<0.05, Figure 6C). Immunohistochemical analysis showed that VEGF and bFGF were strongly positive expressed in proliferating IHs and localized at cytoplasm or lumen of blood vessel in endothelial cell. Treatment with PRN, led to a marked decrease in VEGF staining (Figure 7C). There was also a reduction in bFGF staining, However, this effect was less pronounced (Figure 7F).
Figure 6.
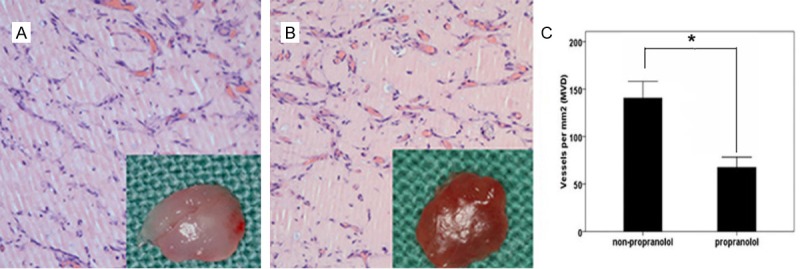
Effect of PRN and immunohistochemical analysis of VEGF and bFGF in the murine IH model. PRN reduces tumorigenesis in the in vivo IH model. A, B. Representative H&E stained section of A. implantation tissue containing HemSCs pretreated with PRN; B. Control tissue with no PRN pretreatment (×400); C. Statistical comparison of MVD in murine tumor tissue (*P<0.05).
Figure 7.
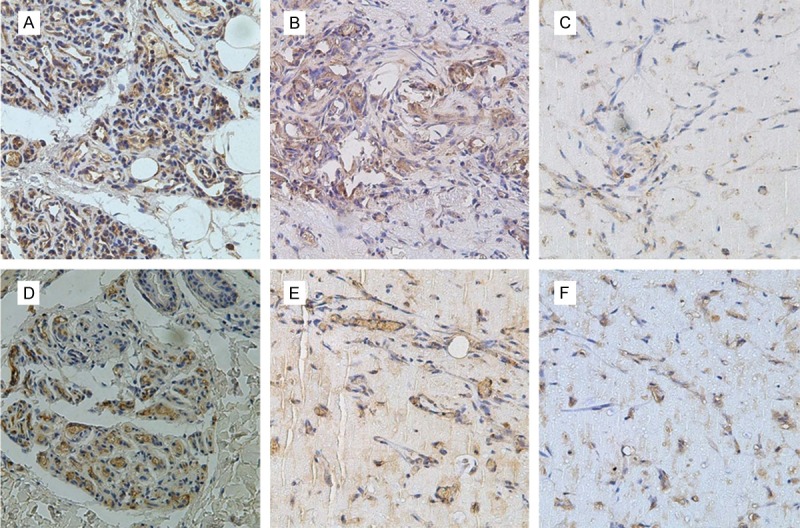
Effect of PRN and immunohistochemical analysis of VEGF and bFGF in the murine IH model. Differential regulation of VEGF and bFGF protein production by PRN in the in vivo model of IH. Immunohistochemical staining for VEGF (A-C) and bFGF (D-F). Representative tissue sections from: (A, D) Proliferating hemangioma tissue; (B, E) Control group without PRN treatment; (C, F) Experimental group with PRN treatment (×400).
Discussion
The therapeutic use of PRN, a nonselective β-adrenergic antagonist, was discovered by serendipitously. This β-blocker is a promising treatment with impressive efficacy. It is generally well tolerated; now replaced corticosteroids as the first-line in treatment of complicated His [6]. Although there is much speculation, at present, very little is known about its mechanisms of PRN action in IHs. However, it is likely to involve three different pharmacological activities. Early effects are vasoconstriction, which is attributed to the blocking of nitric oxide production; intermediate effects involve the inhibition of angiogenesis resulting from the blocking of angiogenic factors such as VEGF and bFGF. Long-term effects are characterized by induction of apoptosis in IH endothelial cells, and result in tumor regression [7].
Effect on proliferation and apoptosis of IH
We found that PRN had no significant effect on proliferation of HemSCs. Although other studies have suggested that PRN can induce apoptosis of vascular endothelial cells (VECs) [8], we found that PRN could not induce significant apoptosis of HemSCs even at a concentration of 50 μM. The half-maximal inhibition was 133 μM, which was far beyond the normal plasma concentration. The results show that the action of PRN is not acting through the inhibition of HemSC proliferation. In addition, continued HemSC proliferation and lack of apoptosis may account for why IHs rebound after patients stopped taking PRN, because HemSC did not apoptosis, proliferation after withdrawal. Interestingly, HUVECs were more sensitive to PRN compared with HemSCs. This result was in line with that previously reported by Sylvie Lamy et al [9]. The influence of hemangioma endothelial cells in this model requires further study.
Mechanism of action on suppression VEGF and bFGF by PRN
VEGF and bFGF are important proangiogenic growth factors that can stimulate endothelial cell proliferation and differentiation, and promote vasculogenesis [10,11]. Importantly, VEGF and bFGF are the main growth factors which promote vasculogenesis in proliferating hemangiomas [12,13]. We observed high levels of VEGF and bFGF in proliferating IH tissue. We show that even a low concentration, PRN is capable of down-regulating the expression of VEGF mRNA and decreasing VEGF protein levels in HemSCs. It should be noted that we also observed that the proportion of VEGF-positive HemSCs was very low in IH tissue. This is likely an indication that high expression of VEGF is a more widespread phenomenon, and VEGF is expressed by other cell types. We found that PRN can also down-regulate the expression of bFGF by HemSCs, however this effect is less pronounced than that for VEGF expression. Specifically, we show in our murine IH model that the expression of VEGF was lower than the expression of bFGF after PRN treatment. This suggests that PRN may be exerting its’ effect primarily through the inhibition of VEGF [14], and its pro-angiogenic activities. However, it is possible that PRN may additionally be acting through the down-regulation of bFGF [15]. Several studies have demonstrated that PRN can inhibit the expression of bFGF, but the mechanism is not established [16]. Yamashita et al [17] found that the norepinephrine can increase the expression of bFGF mRNA,and that effect can be partially inhibited by PRN. Another study confirmed that the induction of bFGF expression by β-adrenaline receptor agonists can be suppressed by PRN in the central nervous system of adult rats [18]. These findings provide insight into the mechanism of action of PRN in IH. Future work will examine the potential signaling pathways involved.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (81271163, 81070846).
Disclosure of conflict of interest
None.
References
- 1.Léauté-Labrèze C, Prey S, Ezzedine K. Infantile haemangioma: part I. Pathophysiology, epidemiology, clinical features, life cycle and associated structural abnormalities. J Eur Acad Dermatol Venereol. 2011;25:1245–1253. doi: 10.1111/j.1468-3083.2011.04102.x. [DOI] [PubMed] [Google Scholar]
- 2.Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 3.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 6.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating Infantile Haemangiomas. J Plast Reconstr Aesthet Surg. 2011;64:445–451. doi: 10.1016/j.bjps.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into themolecular mechanisms of action. Br J Dermatol. 2010;163:269–274. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 8.Sommers Smith SK, Smith DM. Beta blockade induces apoptosis in cultured capillary endothelial cells. In Vitro Cell Dev Biol Anim. 2002;38:298–304. doi: 10.1290/1071-2690(2002)038<0298:BBIAIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Lamy S, Lachambre MP, Lord-Dufour S, Béliveau R. Propranolol suppresses angiogenesis in vitro: Inhibition of proliferation, migration and differentiation of endothelial cells. Vascular Pharmacology. 2010;53:200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- 11.Wolter NE, Wolter JK, Enepekides DJ, Irwin MS. Propranolol as a novel adjunctive treatment for head and neck squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2012;41:334–344. [PubMed] [Google Scholar]
- 12.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 13.Frieden IJ, Haggstrom AN, Drolet BA, Mancini AJ, Friedlander SF. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas. Pediatr Dermatol. 2005;22:383–406. doi: 10.1111/j.1525-1470.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang J, Most D, Bresnick S, Mehrara B, Steinbrech DS, Reinisch J, Longaker MT, Turk AE. Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plast Reconstr Surg. 1999;103:1–9. doi: 10.1097/00006534-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Przewratil P, Sitkiewicz A, Wyka K, Andrzejewska E. Serum levels of vascular endothelial growth factor and basic fibroblastic growth factor in children with hemangiomas and vascular malformations-preliminary report. Pediatr Dermatol. 2009;26:399–404. doi: 10.1111/j.1525-1470.2009.00910.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Zheng J. Signaling regulation of fetoplacental angiogenesis. J Endocrinol. 2012;212:243–255. doi: 10.1530/JOE-11-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita H, Sato N, Kizaki T, Oh-ishi S, Segawa M, Saitoh D, Ohira Y, Ohno H. Norepinephrine stimulates the expression of fibroblast growth factor 2 in rat brown adipocyte primary culture. Cell Growth Differ. 1995;6:1457–1462. [PubMed] [Google Scholar]
- 18.Follesa P, Mocchetti I. Regulation of basic fibroblast growth factor and nerve growth factor mRNA by β-adrenergic receptor activation and adrenal steroids in rat central nervous system. Mol Pharmacol. 1993;43:132–138. [PubMed] [Google Scholar]


