Abstract
The Forkhead Box L1 (Foxl1) transcription factor regulates epithelial proliferation and development of gastrointestinal tract, and has been implicated in gastrointestinal and pancreatic tumorigenesis. However, the role of Foxl1 in renal cancer development and progression remains to be elucidated. The study was conducted to investigate the expression of Foxl1 and its prognostic significance in clear cell renal cell carcinoma (ccRCC). Meanwhile, the function of Foxl1 in human ccRCC was further investigated in cell culture models. Methods: Real-time quantitative PCR, western-blot, immunohistochemistry were used to explore Foxl1 expression in primary ccRCC clinical specimens and ccRCC cell lines. Foxl1 expression was up-regulated by over-expression vector in 786-O and ACHN cells, proliferation, cell cycle, migration and invasion were assayed. Results: Foxl1 expression was down-regulated in the majority of the ccRCC clinical tissue specimens at both mRNA and protein levels. Clinic pathological analysis showed that Foxl1 expression was significantly correlated with tumor stage, lymph node metastasis, distant metastasis, clinical TNM stage (cTNM) and histological grade of renal cancer. The Kaplan-Meier survival curves revealed that low Foxl1 expression was associated with poor prognosis in ccRCC patients. Foxl1 expression was an independent prognostic marker of overall ccRCC patient survival in a multivariate analysis. Mechanistic analyses demonstrated that over-expression of Foxl1 inhibits tumor cell growth, migration and invasion in renal cancer cells. Conclusions: These results suggest that Foxl1 expression is a candidate predictor of clinical outcome in patients with resected ccRCC and it plays an inhibitory role in renal tumor progression.
Keywords: Foxl1, clear cell renal cell carcinoma, prognosis
Introduction
Renal cell carcinoma (RCC) is the most lethal urologic tumor and the sixth leading cause of cancer deaths in Western countries. Each year, around 200,000 patients are diagnosed with this malignancy resulting in approximately 100,000 deaths, and its incidence is increasing steadily in recent years [1,2]. RCC is represented by 80% by clear cell RCC (ccRCC), originating from the renal proximal tubule [3]. Nearly 25-30% of patients with RCC have evidence of metastases at initial presentation [4,5]. Although radical nephrectomy is effective to cure early and local RCCs, 30% of patients develop metastatic disease after surgery [6]. Patients with metastatic RCC face a dismal prognosis and have limited therapeutic options. Median survival in a recent cohort was only 1.5 years with fewer than 10% of patients surviving for 5 years [7]. Therefore, it is of paramount importance to better understand the pathogenesis of aggressive RCC in order to develop effective strategies for the prevention and treatment of RCC.
Foxl1 proteins belong to the forehead box (Fox) family of transcription factors. Fox family shares a highly conserved 100-aa DNA binding domain (the forkhead box) and comprises more than 100 members in humans, classified as FoxA to FoxR on the basis of sequence similarity [8]. Fox proteins are at the junction of multiple signaling pathways and play critical roles in a variety of physiological and pathological processes inc-luding cancer. For example, FoxO transcription factor, can lead to cell cycle arrest and apoptosis in pancreatic cancer [9]. In addition, FoxOs deficiency in genetic mice led to the development of thymic lymphomas and hemangiomas, indicating that the FoxOs are tumor suppressors [10,11]. In contrast to FoxOs, FoxM1 has been shown to have pro-proliferative function and increased expression of FoxM1 gene was often found in various human cancers [12]. In hepatocellular carcinoma, over-expression of FoxM1 is associated with aggressive tumor features and poor prognosis [13]. Siomycin A as an inhibitor of FoxM1 can specifically target FoxM1 to induce apoptosis in cancer cells and down regulation of FoxM1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells [14,15].
Foxl1 has been implicated in the regulation of epithelial cell proliferation in gastrointestinal tracts. Loss of Foxl1 led to a marked increase in cellular proliferation of intestinal epithelia in mice, leading to the distortion in the tissue architecture of the stomach and small intestine [16]. The altered proliferation rate in Foxl1-null mutant mice is correlated with an activated Wnt/β-catenin pathway as demonstrated by increased nuclear translocation of β-catenin [17]. Foxl1 deficiency accelerates the initiation of gastro-intestinal tumor and increases tumor load in ApcMin mice, Foxl1 is the first mesenchymal Modifier of Min and plays a key role in gastrointestinal tumorigenesis [18]. Foxl1 is specifically expressed in low-grade fibromyxoid sarcoma as compared to other morphologically similar tumor types [19]. Thus far, the biological function of Foxl1 in human cancer remains to be elucidated. In the present study, we investigated the role of Foxl1 in human renal cancer. Our data demonstrated that Foxl1 expression is associated with clinical TNM (cTNM) and histological grade of ccRCC. Further mechanistic studies showed that Foxl1 inhibits cell growth, migration and invasion in renal cancer cells. Our data highlight an important role for Foxl1 in controlling ccRCC progression.
Methods
Patients and surgical specimens
A total of 88 primary ccRCC tissues and matched adjacent non-tumor renal tissues were obtained from patients who underwent radical nephrectomy in the Department of Urology, Shanghai Tenth People’s Hospital of Tongji University between 2006 to 2008. None of the patients had received chemotherapy or radiotherapy before surgery. After surgical resection, tumor specimens and corresponding adjacent non-tumor tissues were collected and stored in liquid nitrogen until use. Parts of each sample were fixed in formalin, embedded in paraffin and stored in the Department of Pathology, Shanghai Tenth People’s Hospital of Tongji University. 63 of these 88 patients were men and 25 were women. The median age of the patients was 61 years (range, 32-79 years). Clinicopathological characteristics in our study are presented in Table 1. Paraffin-embedded tumor specimens and paired adjacent non-tumor specimens were carefully collected immediately after nephrectomy operation. All patients were followed up until September 2011 with a median observation time of 35 months. For the use of these clinical materials for research purposes, prior patient’s consent and approval from the Institute Research Ethics Committee were obtained.
Table 1.
Clinical-pathological characteristics of renal cell carcinoma patients
| Characteristics | N=88 | % |
|---|---|---|
| Gender | ||
| Male | 63 | 71.6 |
| Female | 25 | 28.4 |
| Age (yrs) | ||
| <65 | 45 | 51.1 |
| >65 | 43 | 48.9 |
| Tumor Size (cm) | ||
| <4 | 41 | 46.6 |
| ≥4 | 47 | 53.4 |
| Histological grade | ||
| I-II | 28 | 31.8 |
| III-IV | 60 | 68.2 |
| cTNM | ||
| I-II | 32 | 36.4 |
| III-IV | 56 | 63.6 |
| T stage | ||
| T1-T2 | 42 | 47.7 |
| T3-T4 | 46 | 52.3 |
| Lymph nodes metastasis | ||
| Absence | 61 | 69.3 |
| Presence | 27 | 30.7 |
| Distant Metastasis | ||
| Absence | 81 | 92.1 |
| Presence | 7 | 7.9 |
| Follow-ups | ||
| Dead | 42 | 47.7 |
| Survival or lost | 46 | 52.3 |
Immunohistochemistry staining
All samples were fixed in 10% formaldehyde solution, embedded in paraffin blocks, cut in 4 μm thick sections, and mounted on glass slides. Each slide was dewaxed in xylene and rehydrated in grade alcohol, followed by boiling in 10 mmol/L of citrate buffer (PH 6.0) for antigen retrieval. After inhibition of endogenous peroxidase activities for 30 minutes with methanol containing 0.3% H2O2, the sections were blocked with 2% bovine serum albumin for 30 minutes and incubated overnight at 4°C with primary mouse monoclonal anti-Foxl1 antibody (Abnova, Walnut, CA). After washing thrice with PBS, the slides were incubated with horseradish peroxidase-conjugated rabbit-anti-mouse IgG for 30 minutes, followed by reaction with diaminobenzidine and counterstaining with Mayer/hematoxylin. Negative control was done by omission of the primary antibody and substituting it with nonspecific mouse IgG.
Evaluation of immunohistochemical staining
The evaluation of the immunohistochemical staining was performed independently by two authors without knowledge of the clinicopathological information. The immunoreactive scores besides Foxl1 were determined by the sum of extension and intensity as literature reported previously [20]. The intensity of staining was scored using the following scale: 0, no staining of the tumor cells; +, mild staining; ++, moderate staining and +++, marked staining. The area of staining was evaluated and recorded as a percentage: 0, less than 5%; +, 5%-25%; ++, 26%-50%; 3+, 51%-75% and 4+, more than 75%. The combined scores were recorded and graded as follows: -, 0; +, 1-2; ++, 3-5; +++, 6-7. Additionally, for statistical analysis, the - and 1+ cases were pooled into the low-expression group, and the 2+ and 3+ cases were pooled into the high-expression group.
Cell lines
Immortalized normal human proximal tubule epithelial cell line HK-2 was purchased from the American Type Culture Collection (ATCC, USA). Human RCC cell lines 786-O and ACHN were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (CCCAS, China). HK-2 cells were cultured in KSFM medium (Gibco), and other cells were cultured in RPMI-1640 medium (HyClone) with 10% fetal bovine serum (Gibco), 50 U/ml of penicillin and 50 μg/ml of streptomycin. All cells were cultured in a sterile incubator maintained at 37°C with 5% CO2.
Gene over-expression
Plasmid pCMV-Foxl1 was used for Foxl1 over-expression, Plasmid pCMV-Control was used for transfection as the negative control. Cells were transfected using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). Following transfection, the mRNA and protein levels were assessed 48 hours later.
Real-time quantitative PCR
Total RNA was isolated from tissues and transfected cells using TRIzol according to the manufacturer’s protocol (Invitrogen). The concentration and quality of the extracted total RNA were determined by measuring OD260 and the OD260: OD280 ratio. The first strand cDNA was synthesized using SuperScript II RNase H Reverse Transcriptase and Oligo (DT) primer from 2 μg of total RNA, according to the manufacturer’s instructions (Invitrogen). The PCR amplification were performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, on a Applied Biosystems 7900 HT (Applied Biosystems) with 1.0 μl of cDNA and SYBR Green Real-time PCR Master Mix (TaKaRa). At the completion of cycling, the melting curve analysis was performed to establish the specificity of the PCR products. Data was collected and analyzed by SDS 2.3 Software (Applied Biosystems). The expression level of each candidate gene was internally normalized against that of the GAPDH. The relative quantitative value was expressed by the 2-ΔΔCt method, representing the amount of the candidate gene expression with the same calibrators. Each experiment was performed in triplicates and repeated three times. The primers used in real-time quantitative PCR are shown in Table 2.
Table 2.
Primer sequences for real-time quantitative PCR
| Gene | primer | Product size (bp) |
|---|---|---|
| Foxl1 | sense: TTATTTGGCGGACAGTGACA | 153 |
| anti: ACACGGCATCAATCTTTTCC | ||
| Cyclin B1 | sense: GGTTGGGTCGGCCTCTACC | 188 |
| anti: AGCCAGGTGCTGCATAACTGGAA | ||
| Cyclin D1 | sense: TCTACACCGACAACTCCATCC | 167 |
| anti: GTGTTTGCGGATGATCTGTTT | ||
| CDK2 | sense: TGCCTGATTACAAGCCAAGTT | 197 |
| anti: GAGTCGAAGATGGGGTACTGG | ||
| P21 | sense: AGGGGACAGCAGAGGAAGAC | 159 |
| anti: GGCGTTTGGAGTGGTAGAAA | ||
| P27 | sense: CGCTCGCCAGTCCATT | 124 |
| anti: ACAAAACCGAACAAAACAAAG | ||
| MMP2 | sense: TGATCTTGACCAGAATACCATCGA | 142 |
| anti: GGCTTGCGAGGGAAGAAGTT | ||
| MMP9 | sense: CATCCATTCATTCATTCATTGG | 125 |
| anti: AGACATTCAAAAACCAACTGCA | ||
| GAPDH | sense: GGCATTGCTCTCAATGACAA | 142 |
| anti: ATGTAGGCCATGAGGTCCAC |
Western blot assay
Cells and tissues were lysed in lysis buffer containing protease inhibitor. Protein concentration was determined using a Bio-Rad protein assay system (Bio-Rad). Equivalent amounts of proteins were separated by SDS-PAGE, and then transferred to polyvinylidene difluoride membranes (Bio-Rad). After being blocked in Tris buffered saline (TBS) containing 5% non-fat milk, the membranes were incubated with specific primary antibodies (Abnova, Walnut, CA) at 4°C for 12 hours and then with horseradish peroxidase-conjugated anti-mouse antibody for 2 hours at room temperature. ECL detection reagent (Amersham LifeScience, Piscataway, NJ) was used to demonstrate the results.
MTT assay
Cells were plated in 96-well culture plates at about 5 × 103 cells per well and incubated for 1 to 5 days. Then, 20 μl of 5 mg/ml MTT solution was added to each well and incubated for 4 hours at 37°C, the media was removed from each well, and the resultant MTT formazan was solubilized in 150 μl of DMSO. The absorbance values at 570 nm were measured using a micro-plate reader (Bio-Rad). The experiment was repeated three times and each experiment had six replicate wells.
Cell cycle analysis
Cells were harvested 48 hours after transfection with pCMV-Control or pCMV-Foxl1 vectors and fixed in 70% ice-cold ethanol overnight. The cells were then washed with PBS, and stained with propidium iodide (50 mg/ml) in PBS supplemented with RNase (50 mg/ml) in the dark at room temperature for 30 minutes. Tests were performed in triplicate for each sample, and analyses of cell cycle distribution were performed by flow cytometer in accordance with the manufacturer’s guidelines (FACS, BD Bioscience, USA).
Scratch migration assay
Cells were transfected with pCMV-Control or pCMV-Foxl1 vectors were seeded into 12-well plates and allowed to grow to 90-95% confluence. Before scratching, cells were starved for 24 hours in the medium with 1% FBS. Similar sized wounds were introduced to monolayer cells using a sterile white pipette tip. Wounded monolayer cells were washed three times with PBS to remove cell debris and then cultured. The speed of wound closure was monitored and photographed at 48 hours. Each experiment was performed in triplicates.
Matrigel invasion assay
Cell invasion assay was performed using a 24-well transwell chamber with a pore size of 8 μm (Costar, NY, USA). The inserts were coated with 50 μl Matrigel (dilution at 1:2, BD Bioscience, USA). Cells were trypsinized after transfection with pCMV-Control or pCMV-Foxl1 vectors for 48 hours and transferred to the upper Matrigel chamber in 100 μl of serum free medium containing 1 × 105 cells and incubated for 24 hours. The lower chamber was filled with medium that contained 10% fetal bovine serum as chemoattractants. After incubation, the non-invaded cells on the upper membrane surface were removed with a cotton tip, and the cells that passed through the filter were fixed and stained using 0.1% crystal violet. The numbers of invaded cells were counted in five randomly selected high power fields under a microscope. This experiment was performed in triplicates.
Statistical analysis
The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 18.0. Clinical and histopathological information and the results from the immunohistochemical studies were entered into a data-base. The variances of Foxl1 expression among different tissues was analyzed using Mann-Whitney U-test and the clinicopathological data was analyzed with Spearman’s correlation test. 2-tailed Student’s t-tests were used to analyze comparisons between the 2 groups. P-value of <0.05 was regarded as statistically significant.
Results
Foxl1 mRNA and protein expression in primary ccRCC tissue samples
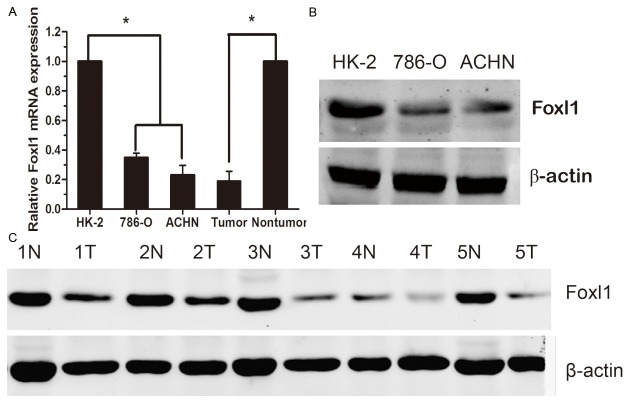
We examined Foxl1 mRNA expression in 88 paired clinical samples from ccRCC patients (tumor tissues and matched adjacent non-tumor tissues) by real-time quantitative PCR. The results revealed statistically significant lower expression of Foxl1 mRNA in tumor tissues, as compared to the matched adjacent non-tumor tissues (P<0.05, Figure 1A). Foxl1 was also low expression at the protein level in tumor tissues, and the Foxl1 protein expression in five representative pairs of samples is shown in Figure 1C.
Figure 1.
The expression of Foxl1 mRNA and protein in the human ccRCC surgical specimens and RCC cell lines. A: The relative mRNA expression of Foxl1 was lower in 88 ccRCC tumor tissues than in matched adjacent non-tumorous tissues and lower in the 786-O, ACHN cells compared with HK-2 cells (P<0.05). B: The Foxl1 protein expression was reduced in the 786-O, ACHN cells compared to HK-2 cells. C: The Foxl1 protein expression was lower in the tumor tissues than in matched adjacent non-tumorous tissues. N, non-tumorous tissues; T, ccRCC tissues. *P<0.05.
Foxl1 mRNA and protein expression in RCC cell lines
We used real-time quantitative PCR and western blot to detect the expression of Foxl1 mRNA and protein in RCC cell lines as well as in an immortalized normal human proximal tubule epithelial cell line (HK-2). As shown in Figure 1A, the 786-O and ACHN showed lower Foxl1 transcript levels relative to the HK-2 cell line. Likewise, Foxl1 protein expression was low expression in the two RCC cell lines compared to the HK-2 cell line (Figure 1B).
Immunohistochemical analysis of Foxl1 expression in ccRCC clinical samples and its relationship to clinicopathological parameters
We analyzed Foxl1 protein level in 88 ccRCC tissues and adjacent non-tumor tissues using an immunohistochemical approach. Foxl1 protein expression in tumors was decreased compared with that in adjacent non-tumor tissues. Foxl1 stained mainly in the nuclear of the cells (Figure 2A). 32 (36.4%) cases showed low Foxl1 expression (Foxl1 - or Foxl1 +), and 56 (63.6%) cases exhibited high Foxl1 expression (Foxl1 ++ or Foxl1 +++).
Figure 2.
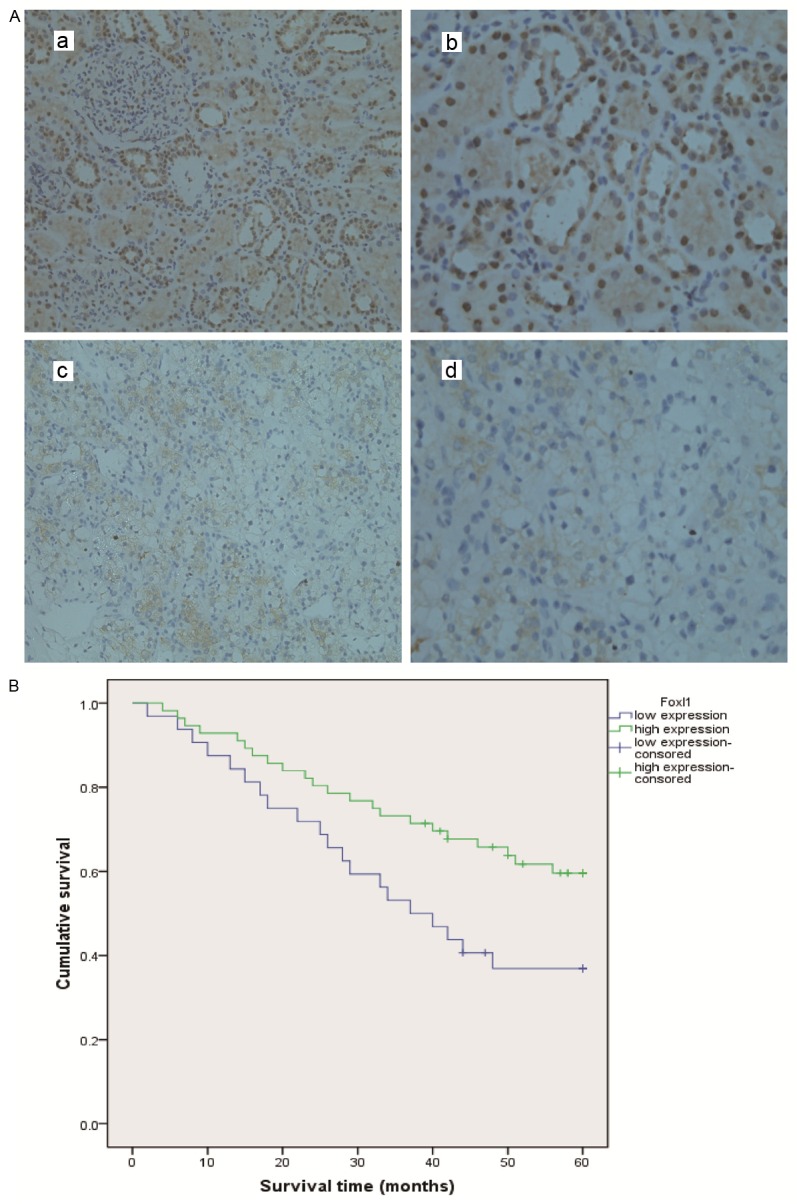
Foxl1 protein expression in renal cancer and patient survival. A: Immunohistochemical analysis of Foxl1 protein expression in 88 patients of ccRCC tissues. a: Immunohistochemistry expression of Foxl1 in adjacent non-tumor tissue (× 200). b: Immunohistochemistry expression of Foxl1 in adjacent non-tumor tissue (× 400). c: Immunohistochemistry expression of Foxl1 in tumor tissue (× 200). d: Immunohistochemistry expression of Foxl1 in tumor tissue (× 400). B: The survival analysis of Foxl1. Patients with lower Foxl1 expression in tumor tissue were closely correlated with poorer overall survival than patients with tumor with higher Foxl1 expression (p<0.05, respectively).
A significant correlation was observed between the lower expression of Foxl1 proteins with tumor stage, lymph node metastasis, distant metastasis, cTNM stage and histological grade. IHC was employed to investigate the association between Foxl1 expression and clinicopathological features in the 88 renal cancer specimens. The expression level of Foxl1 in nuclear was significantly associated with tumor stage (p=0.012), lymph node metastasis (p=0.015), distant metastasis (p=0.019), cTNM stage (p=0.032) and histological grade (p=0.017). There was no significant association between Foxl1 expression and patients’ gender, age and tumor size. Detailed data is shown in Table 3.
Table 3.
Relationship between the expression of Foxl1 proteins and clinicopathological parameters
| Markers | Correlation coefficient (r) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gender | Age | Tumor size | cTNM | T stage | LN metastasis | Distant metastasis | Histological grade | |
| Foxl1 | 0.094 | 0.056 | 0.084 | -0.427a | -0.217a | -0.324a | -0.141a | -0.236a |
p<0.05;
LN: lymph node.
Foxl1 expression and survival analysis: univariate survival analysis
Follow-up information was available for 88 patients until September 2011, within the observation period, there were 42 renal cancer related deaths with a median follow-up time of 23 months (0~56 months). And the remaining 46 patients were still alive or lost to follow-up with a median follow-up time of 53 months ranging (39~60 months). Survival analysis by Kaplan-Meier survival curve and log-rank test demonstrated that patients with higher expression of Foxl1 in tumor tissue had a better overall survival than patients with tumor with lower expression (p<0.05), the 5-years survival rate of patients with higher expression was significantly higher than that of patients with lower expression (60.7% vs. 37.5% Table 4). The survival curve was demonstrated in Figure 2B.
Table 4.
The 5-year survival rate of the Foxl1 expression and other clinicopathological features
| 5-year survival rate | ||||
|---|---|---|---|---|
|
| ||||
| Survival rate | Standard error | p value | ||
| Foxl1 | Low | 0.375 | 0.084 | 0.019 |
| High | 0.607 | 0.054 | ||
| Gender | Male | 0.447 | 0.061 | 0.573 |
| Female | 0.395 | 0.052 | ||
| Age | <65 | 0.537 | 0.067 | 0.017 |
| ≥65 | 0.343 | 0.072 | ||
| Tumor size | <4 cm | 0.568 | 0.078 | 0.003 |
| ≥4 cm | 0.313 | 0.053 | ||
| Histological grade | I-II | 0.576 | 0.084 | 0.015 |
| ≥III | 0.339 | 0.061 | ||
| cTNM | TNM1 | 0.779 | 0.097 | 0.008 |
| TNM2 | 0.474 | 0.053 | ||
Multivariate cox regression analysis
To avoid the influence caused by univariate analysis, the expression of Foxl1 as well as other parameters was examined in multivariate Cox analysis. The Foxl1 was found to be a significant independent prognostic factor for poor overall survival in our study (B=-0.668; p=0.031; Exp (B) =0.513), which indicated that the Foxl1 protein could act as a potential biomarker for prognosis evaluation of renal cancer. Of other parameters, tumor size, histological grade and cTNM were also found to be independent prognostic factors for patient survival (Table 5).
Table 5.
Multivariate Cox’s proportional hazards regression analysis of prognostic factors for renal cancer
| Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 95.0% CI for Exp (B) | ||||||||
|
|
||||||||
| B | SE | Wald | df | Sig. | Exp (B) | Lower | Upper | |
| Foxl1 | -0.668 | 0.310 | 4.640 | 1 | 0.031 | 0.513 | 0.279 | 0.942 |
| Tumor size | 0.059 | 0.031 | 4.237 | 1 | 0.038 | 1.042 | 1.023 | 1.121 |
| Histological grade | 0.527 | 0.216 | 3.316 | 1 | 0.027 | 1.529 | 1.217 | 2.648 |
| Age | 0.034 | 0.023 | 4.376 | 1 | 0.041 | 1.087 | 1.019 | 1.137 |
| cTNM | 0.621 | 0.196 | 6.832 | 1 | 0.012 | 1.671 | 1.172 | 2.642 |
Effects of Foxl1 over-expression on cell growth
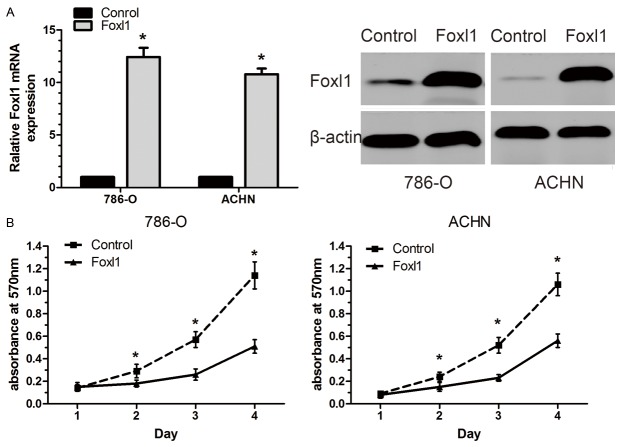
In order to determine whether Foxl1 could be an effective therapeutic target for ccRCC, we employed an over-expression vectors approach to increase Foxl1 endogenous expression in 786-O and ACHN cells. An elevated expression of Foxl1 was demonstrated using real-time quantitative PCR and western blot in cells transfected with pCMV-Foxl1 (Figure 3A). Over-expression of Foxl1 significantly inhibited cell proliferation in both 786-O and ACHN cells (P<0.05, Figure 3B).
Figure 3.
Foxl1 inhibits renal cancer 786-O and ACHN cells growth. A: Increased expression of Foxl1 in transfected renal cancer cells were demonstrated by Real-time quantitative PCR and western blot analysis. B: There were significant decreases in cell growth of Foxl1 over-expressing cells as compared with control cells. The experiments were repeated thrice. *P<0.05.
Effect of Foxl1 over-expression on cell cycle and MMP-2, MMP-9
Cell cycle analysis revealed that Foxl1 over-expression in 786-O and ACHN cells caused an accumulation of cells in the G0-G1 phase and a decrease in the S phase compared with control cells (P<0.05, Figure 4A). To investigate the mechanism underlying the cell cycle arrest, we examined the levels of a few cell cycle regulatory factors and studied the effects of up-regulation of Foxl1. The expression of cycling B1, cycling D1, and cyclin-dependent kinase 2 (Cdk2) at the mRNA levels was found to be decreased in cells transfected with Foxl1 over-expression vector compared with those transfected with control vector (P<0.05, Figure 4B). In contrast, up-regulation of Foxl1 was found to result in an increase in the expression of cyclin-dependent kinase inhibitors such as p21 and p27 (P<0.05, Figure 4B). Taken together, these results indicated that up-regulation of Foxl1 expression suppressed cell cycle progression in ccRCC cells. As shown in Figure 4C, real-time quantitative PCR analysis demonstrated that Foxl1 over-expression significantly decreased MMP-2, MMP-9 mRNA expression compared with control cells (P<0.05). Similar results were observed by western blot analysis (Figure 4D). These results clearly show that tumor progression could be attenuated by the up-regulation of Foxl1.
Figure 4.
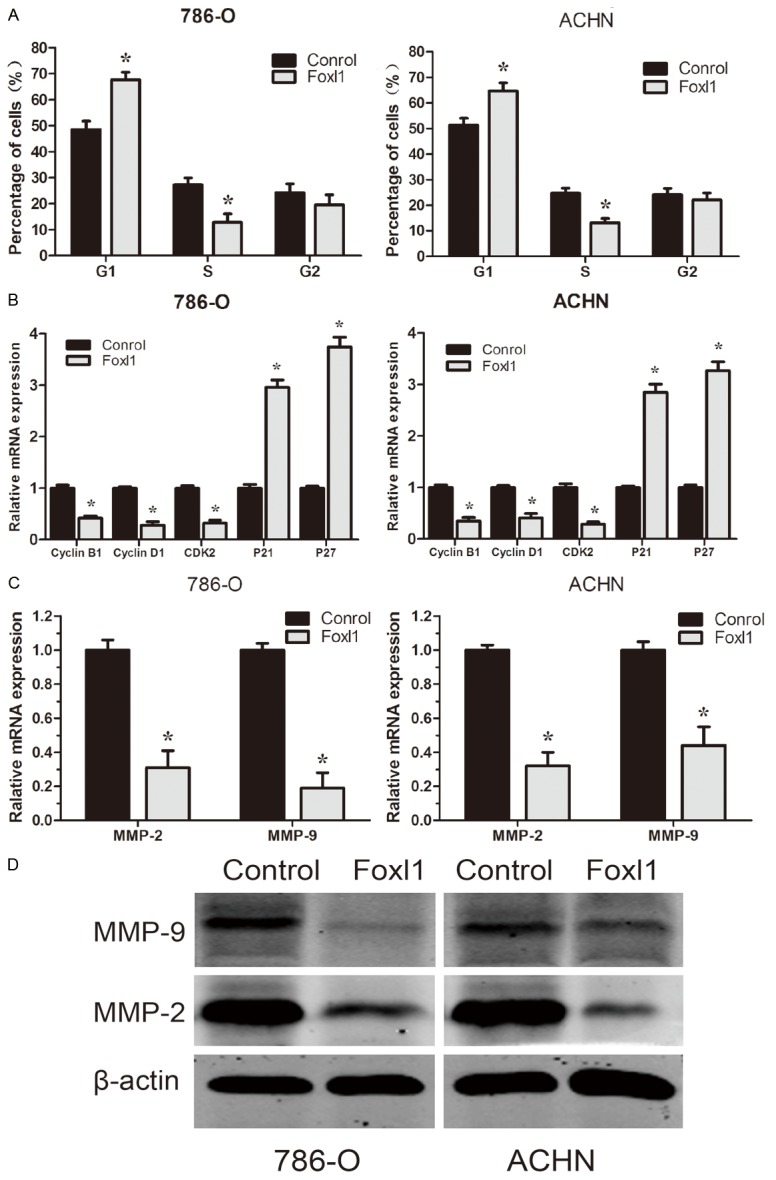
Effect of Foxl1 over-expression on 786-O and ACHN cells cycle and MMPs expression. A: The cell cycle distribution was analyzed using propidium iodide staining and flowcytometry. B: The expression level of cell cycle regulatory factors were detected by real-time quantitative PCR. C: The expression level of MMP-2 and MMP-9 were detected by real-time quantitative PCR. D: The expression level of MMP-2, MMP-9 were detected by Western blot analysis. The experiments were repeated thrice. *P<0.05.
Effect of Foxl1 over-expression on migration and invasion
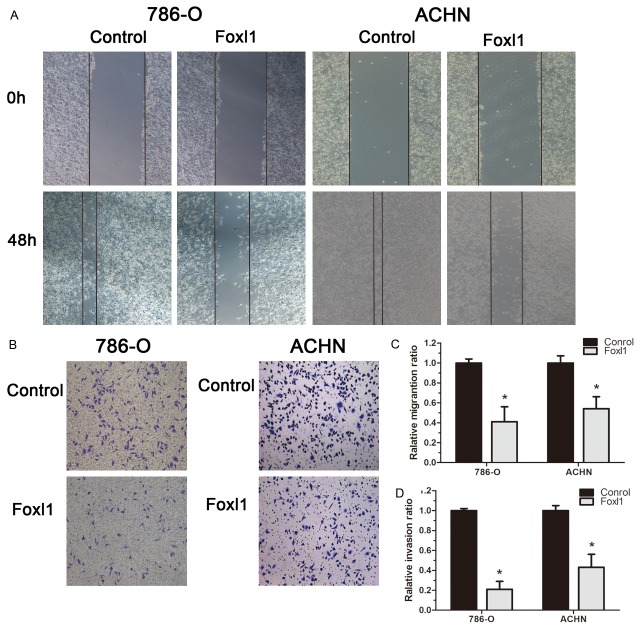
We tested the effect of Foxl1 over-expression on renal cancer cell migration and invasion. In the scratch migration assay, up-regulation of Foxl1 significantly suppressed the migration of both 786-O and ACHN cells (P<0.05, Figure 5A, 5C). Matrigel invasion assay showed the average cell counts crossing matrigel-coated membrane in the control group was more than that in Foxl1 over-expression group both 786-O and ACHN cells, which indicated up-regulation of Foxl1 significantly suppressed the invasion of renal cancer. (P<0.05, Figure 5B, 5D). These results show a critical role of Foxl1 in the inhibition on renal cancer migration and invasion.
Figure 5.
Foxl1 inhibits cell migration and invasion of 786-O and ACHN cells. (A, C) Cell migration was assessed using scratch-healing assays. Confluent monolayer of 786-O and ACHN cells were scratched and healing was monitored by taking photographs at the indicated time points. (B, D) Cell invasion was determined in 786-O and ACHN cells using matrigel invasion assay. The invaded cells were counted under a microscope. The experiments were repeated thrice. *P<0.05.
Discussion
Foxl1 was previously described as a critical transcriptional factor that regulates cell proliferation and development of epithelium in gastrointestinal tracts in mice. We performed Oncomine database analyses on publicly available microarray datasets and found that Foxl1 expression is consistently decreased in multiple myeloma as compared to normal tissues [21,22]. One myeloma dataset also showed that Foxl1 is significantly higher in long survival (live over a year) as compared with short survival group (death within a year) [23]. And a recent study reported Foxl1 was down-regulated and be associated with worse prognosis and to serve as a prognostic marker in human pancreatic cancers [24]. However, Foxl1 expression pattern and biological significance in ccRCC is unknown. In this study, we showed that Foxl1 expression determined by real-time quantitative PCR and Western blot was significantly lower in ccRCC tissues than that in adjacent non-tumor renal tissues. Immunohistochemical analysis also confirmed that tumor tissues exhibited absence or lower Foxl1 expression, in contrast to adjacent non-tumor tissues which displayed abundant Foxl1 expression. To investigate whether Foxl1 expression be associated with the progression of ccRCC, the Foxl1 expression levels and the clinic pathologic characteristics of 88 patients with ccRCC were compared by immunohistochemistry. We found that low Foxl1 expression is significantly correlated with primary tumor stage, lymph node metastasis, distant metastasis, clinical TNM stage (cTNM) and histological grade, suggesting that its expression might be important for the tumorigenesis in ccRCC. Furthermore, reduced Foxl1 expression was identified as an independent worse prognostic factor in ccRCC patients.
We have clearly showed that Foxl1 is lowly expressed in ccRCC cells from patient samples. This prompted us to examine the biological function of Foxl1 in greater detail through in vitro analysis of ccRCC cell lines. Therefore, we first checked its expression level in 786-O, ACHN and HK-2 cell lines and picked up 786-O and ACHN for the further study. We over-expressed the active form of human Foxl1 in the two renal cancer cell lines. We found that over-expression of Foxl1 suppressed cellular growth, and inhibited cell migration and invasion in renal cancer. Thus, our study suggested that Foxl1 plays an inhibitory role in ccRCC.
Abnormal cell proliferation and growth are characteristics of cancer, including ccRCC. Most of the proliferative factors influence cell growth by affecting cell cycle progression. The importance of Foxl1 with respect to the cell cycle is well recognized. In this study, cell cycle analyses revealed that Foxl1 over-expression cells showed higher levels of G1 phase and lower S phase than the control cells. So Foxl1 over-expression inhibited G1 to S transition in cell cycle progression, which might explain the mechanism of Foxl1 on ccRCC cell proliferation. Furthermore, we found that up-regulation of Foxl1 caused a marked reduction in cyclin B1, cyclin D1, and Cdk2 expression, which play important roles in cell cycle progression. We also observed an increased expression of cyclin-dependent kinase inhibitors such as p21 and p27 in Foxl1 over-expression transfected cells, which are known to negatively regulate cell cycle progression. These results suggest that Foxl1 influences the cell cycle progression by negatively regulating the factors that favor cell cycle progression and also by positively influencing the inhibitors of cell cycle in ccRCC cells.
Metastasis is an important aspect of ccRCC. It is known that Matrix metalloproteinases (MMPs) are involved crucially in the processes of tumor cell invasion and metastasis [25,26]. Among these MMPs, MMP-2 and MMP-9 are directly linked with angiogenesis and degradation of the basement membrane collagen, and their expression and activity are correlated with metastatic abilities and prognosis of cancer [27,28]. Here, we showed that up-regulation of Foxl1 by over-expression vector in 786-O and ACHN cells led to reduced expression of MMP-2 and MMP-9. The results suggest that the increase of Foxl1 expression has potential for anti-metastatic therapy.
In order to fully understand the consequences of such up-regulation in the expression and the activity of MMP-2 and MMP-9, we performed scratch migration assay and matrigel invasion assay of ccRCC cells. We found that up-regulation of Foxl1 led to a significant reduction in the migration and invasive potential of 786-O and ACHN cells. These results are consistent with the inactivation of MMP-2, MMP-9 by the up-regulation of Foxl1, which inhibits cancer cell migration and invasion.
Conclusions
In summary, the present study firstly showed that Foxl1 expression was down-regulated in the majority of the ccRCC clinical tissue specimens at both mRNA and protein levels. Lower expression of Foxl1 positively correlates with the aggressive phenotype of ccRCC, and predicts poor survival outcome of patients. We have also presented experimental evidence that up-regulation of Foxl1 in ccRCC cell lines using over-expression vector inhibited cell proliferation and induced cell cycle arrest with reduced expression of cyclin B1, cyclin D1, and Cdk2, and increased expression of p21 and p27. Furthermore, up-regulation of Foxl1 reduced expression and activity of MMP-2 and MMP-9, resulting in the inhibition of migration and invasion. Based on these findings, we conclude that Foxl1 may function as a potential tumor suppressor and serve as a candidate predictor of outcomes in renal cancer.
Acknowledgements
This work was partially supported by grants from the National Natural Science Foundation of China (No. 81000311 and No. 81270831).
Disclosure of conflict of interest
None.
References
- 1.Miyamoto H, Miller JS, Fajardo DA, Lee TK, Netto GJ, Epstein JI. Non-invasive papillary urothelial neoplasms: the 2004 WHO/ISUP classification system. Pathol Int. 2010;60:1–8. doi: 10.1111/j.1440-1827.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 2.Montironi R, Santinelli A, Pomante R, Mazzucchelli R, Colanzi P, Filho AL, Scarpelli M. Morphometric index of adult renal cell carcinoma. Comparison with the Fuhrman grading system. Virchows Arch. 2000;437:82–89. doi: 10.1007/s004280000216. [DOI] [PubMed] [Google Scholar]
- 3.Matsuura K, Nakada C, Mashio M, Narimatsu T, Yoshimoto T, Tanigawa M, Tsukamoto Y, Hijiya N, Takeuchi I, Nomura T, Sato F, Mimata H, Seto M, Moriyama M. Downregulation of SAV1 plays a role in pathogenesis of high-grade clear cell renal cell carcinoma. BMC Cancer. 2011;11:523. doi: 10.1186/1471-2407-11-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B, Zigeuner RE, Artibani W, Guille F, Abbou CC, Salzano L, Gallo C. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer. 2005;104:1362–1371. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 5.Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, Cindolo L, De la Taille A, Tostain J, Mulders PF, Salomon L, Zigeuner R, Prayer-Galetti T, Chautard D, Valeri A, Lechevallier E, Descotes JL, Lang H, Mejean A, Patard JJ. Multi-institutional validation of a new renal cancer-specific survival nomogram. J. Clin. Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z, Chu PG, Woda BA, Liu Q, Balaji KC, Rock KL, Wu CL. Combination of quantitative IMP3 and tumor stage: a new system to predict metastasis for patients with localized renal cell carcinomas. Clin Cancer Res. 2008;14:5579–5584. doi: 10.1158/1078-0432.CCR-08-0504. [DOI] [PubMed] [Google Scholar]
- 7.Patil S, Ishill N, Deluca J, Motzer RJ. Stage migration and increasing proportion of favorable-prognosis metastatic renal cell carcinoma patients: implications for clinical trial design and interpretation. Cancer. 2010;116:347–354. doi: 10.1002/cncr.24713. [DOI] [PubMed] [Google Scholar]
- 8.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arden KC. FoxOs in tumor suppression and stem cell maintenance. Cell. 2007;128:235–237. doi: 10.1016/j.cell.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–4333. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun HC, Li M, Lu JL, Yan DW, Zhou CZ, Fan JW, Qin XB, Tang HM, Peng ZH. Overexpression of Forkhead box M1 protein associates with aggressive tumor features and poor prognosis of hepatocellular carcinoma. Oncol Rep. 2011;25:1533–1539. doi: 10.3892/or.2011.1230. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 15.Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano-Maruyama M, Hase K, Fukamachi H, Kato Y, Koseki H, Ohno H. Foxl1-deficient mice exhibit aberrant epithelial cell positioning resulting from dysregulated EphB/EphrinB expression in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G163–170. doi: 10.1152/ajpgi.00019.2006. [DOI] [PubMed] [Google Scholar]
- 17.Perreault N, Katz JP, Sackett SD, Kaestner KH. Foxl1 controls the Wnt beta-catenin pathway by modulating the expression of proteoglycans in the gut. J Biol Chem. 2001;276:43328–43333. doi: 10.1074/jbc.M104366200. [DOI] [PubMed] [Google Scholar]
- 18.Perreault N, Sackett SD, Katz JP, Furth EE, Kaestner KH. Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev. 2005;19:311–315. doi: 10.1101/gad.1260605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Möller E. Vol. 2010. Lund University, Faculty of Medicine Doctoral Dissertation Series; 2010. EWSR1 and FUS fusion genes in tumorigenesis. [Google Scholar]
- 20.Lynch HT, Smyrk TC. Identifying hereditary non-polyposis colorectal cancer. N Engl J Med. 1998;338:1537–8. doi: 10.1056/NEJM199805213382109. [DOI] [PubMed] [Google Scholar]
- 21.Agnelli L, Mosca L, Fabris S, Lionetti M, Andronache A, Kwee I, Todoerti K, Verdelli D, Battaglia C, Bertoni F, Deliliers GL, Neri A. A SNP microarray and FISH-based procedure to detect allelic imbalances in multiple myeloma: an integrated genomics approach reveals a wide gene dosage effect. Genes Chromosomes Cancer. 2009;48:603–614. doi: 10.1002/gcc.20668. [DOI] [PubMed] [Google Scholar]
- 22.Zhan F, Barlogie B, Arzoumanian V, Huang Y, Williams DR, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Zangari M. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109:1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, Stewart JP, Zhan F, Khatry D, Protopopova M, Protopopov A, Sukhdeo K, Hanamura I, Stephens O, Barlogie B, Anderson KC, Chin L, Shaughnessy JD Jr, Brennan C, Depinho RA. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, He P, Gaedcke J, Ghadimi BM, Ried T, Yfantis HG, Lee DH, Hanna N, Alexander HR, Hussain SP. FOXL1, a novel candidate tumor suppressor, inhibits tumor aggressiveness and predicts outcome in human pancreatic cancer. Cancer Res. 2013;73:5416–5425. doi: 10.1158/0008-5472.CAN-13-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 27.Rink M, Chun FK, Robinson B, Sun M, Karakiewicz PI, Bensalah K, Fisch M, Scherr DS, Lee RK, Margulis V, Shariat SF. Tissue-based molecular markers for renal cell carcinoma. Minerva Urol Nefrol. 2011;63:293–308. [PubMed] [Google Scholar]
- 28.Liu Z, Li L, Yang Z, Luo W, Li X, Yang H, Yao K, Wu B, Fang W. Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer. 2010;10:270. doi: 10.1186/1471-2407-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]