Abstract
Adipose tissue-derived mesenchymal stem cells (ADSCs) are of great interest as a cellular therapeutic agent for regenerative and immunomodulatory purposes. The aim of this study was to investigate whether ADSCs transplantation could promote nerve repair in rats of cerebral ischemia-reperfusion (I/R) injury. We isolated and cultured human ADSCs, and then measured cell surface antigens by flow cytometry and immunofluorescence. Healthy SD rats were randomly divided into sham group, MCAO group, MCAO+vehicle group and MCAO+ADSCs group. Cerebral ischemia-reperfusion injury was induced by middle cerebral artery occlusion (MCAO). Then the human ADSCs were transplanted into the brain of rats 24 h after MCAO. The mRNA level of BDNF (brain derived neurotrophic factor, BDNF), NGF (nerve growth factor, NGF) and bFGF (basic fibroblasts growth factor, bFGF) were detected by real-time PCR at different time points (d7, d14, d21 and d28 after MCAO). Meanwhile, the neurological deficit scores were estimated. The neurological deficit of rats in MCAO+ADSCs group attenuated at d7 in contrast to the MCAO+vehicle group (P<0.05). Subsequently, they were dramatically ameliorated with the time especially at d28. At d7, d14, d21 and d28 after ADSCs transplantation, BDNF, NGF and bFGF mRNA in MCAO+ADSCs group were strikingly higher than those in MCAO+vehicle group, and these two groups both reached the peak at d14. The western blotting results showed that BDNF and Bcl-2 expressed higher in MCAO+ADSCs group than MCAO+vehicle group. Therefore, our current results suggest that ADSCs promote nerve repair after injury through elevating the expression of neurotrophic factors and inhibiting the apoptosis of neural cells.
Keywords: Adipose-derived stem cells, cerebral ischemia-reperfusion injury, BDNF, NGF, bFGF, nerve repair
Introduction
Stroke is a significant health problem and the third leading cause of death in the worldwide [1]. Ischemia-reperfusion (I/R) injury resulting from stroke leads to metabolic distress, oxidative stress and inflammation, making it likely that multiple therapeutic intervention strategies may be needed for successful treatment. However, limited advances have been made in developing therapies to confront the harmful effects of ischemic stroke, with the only available treatment being thrombolysis with tissue plasminogen activator (tPA) [2,3]. Unfortunately, due to the narrow therapeutic window (<4.5 h) and safety concerns, less than 5% of all patients are treated with tPA. Thus, the development of treatment strategies for cerebral ischemic injury is a high priority.
Mesenchymal stem cells (MSC) have emerged as cells with great clinical potential. It has demonstrated the immunosuppressive and regenerative capacities of MSC in vitro studies. Currently, MSC have been evaluated as a cell therapeutic agent in many medical fields including graft versus host disease, solid organ transplantation, and Crohn’s disease [4-6]. As one kind of adult mesenchymal stem cells (MSCs), human bone marrow-derived mesenchymal stem cells (BMSCs) have been widely studied, but harvesting these cells involves a highly invasive procedure [7]. Furthermore, the quantities, differentiation potential and frequency of MSCs derived from bone marrow decline with increasing age [7-9]. Therefore, an alternative cell source is in urgent demand [10,11]. Adipose-derived stem cells (ADSCs) have similar phenotypic and gene expression profiles to BMSCs [12,13], but there are unique advantages that they can be harvested easily by a safe and conventional liposuction procedure from subcutaneous fat tissue. The frequency of ADSCs in adipose tissue is much higher than that of MSCs in bone marrow [14], and ADSCs proliferate significantly faster than BMSCs [15,16].
Therefore, the present study was undertaken to investigate whether the implantation of ADSCs can alleviate the ischemia-reperfusion injury and promote the nerve repair in rats, and clarify the mechanism of this process.
Materials and methods
The isolation of human ADSCs
Human ADSCs were isolated from the upper eyelid orbital fat tissues of healthy adults during double eyelid operations from patients at age within 18-30 years. All donors gave informed consent for harvesting of their adipose tissue. Donors with infectious or systemic diseases or malignancies were excluded in the study. Adipose tissue was washed extensively with phosphate-buffered saline (PBS) three times. And then the tissue was minced and digested at 37°C for 1 h with equal volume of 0.5 mg/ml collagenase type IV (Sigma, USA) and 0.25% trypsin (BD, USA). Subsequently, the suspension was centrifuged to separate the floating adipocytes from the stromal vascular fraction. Then the cells in the stromal vascular fraction were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), and kept at 37°C, 5% CO2, 20% O2, and 95% humidity. After 24 h, the non-adherent cells were removed. The adherent cells were continuously cultured and used for the experiment between passages 3 and 5.
Flow cytometry
Human ADSCs (n=6) within 3-5 passages the initial plating of the primary culture were digested with 0.25% trypsin and washed with PBS. Then the cells were incubated with mouse anti-human CD90-FITC monoclonal antibody (Biolegend, USA), CD105-PE monoclonal antibody (R&D Systems, USA), CD45-PE monoclonal antibody (Biolegend, USA) or CD34-APC monoclonal antibody (Biolegend, USA). Meanwhile, the isotypic control was used. After incubation in darkness for 30 min at room temperature, the cells were analyzed immediately by a flow cytometer (FACS Calibur, BD). The experiments were repeated three times.
Immunofluorescence
Human ADSC cells of third passage (1×105 cells/well) were seeded in 24-well plate and cultured for 24 h. Then cells were washed and fixed with phenolformaldehyde (4%; 20 min, 4°C). Samples were blocked with 2% BSA (in PBS), and then they were incubated with rabbit anti-human CD90 mAb (1:100, Santa Cruz Biotechnology, USA), rabbit anti-human CD105 mAb (1:100, Santa Cruz Biotechnology), rabbit anti-human CD34 mAb (1:200, Cell Signaling Technology, USA) and rabbit anti-human CD45 mAb (1:100, Santa Cruz Technology) overnight at 4°C. Subsequently, the cells were washed in PBS, and then incubated with fluorescein-isothiocyanate (FITC)-conjugated rabbit secondary mAb. Florescence images were captured by Leitz DMRX microscope. The experiments were repeated three times.
Animals
Ninety-six adult male Sprague-Dawley rats (200-250 g) were purchased from Shandong University and housed in a temperature-controlled room with free access to food and water under 12 hr light-dark conditions. The animals were randomly separated into four groups: sham group, MCAO group, MCAO+vehicle group and MCAO+ADSCs group. All procedures were performed in accordance with protocols approved by the Institutional Committee for Animal Care and Use of Weifang Medical University.
MCAO surgical procedure
Sprague-Dawley rats were anesthetized before surgery by intraperitoneal injection of 10% chloral hydrate at a dose of 350 mg/kg. The right MCA (middle cerebral artery) was occluded as described by Longa et al [17] with minor modifications. Briefly, the right common carotid artery was exposed, and a 4-0 monofilament nylon thread coated with silicon was then inserted from the external into the internal carotid artery until the tip occluded the origin of the MCA. After closure of the operative sites, the animals were allowed to awake from the anesthesia. The occluder was left in place for 1 h and then removed. And then the reperfusion started. Rectal temperature was recorded and maintained at 37±0.5°C. An observer blinded to the identity of the groups assessed neurological deficits after reperfusion (before euthanization) by the forelimb akinesia test, whereas the spontaneous rotational test was used as a criterion for evaluating the ischemic insult [18]. Animals not showing behavioral deficits at these time points after reperfusion were excluded from the study.
Intracranial transplants of human ADSCs
Twenty-four hours after the MCAO, the ADSCs were thawed into PBS. The cells were washed and centrifuged three times (1000 rpm for 7 min). Viability was determined using the trypan blue dye exclusion method, and cell concentration was adjusted to 5×104 cells/μl. In the vehicle and ADSCs groups, the rats were re-anesthetized and held on a stereotactic frame. Then 25 μl Hamilton microinjector was fixed to the frame. The cell suspension (5×104/μl) was transplanted into the right corpus striatum (7 μl) and cerebral cortex (3 μl) respectively, and the injection time was no less than 10 min. The needle was retained for at least 5 min after the injection, and the hole was sealed off by max so that the fluid could not run out. The MCAO+vehicle group was injected PBS and the procedure was same to the MCAO+ADSCs group.
Neurobehavioral evaluation
An observer blinded to the identity of the groups assessed neurological deficits at 24 h, d7, d14, d21 and d28 after MCAO in all groups. According to Longa [17], the neurologic findings were scored on a five-point scale: a score of 0 indicated no neurologic deficit, a score of 1 (failure to extend left forepaw fully) a mild focal neurologic deficit, a score of 2 (circling to the left) a moderate focal neurologic deficit, and a score of 3 (falling to the left) a severe focal deficit; rats with a score of 4 did not walk spontaneously and had a depressed level of consciousness.
Quantitative real time polymerase chain reaction
After the last behavioral tests, the brains were removed and cryopreserved in liquid nitrogen. For detection of the growth factors BDNF, NGF and bFGF in the rat brain after transplantation of ADSC cells, real time PCR was carried out. RNA was extracted from brain tissue using RNase Miniprep Kit (Qiagen, USA) according to manufacturer’s protocol. RNA was used for reverse transcription using RevertAid™ first strand cDNA synthesis kit with oligo (dT) primers (Fermentas, Lithuania). Quantitative polymerase chain reaction (qPCR) was performed using primers specific for rat BDNF, NGF and bFGF mRNAs. Primers for real time PCR were as follows: BDNF (169 bp): sense: 5’-GTG TGATATTAGCGAGTGG-3’, antisense: 5’-GCAG CCTCCCTTGGTGTAAC-3’, NGF (122 bp): sense: 5’-GCACCACGAACACCTT-3’, antisense: 5’-GCCTCTTCTTGTAGCCTTCCT-3’, bFGF (161 bp): sense: 5’-CACTTACACCTCCAAGAAGCAT-3’, antisense: 5’-AGAACACTCAGAACAGACTCCT-3’, β-actin (153 bp): sense: 5’-TGTTGCCCTAGACTTCGAGCA-3’, antisense: 5’-GGACCCAGGAAGGAAGGCT-3’. The experiment was duplicated three times.
Western blotting
Brain tissues (n=3 for each group) were obtained from the entire MCA territory at d7, d14, d21 and d28 after MCAO. The tissues were homogenized using a polytron homogenizer, then sonicated for 10 s three times at 4°C. And then the lysates were centrifuged at 12000 rpm for 25 min at 4°C. The supernatant was estimated by the method of Bradford. The supernatant (30 μg protein) was subjected to SDS-PAGE and electrophoretically transferred to PVDF membranes (0.45 μm; Hybond-P, Amersham, Buckinghamshire, HP, UK). Blots were blocked in a blocking buffer, containing 5% bovine serum, 0.1% Tween 20 in 0.1 M PBS (pH 7.4), and incubated with rabbit anti-BDNF (1:200, Santa Cruz Biotechnology), rabbit anti-Bcl-2 (1:200, Santa Cruz Biotechnology) and rabbit anti-actin (1:500, Santa Cruz Biotechnology) at 4°C overnight. Blots were subsequently washed 4 times with TBST and incubated with secondary peroxidase conjugated goat anti-rabbit IgG (Amersham) for 2 hours. The band with peroxidase activity was detected using film exposure with enhanced chemiluminescence detection reagents (ECL system, Amersham).
Statistics analysis
All values are shown as mean±SD. Data were analyzed by using one-way analysis of variance and least significant difference (equal variances assumed), or Tamhane’s test (equal variances not assumed) was used post hoc for multiple comparisons with Statistical Package for the Social Sciences software version 11.5. Differences were considered as statistically significant at P<0.05.
Results
The morphology of ADSCs in culture
At the beginning, the ADSCs appeared as a spindle shape (Figure 1A). They were cultured in DMEM containing 10% FBS, which proliferated rapidly and reached 80% confluency 3-4 days. After the third passage, ADSCs adopted a fibroblast-like morphology with long cell processes (Figure 1B).
Figure 1.
The phenotype of human ADSCs in culture. Morphology of ADSCs at day 3 (A: original magnification ×50) and day 7 (B: original magnification ×50).
Cell surface antigens on human ADSCs
In order to confirm phenotype and purity of ADSCs, cell surface markers were determined by flow cytometry and immunofluorescence. It showed that ADSCs expressed high level of CD90 and CD105, characteristics of mesenchymal stem cells (Figure 2A). The ADSCs were negative for hematopoietic stem cell makers such as CD34 and CD45. Meanwhile, the flow cytometric analysis manifested that ADSCs expressed the MSC markers such as CD90 and CD105 (Figure 2B). Such an expression profile is consistent with previous reports for porcine ADSCs [19-21].
Figure 2.
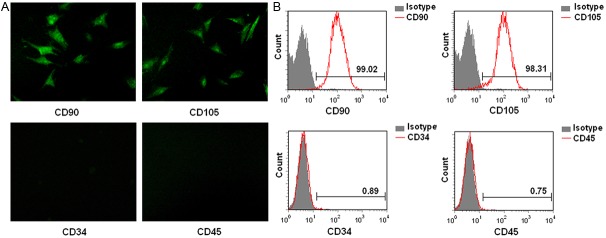
Cell surface antigens of human ADSCs are analyzed by immunofluorescence and flow cytometry. The ADSCs were positive for CD90 and CD105, whereas negative for CD34 and CD45 (A: original magnification ×200). The FACS illustrated that over 95% of ADSCs expressed CD90 and CD105, however, few ADSCs expressed CD34 and CD45 (B).
ADSCs transplantation ameliorates neurological deficits
As shown in Table 1, rats in MCAO group, MCAO+vehicle group and MCAO+ADSCs group represented obvious neurological deficit compared to sham group (P<0.01) before ADSCs transplantation, and there was no significant difference among these three groups. The neurological deficit of rats in MCAO+ADSCs group attenuated at d7 after ADSCs transplantation in contrast to the MCAO+vehicle group (P<0.05). Subsequently, they were dramatically alleviated with time especially at d28 (P<0.01). It demonstrated that ADSCs could alleviate neurological function in rats of ischemia-reperfusion injury.
Table 1.
Neurological deficit scores at different time points after MCAO in all groups
| Groups | Neurological deficit scores | ||||
|---|---|---|---|---|---|
|
| |||||
| 24 h | d7 | d14 | d21 | d28 | |
| Sham | 0 | 0 | 0 | 0 | 0 |
| MCAO | 3.24±0.65** | 2.79±0.54** | 3.11±0.47** | 2.51±0.52** | 2.86±0.51** |
| MCAO+vehicle | 3.41±0.58** | 2.95±0.65** | 2.94±0.53** | 2.71±0.51** | 2.58±0.62** |
| MCAO+ADSCs | 3.52±0.51** | 2.14±0.61**,# | 1.47±0.32**,## | 0.78±0.43*,## | 0.36±0.18## |
P<0.05;
P<0.01 compared to sham group;
P<0.05 compared to MCAO+vehicle group;
P<0.01 compared to MCAO+vehicle group.
Intracranial transplants of ADSCs improve the expression of BDNF, NGF and bFGF mRNA in rats
As shown, BDNF, NGF and bFGF mRNA of MCAO group were significantly higher than those in sham group (P<0.05, P<0.01) (Figure 3). At d7, d14, d21 and d28 after MCAO, BDNF, NGF and bFGF mRNA in MCAO+ADSCs group were strikingly higher than those in MCAO+vechile group (P<0.05, P<0.01). These two groups both reached the peak at d14 and then declined, but the expression in MCAO+ADSCs group was still higher than MCAO+vehicle group at d21 and d28 (P<0.05, P<0.01) (Figure 3). It illustrated that intracranial injection of ADSCs could promote the expression of BDNF, NGF and bFGF. After cerebral ischemia-reperfusion injury, the injured tissue repaired itself, and ADSCs enhanced the intensity and duration of repair, which ameliorated local microenvironment and facilitated nerve repair.
Figure 3.
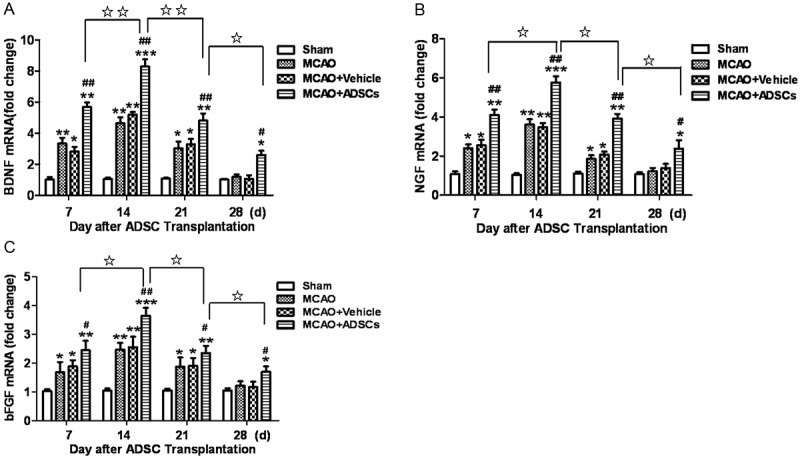
ADSCs transplantation promotes the expression of BDNF, NGF and bFGF mRNA in brain. The human ADSCs were intracranial transplanted after MCAO surgery, and we detected the expression of BDNF, NGF and bFGF mRNA in these groups. It demonstrated that BDNF, NGF and bFGF mRNA in MCAO group were significantly higher than those in sham group. Meanwhile, the expression of BDNF, NGF and bFGF in MCAO+ADSCs group was dramatically higher than that in MCAO+vehicle group. They reached the peak at day 14 after ADSCs transplantation and then decreased with time. Moreover, they were still higher than MCAO+vehicle group at d21 and d28. *P<0.05, **P<0.01 compared to sham group, #P<0.05, ##P<0.01 compared to MCAO+vehicle group, ☆P<0.05, ☆☆P<0.01 compared to MCAO+ADSCs group.
ADSCs transplantation promotes the expression of BDNF and Bcl-2 in rats after MCAO
To clarify the mechanism that human ADSCs alleviated nerve damage and promoted nerve repair, we measured the expression of BDNF and Bcl-2 in groups. It showed that the expression of BDNF and Bcl-2 increased at d7 and d14 in MCAO+vehicle and MCAO+ADSCs group and then decreased from d21. At d7 and d14, the protein level of BDNF in MCAO+ADSCs group was dramatically higher than that in MCAO+vehicle group (P<0.05, P<0.01) (Figure 4A and 4C). In accordance with BDNF, the expression of Bcl-2 in the MCAO+ADSCs group was significantly higher than that in MCAO+vehicle group (P<0.05) (Figure 4B and 4D). Therefore, human ADSCs facilitates nerve repair through improving the expression of BDNF and Bcl-2 as well as its downstream signal pathways.
Figure 4.
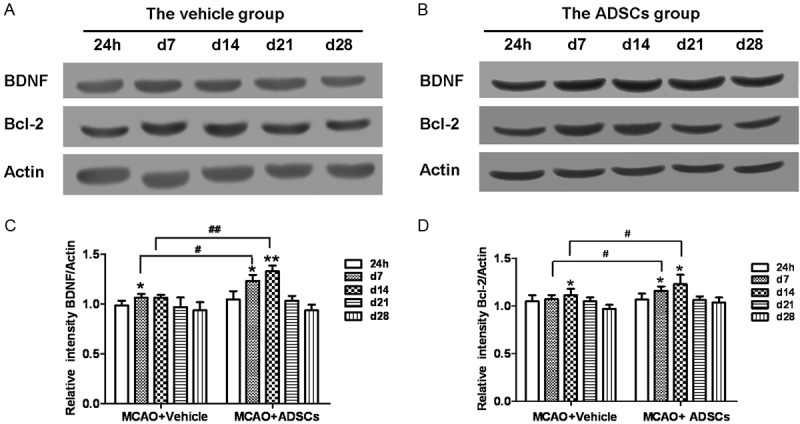
ADSCs transplantation facilitates the protein level of BDNF and Bcl-2 and further improves nerve repair. The expression of BDNF and Bcl-2 was analyzed by western blotting. β-Actin is used as loading control. The results are the means±SD (n=6). The protein levels of BDNF and Bcl-2 in MCAO+ADSCs group were higher than those in MCAO+vehicle group. In addition, BDNF and Bcl-2 in MCAO+ADSCs group increased at d7 and d14 and declines from d21. There was alike in MCAO+vehicle group. *P<0.05, **P<0.01 compared to the protein level at 24 h. #P<0.05, ##P<0.01 compared to MCAO+vehicle group.
Discussion
Cerebral ischemia and reperfusion is a pathological condition characterized by an initial restriction of blood supply to brain and followed by the subsequent restoration of blood flow and concomitant reoxygenation. Ischemia results in an almost immediate loss of oxygen and glucose to the cerebral tissue. However, perhaps surprisingly, restoration of blood flow and reoxygenation is frequently associated with an exacerbation of brain injury [22]. Ischemia and reperfusion injury contributes to pathology in a wide range of conditions [23]. For example, limited oxygen availability (hypoxia) occurs during the ischemic period is related to impaired endothelial cell barrier function [24] due to decreases in adenylate cyclase activity and intracellular cAMP levels and a concomitant increase in vascular permeability and leakage [23]. In addition, ischemia and reperfusion leads to the activation of cell death programs, including apoptosis, autophagy-associated cell death and necrosis [25].
Various MSCs derived from bone marrow, fat and umbilical cord blood have received attention in the field of tissue engineering because of their distinct biologic capability such as transdifferentiation and self-renewal [26]. Adipose tissue-derived mesenchymal stem cells (ADSCs) are capable of differentiating into one or more phenotypes and functional reproduction of damaged tissue [27]. ADSCs share the same properties of stem cells, such as the ability to divide and renew themselves for long periods, and differentiate into specialized cells. Compared with other MSCs, ADSCs can easily be harvested from patients and can be cultured and expanded rapidly. In addition, long-term cultured ADSCs maintain their mesenchymal pluripotency [28]. In this study, we confirmed that human ADSCs expressed characteristic surface markers (CD90 and CD105 positive, and CD34 and CD45 negative), which was consistent with the literature [19-21,29-31]. These results indicate that ADSCs possess the low immunogenicity and can escape from immune response [32,33]. Therefore, the cells may be expanded for a variety of therapeutic applications and offer a rich source for stem cell-based therapy [34].
Previous studies have shown ADSCs can differentiate into Schwann cell [35] and promote peripheral nerve repair [36,37]. On the other hand, it has shown that neurotrophic factors are essential molecules which can promote early peripheral nerve regeneration by literature [38]. Neurotrophins are central to many facets of CNS function, from differentiation and neuronal survival to synaptogenesis and activity-dependent forms of synaptic plasticity [39]. In the mammalian brain, four neurotrophins have been identified: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) and neurotrophin 4 (NT4). BDNF is the most abundant and widely distributed neurotrophin in the mammalian CNS [40]. In this study, we investigated the effect of ADSCs transplantation on nerve repair. In comparison with MCAO+vehicle group, the transplanted ADSCs elevated the expression of BDNF, NGF and bFGF in MCAO+ADSCs group, and they reached the peak at d14 after MCAO (Figure 3).
Ischemia and reperfusion activates various programs of cell death, which can be categorized as necrosis, apoptosis or autophagy-associated cell death [25]. The process of apoptosis includes various changes, such as nuclear and DNA fragmentation, caspase activation and increased expression of apoptosis-associated proteins. Apoptosis occurs mainly through two pathways: extrinsic and intrinsic apoptosis signaling [41]. The anti-apoptotic protein Bcl-2 regulated the intrinsic or mitochondrial pathway [42]. The neurotrophic factor BDNF is involved in growth, differentiation, maturation, and survival of neurons [43]. The animal studies indicate that the BDNF in adult rats supports the survival of the basal forebrain cholinergic neurons, promotes neuronal differentiation and survival, and inhibits brain injuries induced by cerebral ischemia and hypoxia [44]. Our results revealed that ADSCs transplantation promoted the protein level of BDNF and Bcl-2 (Figure 4), which may further regulated the neurons apoptosis and enhanced its repair. Moreover, we confirmed that ADSCs indeed participated in the repair of injured nerve through neurobehavioral evaluation (Table 1). However, we were unable to investigate the differentiation of transplanted cells in vivo, so it is unclear that these high expressed neurotrophic factors were secreted by the transplanted ADSCs directly or by Schwann cells. Nevertheless, we demonstrated that the high level of neurotrophic factors expression was followed by ADSCs transplantation in the brain of rats.
In summary, our study demonstrated that ADSCs promote nerve repair in rats of cerebral ischemia-reperfusion injury through elevating the expression of neurotrophic factors such as BDNF and NGF. Moreover, our results suggest that ADSCs transplantation represents a powerful therapeutic approach for nerve injury. However, the detailed mechanism by which ADSCs promote nerve repair is not explicit yet, it needs further investigation.
Acknowledgements
This work was partly supported by National Natural Science Foundation of China No. 30772268 (to S-J Tang); No. 81272122 (to S-J Tang); Teaching Reform Project of Weifang Medical University No. C2010011 (to S-J Tang).
Disclosure of conflict of interest
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kufner A, Nolte CH, Galinovic I, Brunecker P, Kufner GM, Endres M, Fiebach JB, Ebinger M. Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke. 2013;44:407–413. doi: 10.1161/STROKEAHA.112.662148. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 5.Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, Rabelink TJ. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107–111. doi: 10.5966/sctm.2012-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 7.Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 8.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Cui XD, Gao DY, Fink BF, Vasconez HC, Pu LL. Cryopreservation of human adipose tissues. Cryobiology. 2007;55:269–278. doi: 10.1016/j.cryobiol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Lee TH, Yoon JG. Intracerebral transplantation of human adipose tissue stromal cells after middle cerebral artery occlusion in rats. J Clin Neurosci. 2008;15:907–912. doi: 10.1016/j.jocn.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Gong P, Liao D. In vitro neural/glial differentiation potential of periodontal ligament stem cells. Arch Med Sci. 2010;6:678–685. doi: 10.5114/aoms.2010.17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 13.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–1341. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 14.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 16.Liao D, Gong P, Li X, Tan Z, Yuan Q. Co-culture with Schwann cells is an effective way for adipose-derived stem cells neural transdifferentiation. Arch Med Sci. 2010;6:145–151. doi: 10.5114/aoms.2010.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 19.Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16:963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adiposederived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 21.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 22.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Eckle T. Ischemia and reperfusion-from mechanism to translation. Nat Med. 2011;17:1391–1340. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seong JM, Kim BC, Park JH, Kwon IK, Mantalaris A, Hwang YS. Stem cells in bone tissue engineering. Biomed Mater. 2010;5:062001. doi: 10.1088/1748-6041/5/6/062001. [DOI] [PubMed] [Google Scholar]
- 27.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 28.Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 29.Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GL, van Ham SM, van Milligen FJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 30.Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F, Bani D, Liotta F, Francini F, Perigli G, Serio M, Luconi M. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009;23:3494–3505. doi: 10.1096/fj.08-126946. [DOI] [PubMed] [Google Scholar]
- 31.Fan W, Sun D, Liu J, Liang D, Wang Y, Narsinh KH, Li Y, Qin X, Liang J, Tian J, Cao F. Adipose Stromal Cells Amplify Angiogenic Signaling via the VEGF/mTOR/Akt Pathway in a Murine Hindlimb Ischemia Model: A 3D Multimodality Imaging Study. PLoS One. 2012;7:e45621. doi: 10.1371/journal.pone.0045621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 33.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 34.Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 35.Faroni A, Rothwell SW, Grolla AA, Terenghi G, Magnaghi V, Verkhratsky A. Differentiation of adipose-stem cells into Schwann cell phenotype induces expression of P2X receptors that controlcell death. Cell Death Dis. 2013;4:e743. doi: 10.1038/cddis.2013.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011;28:565–572. doi: 10.3892/ijmm.2011.725. [DOI] [PubMed] [Google Scholar]
- 37.Liu GB, Cheng YX, Feng YK, Pang CJ, Li Q, Wang Y, Jia H, Tong XJ. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci. 2011;7:592–596. doi: 10.5114/aoms.2011.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–53. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, Pang PT, Woo NH. The ying and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 41.Tang S, Hu J, Meng Q, Dong X, Wang K, Qi Y, Chu C, Zhang X, Hou L. Daidzein induced apoptosis via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway in BGC-823 cells. Cell Biochem Biophys. 2013;65:197–202. doi: 10.1007/s12013-012-9418-2. [DOI] [PubMed] [Google Scholar]
- 42.Arai M, Sasaki A, Saito N, Nakazato Y. Immunohistochemical analysis of cleaved caspase-3 detects high level of apoptosis frequently in diffuse large B-cell lymphomas of the central nervous system. Pathol Int. 2005;55:122–129. doi: 10.1111/j.1440-1827.2005.01808.x. [DOI] [PubMed] [Google Scholar]
- 43.Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 44.Moro K, Shiotani A, Watabe K, Takeda Y, Saito K, Mori Y, Ogawa K. Adenoviral gene transfer of BDNF and GDNF synergistically prevent motomeuron loss in the nucleus ambiguous. Brain Res. 2006;1076:1–8. doi: 10.1016/j.brainres.2005.12.119. [DOI] [PubMed] [Google Scholar]


