Abstract
Fractalkine (FKN) is involved in the immunopathogenesis of inflammatory diseases, including endometriosis. Our objective was to investigate the role of FKN in the cross-talking between endometrial stromal cells (ESCs) and U937 (macrophage line) in the endometriotic milieu. We have found that FKN levels in peritoneal fluid and ESCs positively correlate with the progress of endometriosis. The expression of CX3CR1 in the normal ESCs were significantly lower than that in eutopic and ectopic ESCs from women with endometriosis. CX3CR1 expression in U937 was higher than that in ectopic ESCs. FKN secreted by eutopic ESCs could change the balance between the release of IL10 and IL12 of macrophages with the upregulation of IL10 production and downregulation of IL12 production. Moreover, FKN could induce M2 polarization of macrophage with decreased expression of CD86. FKN could increase the expression of matrix metalloproteinase 9 and decrease the expression of tissue inhibitor of metalloproteinase1 and 2, and promote the invasiveness of ESCs by activating p38MAPK and integrinβ1 signal pathway. In conclusion, the higher levels of FKN secreted by eutopic ESCs facilitate the onset and progression of endometriosis by inducing M2 polarization of macrophage which in turn enhances invasiveness of ESCs.
Keywords: Fractalkine, ESCs, macrophage, invasiveness, endometriosis
Introduction
Endometriosis is a very frequent gynecological disorder in fertile women which has a complex, multifactorial etiology and causes severe pelvic pain and even infertility. The most widely accepted theory is that the exfoliated menstrual endometrial cells attach to the peritoneal serous membrane, and their subsequent invasion into the underlying tissue result in endometriotic lesions [1,2]. A growing body of evidence indicates that the primary defect in endometriosis may be located in the eutopic endometrium. Abnormalities inherent to the eutopic endometrium, which are not found in the endometrium of disease-free women, might therefore contribute to the ectopic growth outside the uterine cavity [3,4].
A series of research has shown that chemokines produced in the endometriotic milieu may contribute to a feed-forward cascade of events, which accentuates the recruitment of leukocytes into the peritoneal cavity of patients with endometriosis [5]. Chemokine FKN is the solitary member of a unique CX3C class of chemokines that consists of a transmembrane molecule with an extracellular region containing a conserved CX3C chemokine domain atop a mucin-like stalk [6]. The FKN (also known as CX3CL1) is a specific factor that chemoattracts and activates monocytes and macrophages, which is a major ligand of receptor CX3CR1. FKN is also known to function as a cellular adhesion molecule and to be chemotactic for monocytes and lymphocytes [6-10]. Moreover, Patrick Bellelis et al [11] have found that FKN expression is statistically significantly greater in the foci of endometriosis compared with the eutopic endometrium in patients and controls, and our previous data agreed with these results.
An increased number of active macrophages have been found in peritoneal fluid of patients with endometriosis. These macrophages seem to have phenotypic and functional alterations leading to poor phagocytotic capacity, which is closely related to the severity of endometriosis [12]. Our previous work has indicated that macrophages are involved in ectopic adhesion, implantation, and growth of the endometriotic tissue instead of clearing [13,14]. Therefore, the peritoneal macrophages may contribute to the development of endometriosis. Macrophages are broadly classified into classically activated macrophages (M1 macrophages) and alternatively activated macrophages (M2 macrophages), according to their roles. M1 macrophages are potent effecter cells that kill microorganisms and produce primarily proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, and IL-12. In contrast, M2 macrophages reduce these inflammatory and adaptive Th1 responses by producing anti-inflammatory factors (IL-10, TGF-β and IL-1 receptor antagonist), and promote angiogenesis, tissue remodeling, and repair. Macrophages are plastic cells, because they can switch from an activated M1 state back to M2, and vice versa, upon the induction of specific signals [15].
The initial phase of endometriosis is an invasion event that requires ECM breakdown and repair of tissues, such as an increased activity of these enzymes (MMP1, MMP2, and MMP9) [16,17]. Indeed, MMPs and TIMPs levels have been correlated to the development and progression of endometriosis [17,18]. In addition, integrins mediate the cell-cell and cell-matrix interaction, and regulate various cellular functions including motility, migration, death, metastasis, and proliferation [19], and are also related to the progression of uterine adenomyosis [20].
Thus, the present study was undertaken to investigate whether FKN in the endometriotic milieu can induce M2 polarization of macrophage, and the effect of educated macrophage on the invasiveness of ESCs in endometriosis so as to elucidate mechanisms by which the FKN is involved in the onset and progression of endometriosis.
Material and methods
Tissue collection, cell isolation, and culture
All tissue samples were obtained with informed consent in accordance with the requirements of the research ethics committee in Shanghai ninth Hospital affiliated to Jiaotong University School of Medicine. Samples of endometriotic peritoneal surface lesion (n=6) and ovarian lesion (n=6) were obtained from women age 31-43 years undergoing laparoscopy for pain or other benign indications. The patients with endometriosis were classified according to the revised American Fertility Society (AFS) classification: five in Stage 1 and seven in Stage 2. Endometrial tissues were obtained from fertile women with (n=12) or without (n=6) endometriosis as control. The samples were obtained by Pipelle biopsy during diagnostic laparoscopy or by uterine curettage for benign indications. The absence of visible endometriosis as the control was confirmed by the surgeon performing the operation. None of the women had received hormonal medication in the 3 months before the surgical procedure. All the samples were obtained in the proliferative phase of the cycle, which was confirmed histologically according to established criteria.
The endometrial tissues were collected under sterile conditions and transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). The ESCs were isolated according to methods described previously [14]. The endometrial tissues were digested with collagenase type IV (0.1%; Sigma, USA) for 30 min at 37°C with constant agitation for recovering ESCs. The tissue pieces were filtered through sterile gauzes pads (pore diameter sizes: 200 mesh) to remove cellular debris. Following gentle centrifugation, the supernatant was discarded and the cells were resuspended in DMEM/F-12. The ESCs were separated from epithelial cells by passing them over sterile gauzes pads (pore diameter sizes: 400 mesh). The filtrated suspension was layered over Ficoll and centrifuged at 800 g for 20 min to further remove leukocytes and erythrocytes, and the middle layer was collected and then washed with D-Hanks solution. The ESCs were placed in a culture flask and allowed to adhere for 20 min. The adherent stromal cells were cultured as a monolayer in flasks with DMEM/F-12 supplemented with 10% FCS and 20 mmol/l HEPES and incubated in a humidified incubator with 5% CO2 at 37°C. This method supplied 95% vimentin positive and cytokeratin-negative ESCs.
Collection and preparation of peritoneal fluid
Peritoneal fluid was aspirated from the pelvic cavity at the beginning of the standard laparoscopic procedure under general anesthesia. Samples of peritoneal fluid contaminated with blood were excluded from the study. The samples were centrifuged at 1500 rpm for 10 min. The supernatant was frozen at -80°C for subsequent determination of FKN concentrations.
ELISA
The peritoneal fluid and cell culture supernatants were harvested, centrifuged to remove cellular debris, and stored at -80 until assay by ELISA. Each experiment was carried out in triplicate and repeated three times. The FKN, IL-10 and IL-12 concentration in the peritoneal fluid or cell culture supernatant was qualified by ELISA (R&D Systems) according to the manufacturer’s instructions.
Western blots
Western blot analysis was performed by antihuman CX3CR1 polyclonal antibody (Abcam, Cambridge, UK), and was resolved on SDS-PAGE and transferred to immobilon PVDF membranes. After being soaked in blocking buffer, the membrane was incubated with primary antibody overnight at 4°C. The blots were developed using the HRP-linked secondary antibody and a chemiluminescent detection system. The experiments were repeated three times.
Indirect coculture of ESCs and U937
ESCs at 1×105 cells per well were plated in the upper compartment of Costar transwell cell culture chamber inserts (0.4 um, 12 mm diameter), and U937 cells were in the lower compartment. Each experiment was carried out in triplicate wells per time and repeated three times.
Flow cytometry analysis
U937 cells were indirectly cocultured with ESCs incubated with or without 10 μg/ml anti-FKN neutralizing antibody (R&D systems) for 72 h, and were then stimulated with LPS (10 ng/ml) for 24 h. Then the cells were incubated for 30 minutes at room temperature with 80 ul PBS containing 0.2% BSA (PBS-BSA) supplemented with 20 ul antihuman CD86, and HLA-DR antibody (eBioscience, San Diego, CA, USA). After that the cells were washed with PBS-BSA, and were analyzed by a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA).
Matrigel invasion assay
The invasion of the ESCs (n=6) from the eutopic endometrium with or without endometriosis across matrigel was evaluated objectively in an invasion chamber, based on our previous procedure [21]. Briefly, the cell inserts (8 um pore size, 6.5mm diameter, Corning) coated with 15-25 ul of matrigel were placed in a 24-well plate. The primary ESCs of 1×105 were plated in the upper chamber (the media contained 1% charcoal stripped FCS). Recombinant human FKN, P38MAPK inhibitor SB203580 (30 uM), or anti-FKN neutralizing antibody were added. The lower chamber (the media was contained 5% charcoal stripped FCS) was filled with 1×105 of U937. The cells were then incubated at 37°C for 48 hours. The inserts were removed, washed in PBS, and the non-invading cells together with the matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10 minutes at room temperature and stained with hematoxylin. The result was observed under Olympus BX51 + DP70 microscope (Olympus). The cells migrating to the lower surfaces were counted at a magnification of ×200. The cells migrating to the lower surfaces were counted in five predetermined fields. Each experiment was carried out in triplicate, and repeated three times.
In-cell Western
According to the description by Egorina [22] and our previous procedure [21], we used a newly set up assay called in-cell Western to determine the in-cell protein level of interest. The procedure was as follows: 2×104 cells/well eutopic ESCs (n=6) co-cultured with supernatant of U937 were incubated with or without vehicle or recombinant human FKN (R&D systems), or anti-human FKN with vehicle as control , and then cells were immediately fixed with 4% formaldehyde in PBS for 20 min at room temperature. After washing with 0.1% Triton, the cells were blocked by adding 150 ml of LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE, USA) for 90 min at room temperature. The cells were incubated with mouse antihuman phospho-p38 (1:50, Santa Cruz Biotechnology), rabbit anti-human p38 (1:80, Santa Cruz Biotechnology), integrinβ1 (10 ug/ml, R&D Systems), mouse anti-human phospho-Erk1/2 (1:50, Santa Cruz Biotechnology), rabbit anti-human Erk1/2 (1:80, Santa Cruz Biotechnology), rabbit anti-human phospho-Akt (1:50, Santa Cruz Biotechnology), goat anti-human Akt (1:80, Santa Cruz Biotechnology), mouse anti-human MMP2 (20 ug/ml, R&D Systems), MMP9 (20 ug/ml, R&D Systems), tissue inhibitor of metalloproteinase (TIMP1) (15 ug/ml, R&D Systems) or TIMP2 (15 ug/ml, R&D Systems) antibody. To assess the housekeeping protein actin, rabbit or goat anti-human actin (Abcam) was added to each well at the same time as an internal control. After overnight treatment at 4°C, the wells were incubated with corresponding second IRDye 700DX-conjugated affinity purified (red fluorescence) anti-mouse and IRDye 800DX-conjugated affinity purified (green fluorescence) anti-goat. However, for the survivin detection group, the wells were incubated with corresponding second IRDye 700DX-conjugated affinity purified (red fluorescence) anti-rabbit and IRDye 800DX-conjugated affinity purified (green fluorescence) anti-goat, fluorescence antibody recommended by the manufacturer (Rockland, Inc., Gilbertsville, PA, USA). This procedure must be carried out in the dark. Images of target gene were obtained using the Odyssey Infrared Imaging System (LI-COR Biosciences). The expression level of the correspondent molecules was calculated as the ratio of the intensity of target gene to actin. The experiments were carried out in triplicate, and repeated three times.
Statistical analysis
All values are shown as the mean + SD. Data were analyzed by using one-way analysis of variance and least significant difference (equal variances assumed), or Tamhane’s test (equal variances not assumed) was used post hoc for multiple comparisons by Statistical Package for the Social Sciences software version 11.5. Differences were considered as statistically significant at P<0.05.
Results
FKN levels in peritoneal fluid and ESCs positively correlate with the progress of endometriosis
FKN concentration in peritoneal fluid and supernatant of ESCs were detected by ELISA. Compared with peritoneal fluid from health control, FKN concentration was significantly increased in peritoneal fluid from women with endometriosis (stage I-II, P<0.05 and stage III-IV, P<0.01) (Figure 1A). Moreover, FKN concentration in the supernatant of ectopic ESCs was significantly higher than that of the eutopic ESCs, and the latter was further significantly higher than that of the ESCs from the normal fertile phase (P<0.05 or P<0.01; Figure 1B).
Figure 1.
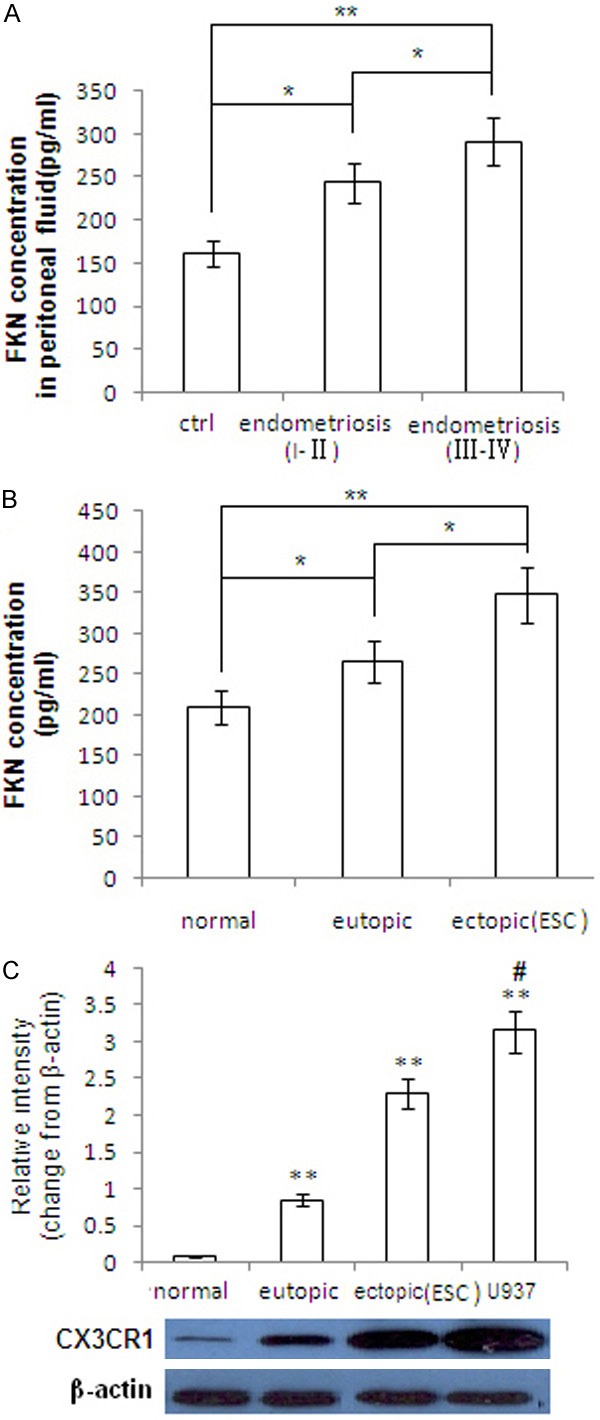
The concentration of FKN in peritoneal fluid and ESCs and CX3CR1 expression on ESCs and macrophage. A. ELISA assay was performed to analyze the level of FKN in peritoneal fluid from the healthy control and endometriosis (r-AFS stage I-II, and stage III-IV). B. Supernatant of primary ESCs from normal endometrium, eutopic endometrium and ectopic lesions in endometriosis were analyzed by ELISA. C. Expression of CX3CR1 was analyzed by western blot. Error bars depict the standard error of the mean. *P<0.05 compared with the normal control, **P<0.01 compared with the normal control. #P<0.05 compared with the ectopic ESCs.
We analyzed CX3CR1 protein expression in the ESCs and macrophage by western blot. As shown in Figure 1C, CX3CR1 expression in the ectopic ESCs was significantly higher than that of the eutopic ESCs, while the latter was significantly higher than that of normal ESCs of the fertile phase (P<0.05 or P<0.01). CX3CR1 expression in U937 cells was higher than that in ectopic ESCs (P<0.05).
These data suggest that there are positive correlation between the FKN concentration and the progress of endometriosis. In addition, similar correlation was seen between the expression of CX3CR1 and the degree of disease progress.
FKN derived from eutopic ESCs with endometriosis induces M2 polarization of macrophage
To investigate the effect of FKN secreted by eutopic ESCs on polarization of macrophages, U937 cells were indirectly co-cultured with ESCs incubated with or without 10 ug/ml FKN neutralizing antibody for 72 h, and were then stimulated with LPS (10 ng/ml) for 24 h. The results revealed that FKN derived from eutopic ESCs could change the balance between the release of IL10 and IL12 of macrophages with the upregulation of IL10 production and downregulation of IL12 production (P<0.05 or P<0.01; Figure 2A and 2B). FKN could induce M2 macrophage polarization with decreased expression of CD86 (P<0.05; Figure 2C). These results above indicate that FKN secreted by ESCs can induce the M2 polarization of macrophages.
Figure 2.
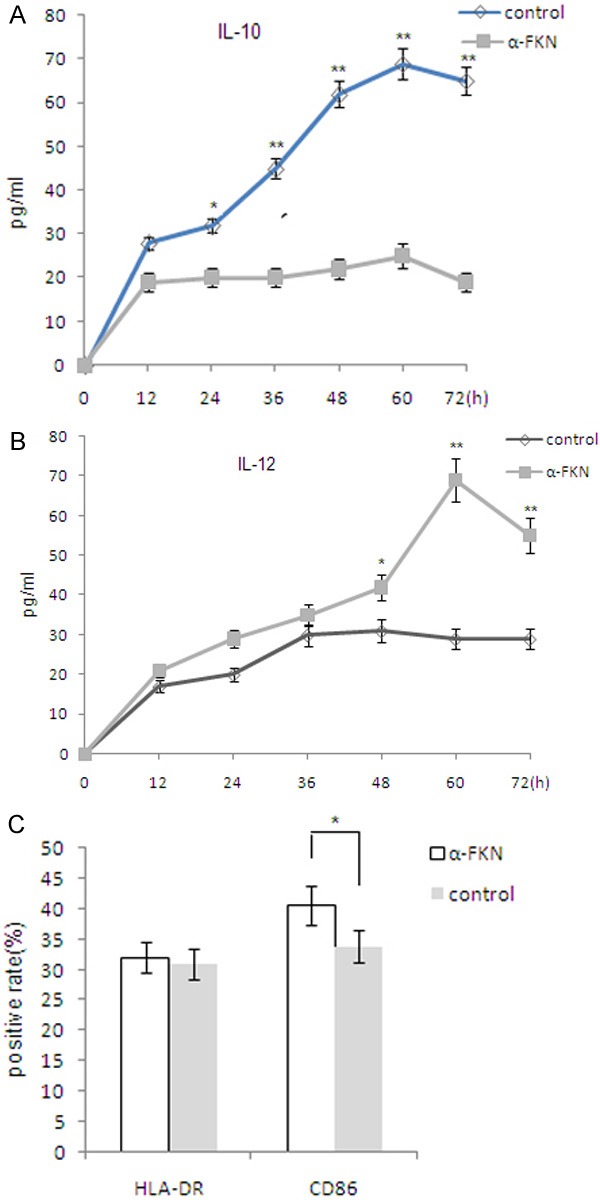
FKN derived from eutopic ESCs with endometriosis induces M2 polarization of macrophage. A. U937 (1×105 cells per well of 96 well plates) was stimulated with LPS (10 ng/ml) for 24 h after indirectly co-cultured with eutopic ESCs incubated with or without 10 ug/ml FKN neutralizing antibody for 0-72 h, followed by an analysis of the secretion of IL-10 and IL-12 by ELISA. B. U937 cells were indirectly co-cultured with ESCs incubated with or without 10 ug/ml FKN neutralizing antibody for 72 h, and were then stimulated with LPS (10 ng/ml) for 24 h. C. Expressions of HLA-DR and CD86 in U937 cells were determined by flow cytometry for correspondent positive rate of the surface molecules. Eutopic ESCs=ESCs from eutopic endometrium with endometriosis. Data are expressed as mean ± SD. *P<0.05 compared with the normal control, **P<0.01 compared with the normal control.
The FKN-induced M2 macrophages enhances the invasiveness of ESCs by activating P38MAPK and integrinβ1 signal pathway
To further probe into modulation of FKN-induced M2 macrophages on biological behavior of ESCs, we evaluated its effect on invasiveness of ESCs. After the eutopic ESCs were cultured alone or co-cultured with or without U937 cells which were pretreated with or without rhFKN for 24 hours, these cells were treated with or without FKN neutralizing antibody or SB203580 or integrinβ1 neutralizing antibody for another 24 hours, then Matrigel invasion assay was used to analyze the invasiveness of ESCs. Recombinant human FKN (rhFKN) significantly increased the invasiveness of the eutopic ESCs after treatment for 48 hours (P<0.01). Either FKN neutralizing antibody or P38MAPK inhibitor SB203580 or integrinβ1 neutralizing antibody decreased the invasiveness of ESCs (P<0.01). Our results have demonstrated that rhFKN can enhance the invasiveness of ESCs by activating P38MAPK and integrinβ1 signal pathway, and the abnormal high FKN in eutopic ESCs may be the characteristics of endometrium with great potential for the formation of endometriotic foci (Figure 3).
Figure 3.
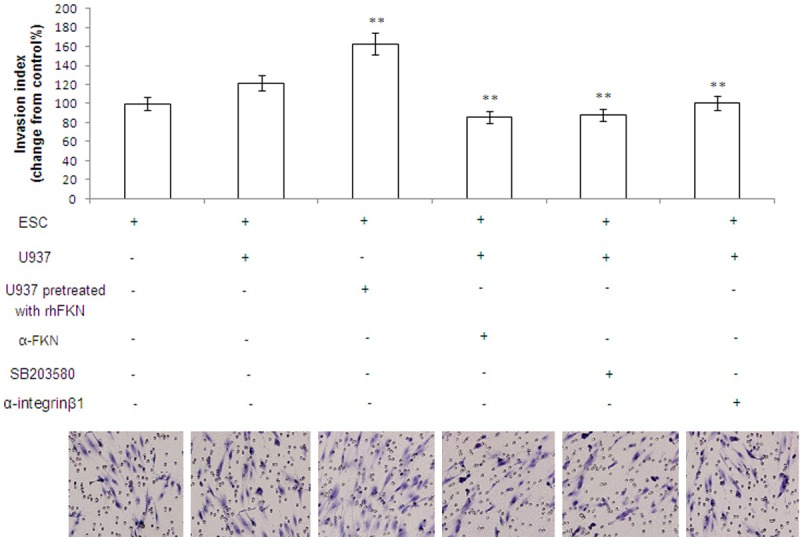
The FKN-induced M2 macrophages enhances the invasiveness of ESCs by activating P38MAPK and integrinβ1 signal pathway. After the eutopic ESCs were cultured alone or co-cultured with or without U937 cells which were pretreated with or without rhFKN for 24 hours, these cells were treated with or without FKN neutralizing antibody or SB203580 or integrinβ1 neutralizing antibody for another 24 h, then Matrigel invasion assay was used to analyze the invasiveness of ESCs. Original magnification: ×200.
Fractalkine up-regulates the expression of MMP9 and down-regulates the expression of TIMP1 and TIMP2 and activates the P38MAPK and integrinβ1 signal pathway
Data (Figure 4A) show that FKN raised the proportion of phospho-p38 to total p38 (p<0.01) and expression of integrinβ1 (p<0.01). In contrast, neutralizing antibody to FKN could reverse the activation of integrinβ1 and p38MAPK signals induced by FKN (P<0.01). However, FKN did not influence the phosphorylation level of Erk1/2 and Akt when compared with the vehicle control (P>0.05). It has been clearly demonstrated in Figure 4B that FKN can obviously enhance the expression of MMP9 (P<0.01) and inhibit the expression of TIMP1 (P<0.01) and TIMP2 (P<0.01), but has no effect on the expression of MMP2 in the ESCs. These results indicate that the increased FKN secretion in ESCs may lead not only the abnormal increase in invasion through enhancing MMP9 expression and suppressing the TIMP1 and TIMP2 expression but also the abnormal enhanced adhesion of integrinβ1, which is attributed to the onset and development of endometriosis.
Figure 4.
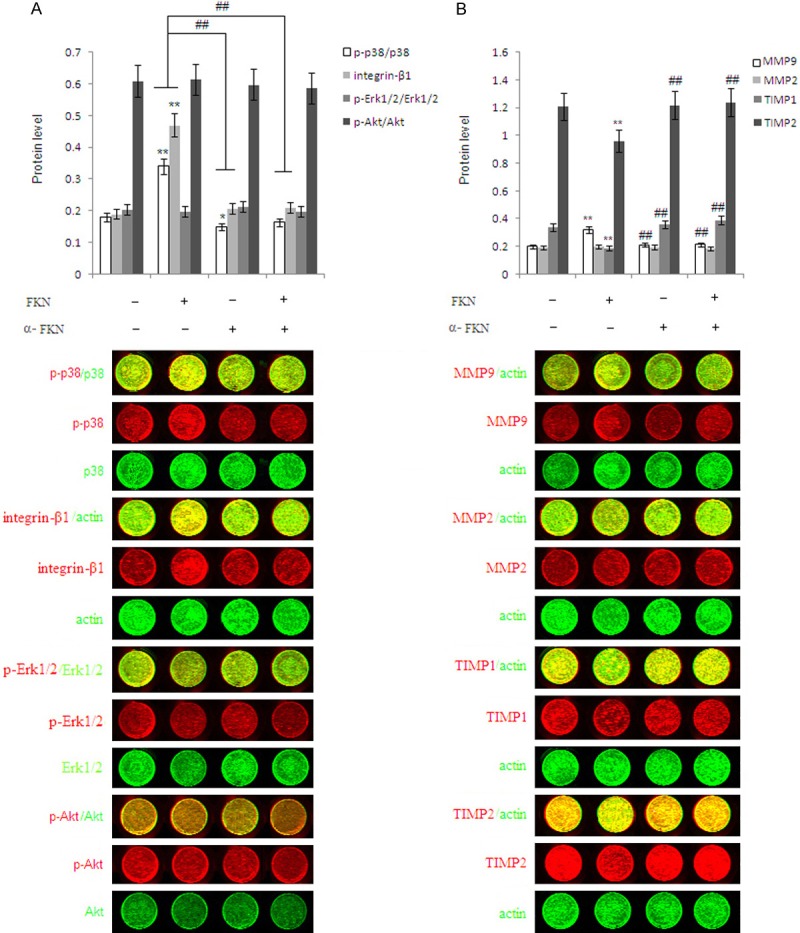
Fractalkine up-regulates MMP9 expression and up-regulates TIMP1 and TIMP2 expression and activates the P38MAPK and integrinβ1 signal pathway. Eutopic ESCs was directly co-cultured with S-U937 with or without rhFKN and/or FKN neutralizing antibody for 48 h, with vehicle as controls. A. In-cell Western assays were performed to examine the phosphorylation level of p38, Erk1/2, Akt, integrinβ1 in ESCs. B. MMP2/9, and TIMP1/2 expression were analyzed in ESCs, respectively. All data represents mean ± SD. S-937: supernatant of U937 cells . *P<0.05 compared to the vehicle control. **P<0.01 compared to the vehicle control. #P<0.05 compared to the FKN treatment. ##P<0.01 compared to the FKN treatment.
Discussion
An abnormal peritoneal microenvironment is thought to be a particularly relevant ‘permissive’ condition for implantation and growth of refluxed endometrium [23]. Phenotypic and functional alterations in peritoneal macrophages are also found associated with endometriosis [24]. In agreement with our previous studies, the immuno-inflammatory microenvironments mediated by numerous cytokines and growth factors, especially for chemokine, are now believed to play an important role in the progression of endometriosis [14,21]. FKN is expressed in neurons, endothelial cells, hepatocytes, and vascular smooth muscle cells [25-29]. The extracellular domain of FKN can be cleaved through the action of the extracellular proteases Adam 10 and 17 to produce a soluble form of FKN [30,31]. Soluble FKN can exert paracrine effects in the extracellular space and can also enter the circulation to potentially cause endocrine effects on distant tissues [32]. In the present study we have further found that FKN concentration in peritoneal fluid is positive correlation with the progress of endometriosis. The secretion of FKN in ESCs from ectopic lesion was higher than that from eutopic endometrium with and without endometriosis. In addition, CX3CR1 expression in U937 cells was higher than that in ectopic ESCs. Therefore, we hypothesized FKN from ESCs may regulate the dialogue between ESCs and macrophage, which was involved in the development of endometriosis.
Alteration of the balance between the two subclasses of M1 and M2 macrophages might be involved in the pathogenesis of pelvic endometriosis, which might in turn cause an increase in the local production of factors promoting angiogenesis and implantation of endometrial cells. In this study, we have found that FKN secreted by ESCs can induce the formation of M2 polarization of macrophages with CD86low phenotype and increased IL10/IL12 ratio. During their exposure to the ectopic milieu, the newly recruited macrophages may be induced tolerant. Our results give a new insight into the formation of M2 polarization of macrophages in endometriosis. rhFKN can change the balance of M1 and M2 macrophage, creating the conditions that redound to endometriosis progression. Therefore, the regulation of the M2 polarization of macrophage phenotype may shed light on developing a new therapeutic regimen for endometriosis.
The adherence and invasion of the retrograded endometrial cells into the peritoneum is a key step for the early stage of endometriosis, and the retrograded ESCs are responsible for the adherence and implantation of endometrium to peritoneum in the early stage of endometriosis [33]. In our matrigel invasion assay, we found that indirect coculture of ESCs and macrophage promotes the invasiveness of ESCs through FKN secretion. Our results have demonstrated that FKN may act in paracrine and indirect mechanisms to support endometriosis progression. One mechanism by which FKN may affect endometriosis progression is its capacity to upregulate the expression of MMP9 and downregulate the expression of TIMP1 and TIMP2 in ESCs. Meanwhile, we have found that SB203580 and integrinβ1 antibody can reverse the enhancement of invasiveness of ESCs induced by FKN. These observations indicate that the ESCs-derived FKN stimulates invasion of ESCs partially through regulating p38MAPK and integrinβ1 signal pathway.
Several researchers have demonstrated that FKN regulate cell-survival and immune-modulatory responses through the activation of the MAPK/Erk1/2 pathway [34,35]. Other studies suggest that Inhibitors of p38MAPK, JAKSTAT, NF-κB, and AP-1 significantly reduced CX3CL1 and CX3CR1 expression. Knockdown of STAT1 and STAT3 with decoy oligodeoxynucleotide and the silencing of p65 with short interfering RNA decreased CX3CL1 and CX3CR1 expression. Anti-TLR4 antibody and pertussis toxin also reduced CX3CL1 and CX3CR1 protein expression [36]. We analyzed the phosphorylation level of p38, Erk1/2, Akt and integrinβ1, after treated with recombinant human FKN, and/or FKN neutralizing antibody. Based on our results, it can be concluded that ESCs-derived FKN can activate p38MAPK and integrinβ1 signals, but not influence Erk1/2 and Akt signals.
In conclusion, based on our results, a hypothetical model may be proposed to elucidate the onset and progression of endometriosis. The endometrium with the abnormal high level of FKN secretion regurgitates into the peritoneal cavity, recruits more macrophages into the ectopic milieu and induces M2 polarization of macrophage, which cannot effectively clear the ectopic endometrium. On the other hand, the educated M2 macrophage enhances the invasiveness of ESCs through p38MAPK and integrinβ1 signal pathway, and further give rise to the formation of endometriotic foci. In this progression, upregulation of MMP9 and downregulation of TIMP1 and TIMP2 as effective molecules are involved in the invasiveness increase of ESCs. Therefore, interference with chemokine regulatory loops such as FKN may represent a novel therapeutic strategy to reduce the growth of endometriosis.
Acknowledgements
This work was supported by Foundation for shanghai ninth hospital affiliated to JiaoTong University School of Medicine (No. JY2011A02 to Y.W.); National Natural Foundation of China (NSFC) (31071275 to QF Lyu, 81270749 to YP Kuang) and the Natural Science Foundation of Shanghai, China (11411950105 to YP Kuang).
Disclosure of conflict of interest
None.
References
- 1.Witz CA, Monotoya-Rodriguez IA, Schenken RS. Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil Steril. 1999;71:56–60. doi: 10.1016/s0015-0282(98)00400-2. [DOI] [PubMed] [Google Scholar]
- 2.Nisolle M, Casanas-Roux F, Donnez J. Early-stage endometriosis: adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril. 2000;74:306–312. doi: 10.1016/s0015-0282(00)00601-4. [DOI] [PubMed] [Google Scholar]
- 3.Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13:467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132:107–110. doi: 10.1016/j.ejogrb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Akoum A, Jolicoeur C, Boucher A. Estradiol amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis. J Clin Endocrinol Metab. 2000;85:896–904. doi: 10.1210/jcem.85.2.6348. [DOI] [PubMed] [Google Scholar]
- 6.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 7.Kanazawa N, Nakamura T, Tashiro K, Muramatsu M, Morita K, Yoneda K, Inaba K, Imamura S, Honjo T. Fractalkine and macrophage-derived chemokine: T cell-attracting chemo kines expressed in T cell area dendritic cells. Eur J Immunol. 1999;29:1925–1932. doi: 10.1002/(SICI)1521-4141(199906)29:06<1925::AID-IMMU1925>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 10.Chapman GA, Moores KE, Gohil J, Berkhout TA, Patel L, Green P, Macphee CH, Stewart BR. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur J Pharmacol. 2000;392:189–195. doi: 10.1016/s0014-2999(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 11.Bellelis P, Barbeiro DF, Rizzo LV, Baracat EC, Abrão MS, Podgaec S. Transcriptional changes in the expression of chemokines related to natural killer and T-regulatory cells in patients with deep infiltrative endometriosis. Fertil Steril. 2013;99:1987–1993. doi: 10.1016/j.fertnstert.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Raiter-Tenenbaum A, Baranao RI, Etchepareborda JJ, Meresman GF, Rumi LS. Functional and phenotypic alterations in peritoneal macrophages from patients with early and advanced endometriosis. Arch Gynecol Obstet. 1998;261:147–157. doi: 10.1007/s004040050214. [DOI] [PubMed] [Google Scholar]
- 13.Shi YL, Luo XZ, Zhu XY, Li DJ. Combination of 17 beta-estradiol with the environmental pollutant TCDD is involved in pathogenesis of endometriosis via up-regulating the chemokine I-309-CCR8. Fertil Steril. 2007;88:317–325. doi: 10.1016/j.fertnstert.2006.11.129. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Wang Y, Zhou WH, Wang L, He YY, Li DJ. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum Reprod. 2008;23:1614–1626. doi: 10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
- 15.Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 17.Wu MH, Shoji Y, Wu MC, Chuang PC, Lin CC, Huang MF, Tsai SJ. Suppression of matrix metalloproteinase-9 by prostaglandin E2 in peritoneal macrophage is associated with severity of endometriosis. Am J Pathol. 2005;167:1061–1069. doi: 10.1016/S0002-9440(10)61195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S, Zhao XW, Wang N, Chen SC, Zhou RM, Li Y. Association of polymorphisms of the MMP-2 and TIMP-2 genes with the risk of endometriosis in North Chinese women. Fertil Steril. 2008;90:2023–2029. doi: 10.1016/j.fertnstert.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 20.Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Hum Reprod. 2007;22:3139–3147. doi: 10.1093/humrep/dem262. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Yu J, Luo XZ, Wang XQ, Li MQ, Wang L, Li DJ. Abnormal regulation of chemokine TECK and its receptor CCR9 in the endometriotic milieu is involved in pathogenesis of endometriosis by way of enhancing invasiveness of endometrial stromal cells. Cell Mol Immunol. 2010;7:51–60. doi: 10.1038/cmi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egorina EM, Sovershaev MA, Osterud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–620. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 23.Koninckx PR, Kennedy SH, Barlow DH. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update. 1998;4:741–751. doi: 10.1093/humupd/4.5.741. [DOI] [PubMed] [Google Scholar]
- 24.Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–1110. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- 25.Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 2010;52:1390–1400. doi: 10.1002/hep.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 27.Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- 28.Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158:855–866. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis:an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 30.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 31.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 32.Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L, Lu Y, Putt ME, Ahima RS, Reilly MP. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60:1512–1518. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75:385–390. doi: 10.1016/s0015-0282(00)01699-x. [DOI] [PubMed] [Google Scholar]
- 34.Brand S, Sakaguchi T, Gu X, Colgan SP, Reinecker HC. Fractalkine-mediated signals regulate cell-survival and Immune-modulatory responses in Intestinal epithelial cells. Gastroenterology. 2002;122:166–177. doi: 10.1053/gast.2002.30329. [DOI] [PubMed] [Google Scholar]
- 35.Uchida M, Ito T, Nakamura T, Igarashi H, Oono T, Fujimori N, Kawabe K, Suzuki K, Jensen RT, Takayanagi R. ERK pathway and sheddases play an essential role in ethanol-induced CX3CL1 release in pancreatic stellate cells. Lab invest. 2013;93:41–53. doi: 10.1038/labinvest.2012.156. [DOI] [PubMed] [Google Scholar]
- 36.Gan AM, Butoi ED, Manea A, Simion V, Stan D, Parvulescu MM, Calin M, Manduteanu I, Simionescu M. Inflammatory effects of resistin on human smooth muscle cells: up-regulation of fractalkine and its receptor, CX3CR1 expression by TLR4 and Gi-protein pathways. Cell Tissue Res. 2013;351:161–174. doi: 10.1007/s00441-012-1510-9. [DOI] [PubMed] [Google Scholar]


