Abstract
Oxaliplatin is currently approved for patients with metastatic colorectal cancer (mCRC). Its uptake and consequent cytotoxicity is determined by the levels of organic cation transporter 2 (OCT2). In addition, tumor budding (TB) is associated with high malignant potential. However, the impact of the levels of OCT2 and TB on clinicopathological findings and the prognosis of mCRC patients treated with oxaliplatin-based chemotherapy remains unclear. Here, 80 mCRC patients were retrospectively assessed. Immunohistochemistry was performed to determine the levels of OCT2 and TB. High levels of OCT2 (47/80, 59%) were detected at the invasion front and were associated with depth of invasion (P=0.03), whereas high levels of TB (40/80, 50%) were associated with extensive lymphatic invasion (P=0.03). In univariate analysis, high OCT2 levels were significantly correlated with longer progression-free survival (PFS) (P=0.02) whereas high TB levels were associated with shorter PFS (P=0.01). In combined analysis, patients with 2 favorable factors (high OCT2/low TB) had longer PFS than those with 1 (P=0.03) or 0 (P<0.001) favorable factors. Multivariate analysis confirmed that the OCT2 level (P=0.007), TB level (P=0.004), and combined OCT2/TB status (P=0.001) were independent predictors for PFS. These results suggest that high levels of OCT2 indicate severe invasion, but also better prognosis in mCRC patients treated with oxaliplatin-based chemotherapy, possibly because of its role in oxaliplatin susceptibility. Combined analysis of OCT2 and TB status may guide the selection of patients for successful oxaliplatin-based chemotherapy.
Keywords: Organic cation transporter 2, tumor budding, oxaliplatin, progression-free survival, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer worldwide and accounts for 9% of all new cancer cases and cancer deaths [1]. Twenty percent of all CRC patients are diagnosed with metastatic CRC (mCRC). While the overall 5-year survival rate for CRC patients is 64%, this rate drops to 12% in mCRC patients, despite recent advances in combined systemic chemotherapies [1].
Platinum compounds such as cisplatin, carboplatin, and oxaliplatin are among the most active anticancer drugs and are widely used in combination with other anticancer drugs or radiation. Oxaliplatin, a diaminocyclohexane-containing platinum agent, differs in its spectrum of activity and resistance profile from those of cisplatin and carboplatin [2,3]. Combination therapy with infusional 5-fluorouracil (5-FU)/leucovorin (LV) plus oxaliplatin (FOLFOX) is currently one of the standard first-line regimens for mCRC [4], for which cisplatin and carboplatin treatments are essentially ineffective [2,3]. The identification of prognostic markers that can predict benefit from first-line oxaliplatin-based chemotherapy is one approach towards personalized therapy of mCRC.
Facilitating increases in intracellular drug accumulation through solute carrier (SLC) transporters is an important mechanism for enhancing the efficacy of anticancer drugs [5]. The organic cation transporters (OCTs) that belong to the SLC22A family mediate intracellular uptake of various structurally diverse organic cations including endogenous compounds, xenobiotics, and clinically used drugs [6,7]. In vitro studies have demonstrated that OCT2, also referred to as SLC22A2, is a critical determinant of the uptake and consequent cytotoxicity of platinum compounds, particularly oxaliplatin [8-10]. To our best of knowledge, however, the clinicopathological role of OCT2 in CRC remains to be elucidated.
The morphological features of tumor cells at the invasion front differ from those at the tumor center. The presence of isolated cells or small cell clusters in the stroma at the invasive front indicates a process that is closely related to the disintegration of cell adhesion molecules called, “tumor budding” (TB) [11]. A high level of TB has been associated with high tumor grade, vascular and lymphatic invasion, lymph node metastasis, and poor prognosis in CRC patients [11-17]. In a preliminary examination to determine the optimal immunohistochemical conditions to detect OCT2, we observed strong OCT2 positivity at the TB sites in CRC tissues. Therefore, this study aimed to address the impact of the OCT2 and TB levels on clinicopathological findings and the prognosis of mCRC patients treated with oxaliplatin-based chemotherapy.
Materials and methods
Patients and tissue samples
We evaluated 80 mCRC patients who were treated at the Fujita Health University Hospital from 2007 to 2011. Eligibility criteria for study enrollment were first-line chemotherapy with FOLFOX or FOLFOX plus bevacizumab (FOLFOX/BV), no prior anti-epidermal growth factor receptor treatment, no preoperative chemotherapy and/or radiotherapy, and availability of paraffin-embedded tissue specimens of the resected primary tumors.
The clinicopathological findings of the patients are summarized in Table 1. Patient ages ranged from 27 to 79 years (median age, 62 years). Depth of invasion (pT factor) and lymph node metastasis (pN factor) were classified according to the tumor-node-metastasis (TNM) classification of the International Union against Cancer [18]. Lymphatic and venous invasion were categorized according to the Japanese Classification of Colorectal Carcinoma: no invasion (ly0, v0), minimal invasion (ly1, v1), moderate invasion (ly2, v2), and severe invasion (ly3, v3) [19]. Histological classification (well-differentiated, moderately differentiated, poorly differentiated, and mucinous adenocarcinoma) was also defined according to the Japanese Classification of Colorectal Carcinoma.
Table 1.
Clinicopathological findings of mCRC patients and their association with OCT2 and TB levels
| Variables | n | OCT2 | TB | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Low | High | P value | Low | High | P value | ||
| Age (years) | |||||||
| <60 | 26 | 12 | 14 | 0.63 | 14 | 12 | 0.81 |
| ≥60 | 54 | 21 | 33 | 26 | 28 | ||
| Gender | |||||||
| Male | 53 | 23 | 30 | 0.64 | 28 | 25 | 0.64 |
| Female | 27 | 10 | 17 | 12 | 15 | ||
| Location of primary tumor | |||||||
| Colon | 56 | 25 | 31 | 0.46 | 28 | 28 | 1.00 |
| Rectum | 24 | 8 | 16 | 12 | 12 | ||
| Histological differentiation | |||||||
| Well/moderate | 75 | 31 | 44 | 1.00 | 38 | 37 | 1.00 |
| Poor/mucinous | 5 | 2 | 3 | 2 | 3 | ||
| Depth of invasion | |||||||
| pT2/pT3 | 41 | 22 | 19 | 0.03* | 24 | 17 | 0.18 |
| pT4 | 39 | 11 | 28 | 16 | 23 | ||
| Lymph node metastasis | |||||||
| pN0/pN1 | 45 | 18 | 27 | 0.82 | 24 | 21 | 0.65 |
| pN2 | 35 | 15 | 20 | 16 | 19 | ||
| Lymphatic invasion | |||||||
| ly0/ly1 | 19 | 6 | 13 | 0.43 | 14 | 5 | 0.03* |
| ly2/ly3 | 61 | 27 | 34 | 26 | 35 | ||
| Venous invasion | |||||||
| v0/v1 | 23 | 7 | 16 | 0.32 | 14 | 9 | 0.32 |
| v2/v3 | 57 | 26 | 31 | 26 | 31 | ||
Statistically significant;
mCRC, metastatic colorectal cancer; OCT2, organic cation transporter 2; TB, tumor budding.
First-line FOLFOX chemotherapy consisted of oxaliplatin (85 mg/m2) infused over 90 min, l-LV (200 mg/m2) infused during 2 h, followed by a 400 mg/m2 intravenous bolus of 5-FU on day 1 and a 2400 mg/m2 continuous infusion of 5-FU over 46 h every 2 weeks. In the FOLFOX/BV regimen, patients receiving bevacizumab were dosed at 5 mg/kg every 2 weeks on day 1 of FOLFOX. Written informed consent for the administration of chemotherapy as well as the use of tumor tissue for immunohistochemical analysis was obtained from all patients. The ethics committees of Kobe University Graduate School of Health Sciences and Fujita Health University School of Medicine approved this study.
The resected tumors were fixed in 10% formalin and embedded in paraffin wax. Three-micrometer-thick sections were cut and mounted onto aminopropyltriethoxysilane-coated slides. Routine hematoxylin and eosin staining was performed to assess histopathological features under a light microscope. Tissue sections from the deepest invasive sites were chosen.
Immunostaining for OCT2 and cytokeratin
Immunostaining was performed not only for OCT2 but also for pan-cytokeratin, an epithelial cell marker that facilitates the visualization of tumor buds [14,16,17]. Sections were deparaffinized in xylene, rehydrated in a graded series of alcohol, and rinsed with tap water.
The slides were subjected to heat-induced antigen retrieval in a pressure cooker for 10 min at 120°C with optimal soaking solutions: 0.001 mol/L ethylenediaminetetraacetic acid (pH 8.0) for OCT2 and 0.01 mol/L citrate buffer (pH 6.0) for pan-cytokeratin. Blocking of endogenous peroxidase was not performed, because the enzyme is inactivated during the heating treatment [20]. After heating treatment, the sections were cooled in the soaking solution for 30 min at room temperature (RT). The sections were then rinsed with running tap water and then with 0.01 mol/L phosphate-buffered saline (PBS, pH 7.2). After rinsing, the sections were incubated with primary antibodies overnight at RT. The primary antibodies used were a rabbit polyclonal anti-OCT2 (1:400, Sigma-Aldrich, St. Louis, MO, USA) and a mouse monoclonal anti-pan-cytokeratin (clone AE1/AE3, 1:400; Dako, Glostrup, Denmark). The sections were then rinsed in PBS, and incubated accordingly with either anti-rabbit or anti-mouse horseradish peroxidase polymer (Histofine Simple Stain MAX-PO; Nichirei Bioscience, Tokyo, Japan) for 1 h at RT. Subsequently, the reaction products were visualized using a diaminobenzidine solution (Dako). The sections were then rinsed in distilled water, counterstained with Mayer’s hematoxylin, dehydrated through a graded series of alcohol and xylene, and finally coverslipped.
A normal kidney tissue section was used as positive control and staining with PBS containing 1% bovine serum albumin instead of the primary antibody was used as negative control.
Assessment of OCT2 and TB levels
Three investigators (S.T., Y.H., and S.K.) who were blinded to both the clinicopathological findings and outcome data assessed each of the stained sections independently. Discordant results were then discussed until an agreement was reached. Expression levels of OCT2 were evaluated independently at the tumor center and the invasion front. The percentage of positively stained tumor cells was scored from 1 to 4 (1, <10%; 2, 10% to 50%; 3, 51% to 80%; 4, >80% of positive tumor cells), and the staining intensity was graded from 0 to 3 (0, negative; 1, weak; 2, moderate; 3, strong staining). A composite score (0 to 12) was obtained by multiplying the percentage of positively stained cells and intensity. According to the cut-off score determined by receiver operating characteristic (ROC) curve analysis, scores of 0-8 represented a low OCT2 level and scores of 9-12 represented a high OCT2 level.
TB was defined as the presence of tumor buds (dedifferentiated single cells or clusters composed of <5 cells at the invasion front) in the pan-cytokeratin-stained sections, according to the criteria proposed by Ueno et al. [11]. The invasion front was scanned using the low-power (4×) objective lens to identify the area with the highest density of TB. The number of tumor buds was then counted in 10 fields using the high-power (40×) objective lens. Tumors with an average of <10 tumor buds were classified as having a low TB level and those with an average of ≥10 tumor buds were considered as having a high TB level [17].
Assessment of patient prognosis
Because overall survival is generally influenced by many factors including molecular markers and subsequent lines of therapy, we analyzed progression-free survival (PFS) to assess the prognostic significance of OCT2 and TB. PFS was defined as the period from the start of first-line chemotherapy until the first evidence of radiological progression or death.
Statistical analysis
The Wilcoxon’s rank sum test was employed to determine whether the levels of OCT2 or TB were statistically different between the tumor center and the invasion front. The Spearman rank correlation test was used to measure the strength of association between OCT2 and TB levels. The Fisher’s exact test was applied to examine the association of OCT2 and TB levels with clinicopathological parameters.
The Kaplan-Meier method was used to estimate PFS, and the differences between the survival curves were assessed using the log-rank test. A Cox proportional hazard regression model for PFS was used to assess the independence of variables. Results were considered significant at a P value of less than 0.05. Statistical analyses were performed using the SPSS 20.0 software program (SPSS Inc., IL, USA).
Results
OCT2 and TB levels in CRCs
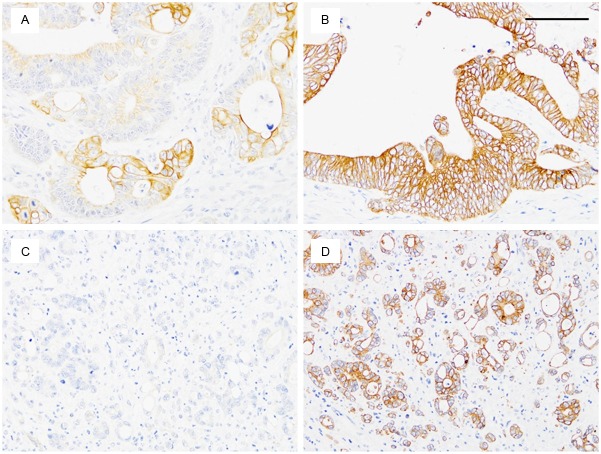
OCT2 immunostaining tended to be strong at the invasion front but weak or absent at the tumor center. High OCT2 levels at the invasion front were observed in 47 tumors (59%) but invariably, the tumor center expressed low OCT2 levels (P<0.001, Figure 1). The remaining 33 tumors (41%) displayed low OCT2 levels both at the tumor center and at the invasion front. Surface epithelial cells in the adjacent normal mucosa displayed weak or moderate OCT2 staining, consistently weaker than that at the invasion front of the tumor tissues. With regard to TB, high levels were observed in 40 tumors (50%). OCT2 levels at the invasion front did not correlate with TB levels (r=0.18, P=0.12).
Figure 1.
Representative immunostaining patterns for organic cation transporter 2 (OCT2). Strong membranous/cytoplasmic expression of OCT2 was found at the invasion front (tumor cell groups surrounded by stroma), whereas the tumor center (asterisk) showed no or weak OCT2 staining. A: Low magnification. B: High magnification of the boxed area. Scale bar, 100 μm.
Correlation of OCT2 and TB levels with clinicopathological findings
We evaluated the correlation of OCT2 and TB levels with clinicopathological findings (Table 1). High OCT2 levels were significantly associated with depth of invasion (more frequently in tumors with serosal invasion, P=0.03), but did not correlate with the other parameters (patient age, gender, tumor location, degree of differentiation, lymph node metastasis, lymphatic and venous invasion, and chemotherapy regimen).
High TB levels were significantly associated with the degree of lymphatic invasion (P=0.03), but not with the other clinicopathological parameters.
Correlation of OCT2 and TB levels with PFS in univariate analysis
All patients could be evaluated for PFS, with the median PFS of patients being 8.3 months (range, 2.4-27.3 months). Contrary to the association with severe invasion, a high OCT2 level was a favorable factor for PFS: median PFS was 12.0 months in patients with high OCT2 levels and 9.0 months in patients with low OCT2 levels (P=0.02) (Table 2, Figure 2A). In contrast, high levels of TB correlated significantly with shorter PFS: median PFS was 9.0 months in patients with high TB levels and 12.0 months in patients with low TB levels (P=0.01) (Table 2, Figure 2B).
Table 2.
Univariate analysis of prognostic factors for PFS in mCRC patients treated with oxaliplatin-based chemotherapy
| Variables | Median PFS month (95% CI) | P value |
|---|---|---|
| Tumor location | ||
| Colon | 11.0 (9.5-12.5) | 0.54 |
| Rectum | 9.0 (5.1-12.9) | |
| Depth of invasion | ||
| pT2/pT3 | 10.0 (8.0-12.0) | 0.43 |
| pT4 | 11.0 (8.5-13.5) | |
| Lymph node metastasis | ||
| pN0/pN1 | 10.0 (7.8-12.2) | 0.73 |
| pN2 | 10.0 (8.3-11.7) | |
| Lymphatic invasion | ||
| ly0/ly1 | 14.0 (9.6-18.4) | 0.45 |
| ly2/ly3 | 10.0 (8.8-11.2) | |
| Venous invasion | ||
| v0/v1 | 11.0 (9.4-12.6) | 0.52 |
| v2/v3 | 10.0 (7.8-12.2) | |
| OCT2 | ||
| Low | 9.0 (6.0-12.0) | 0.02* |
| High | 12.0 (9.8-14.2) | |
| TB | ||
| Low | 12.0 (8.6-15.4) | 0.01* |
| High | 9.0 (6.5-11.5) | |
| Combined OCT2 and TB | ||
| Two favorable factors | 14.0 (11.5-16.5) | <0.001* |
| One favorable factor | 10.0 (8.9-11.1) | |
| No favorable factors | 6.0 (4.2-7.8) |
Statistically significant;
PFS, progression-free survival; mCRC, metastatic colorectal cancer; CI, confidence interval; OCT2, organic cation transporter 2; TB, tumor budding.
Figure 2.
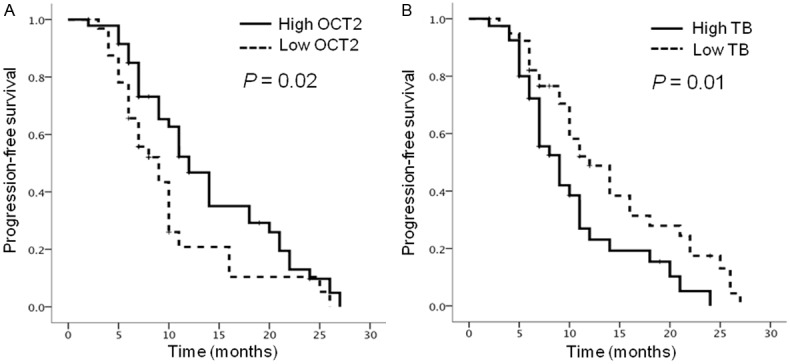
Kaplan-Meier progression-free survival (PFS) curves according to levels of organic cation transporter 2 (OCT2) and tumor budding (TB). A: High levels of OCT2 (solid line) significantly correlated with longer PFS (P=0.02) as compared with low levels of OCT2 (dashed line). B: High levels of TB (solid line) significantly correlated with shorter PFS (P=0.01) as compared with low levels of TB (dashed line).
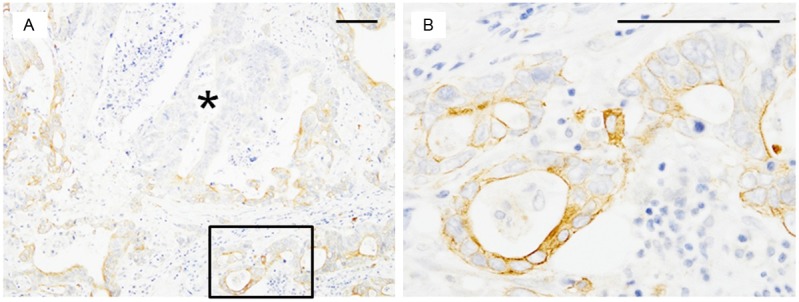
The number of favorable OCT2/TB factors was significantly associated with PFS (P<0.001) (Table 2, Figure 3). Patients with 2 favorable factors (high OCT2/low TB; median PFS of 14.0 months; Figure 4A and 4B) showed significantly longer PFS than those with 1 favorable factor (high OCT2/high TB or low OCT2/low TB; median PFS of 10.0 months; P=0.03) and those with 0 favorable factors (low OCT2/high TB; median PFS of 6.0 months; P<0.001; Figure 4C and 4D). Patients with 1 favorable factor showed significantly longer PFS than those with 0 favorable factors (P=0.005). In contrast, tumor location, depth of invasion, lymph node metastasis, lymphatic invasion, and venous invasion did not correlate with PFS.
Figure 3.
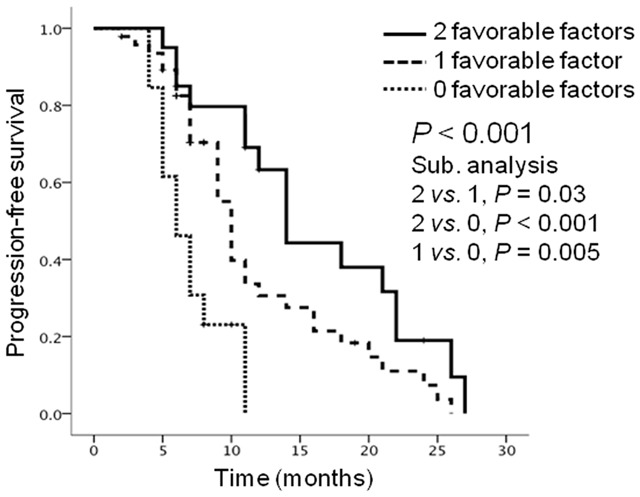
Kaplan-Meier progression-free survival (PFS) curves according to the number of favorable organic cation transporter 2 (OCT2)/tumor budding (TB) factors: 2 favorable factors (high OCT2/low TB) (solid line), 1 favorable factor (high OCT2/high TB or low OCT2/low TB) (dashed line), and 0 favorable factors (low OCT2/high TB) (dotted line). This combined classification was significantly associated with PFS (P<0.001). Patients with 2 favorable factors achieved the longest PFS whereas those with 0 favorable factors had the shortest PFS.
Figure 4.
Representative immunostaining patterns according to patient progression-free survival (PFS). A, C: Organic cation transporter 2 (OCT2). B, D: Pan-cytokeratin. Pan-cytokeratin immunostaining enabled clear visualization of tumor budding (TB). A, B: Immunohistochemical results of a tumor from a patient with a long PFS (14.1 months), showing the most favorable OCT2/TB pattern (high OCT2/low TB). C, D: Immunohistochemical results of a tumor from a patient with a short PFS (7.0 months), showing the least favorable pattern (low OCT2/high TB). Scale bar, 100 μm.
Correlation of OCT2 and TB levels with PFS in multivariate analysis
We first used the Cox proportional hazard regression model with seven variables: tumor location, depth of invasion, lymph node metastasis, lymphatic invasion, venous invasion, OCT2 level, and TB level (Table 3, model 1). This analysis showed that only the OCT2 level (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.24-0.80; P=0.007) and TB level (HR, 2.38; 95% CI, 1.31-4.30; P=0.004) preserved independent prognostic significance.
Table 3.
Multivariate analysis of prognostic factors for PFS in mCRC patients treated with oxaliplatin-based chemotherapy
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Variables | HR (95% CI) | P value | Variables | HR (95% CI) | P value |
| Tumor location | Tumor location | ||||
| Colon vs. Rectum | 0.77 (0.39-1.54) | 0.46 | Colon vs. Rectum | 0.77 (0.39-1.52) | 0.45 |
| Depth of invasion | Depth of invasion | ||||
| pT2/pT3 vs. pT4 | 0.90 (0.47-1.72) | 0.74 | pT2/pT3 vs. pT4 | 0.90 (0.48-1.72) | 0.76 |
| Lymph node metastasis | Lymph node metastasis | ||||
| pN0/pN1 vs. pN2 | 0.94 (0.50-1.77) | 0.86 | pN0 vs. pN1/pN2 | 0.94 (0.51-1.72) | 0.83 |
| Lymphatic invasion | Lymphatic invasion | ||||
| ly0/ly1 vs. ly2/ly3 | 0.87 (0.43-1.81) | 0.72 | ly0/ly1 vs. ly2/ly3 | 0.88 (0.43-1.81) | 0.73 |
| Venous invasion | Venous invasion | ||||
| v0/v1 vs. v2/v3 | 1.15 (0.59-2.23) | 0.68 | v0/v1 vs. v2/v3 | 1.15 (0.60-2.24) | 0.67 |
| OCT2 | Combined OCT2 and TB | ||||
| Low vs. High | 0.44 (0.24-0.80) | 0.007* | Two favorable factors vs. | 0.43 (0.26-0.70) | 0.001* |
| TB | One favorable factor vs. | ||||
| Low vs. High | 2.38 (1.31-4.30) | 0.004* | No favorable factors | ||
Statistically significant;
PFS, progression-free survival; mCRC, metastatic colorectal cancer; HR, hazard ratio; CI, confidence interval; OCT2, organic cation transporter 2; TB, tumor budding.
Next, the combined OCT2 and TB status was evaluated in multivariate analysis (Table 3, model 2). We found that the number of favorable OCT2/TB factors was the sole independent predictor of PFS (HR, 0.43; 95% CI, 0.26-0.70; P=0.001).
Discussion
Significant progress in the treatment of patients with mCRC has been achieved with the development of 5-FU plus oxaliplatin- or irinotecan-containing chemotherapy regimens [4]. To avoid the improper administration of these regimens that would cause unpleasant side effects, it is important to identify predictive factors that can select patients for oxaliplatin-based chemotherapy from irinotecan-based or other combination therapies. One candidate predictive factor is the excision repair cross-complementation group 1 (ERCC1), a key protein that repairs DNA adducts induced by platinum compounds. Indeed, a large number of studies evaluated whether ERCC1 protein and mRNA expression levels as well as DNA polymorphisms were associated with poor outcomes in CRC patients treated with oxaliplatin-based chemotherapy [21,22]. Unfortunately, however, the results of these studies were inconsistent. Therefore, additional work is warranted to further identify biomarkers that are associated with the benefit of oxaliplatin-based chemotherapy.
Facilitated transport systems are involved in the intracellular uptake of platinum drugs. Thus, the level of platinum compound transporters may predict, at least in part, the sensitivity of tumors to platinum drugs [23]. Preclinical studies have indicated that the level of the transporter OCT2 is a critical determinant in the uptake and consequent cytotoxicity of platinum compounds, particularly oxaliplatin [8-10]. In addition, several researchers have emphasized that TB should be used as an index of tumor aggressiveness in CRC patients [11-17]. However, to the best of our knowledge, the impact of the levels of OCT2 and TB on clinicopathological findings and the prognosis of mCRC patients treated with first-line oxaliplatin-based chemotherapy has not been evaluated.
We found that OCT2 has a unique expression pattern in CRC tissues: high levels of OCT2 were detected at the invasion front, whereas the tumor center consistently displayed low OCT2 levels. In addition, clinicopathological correlation analysis indicated that the level of OCT2 was associated with depth of invasion. One study showed that OCT2 mRNA was expressed in 11 of 20 CRC tissues [8]. However, OCT2 mRNA was not expressed in CRC cell lines [8,10]. These data suggest that the upregulation of OCT2 is specific to CRC cells that invade the surrounding tissue. Until now, however, the molecular basis of this phenomenon has not been understood and further studies are required to elucidate the roles of OCT2 in promoting CRC cell invasion.
In agreement with other studies [12,16], a high level of TB was associated with lymphatic invasion. High TB levels have been linked to increased expression of proteins that are closely related to the degradation of the extracellular matrix (ECM) [24]. The levels of ECM degradation proteins such as matrix metalloproteinase-2 [25], urokinase plasminogen activator (uPA), and uPA receptor have been shown to correlate with lymphatic invasion [26]. Thus, TB cancer cells may be producing proteins that degrade the ECM in order to invade the lymphatic vessels.
With regard to the impact of the OCT2 level on PFS in mCRC patients treated with oxaliplatin-based chemotherapy, high OCT2 levels were significantly associated with longer PFS. Furthermore, multivariate analysis confirmed an independent prognostic impact of OCT2. Preclinical data on the influence of OCT2 on the uptake and consequent cytotoxicity of oxaliplatin [8-10] and our present clinical data suggest a better prognosis for mCRC patients with tumors having a high OCT2 level. This improved prognosis may be attributable to the high levels of OCT2 within invading cells that would allow for efficient uptake of oxaliplatin.
Tumors with a high TB level were significantly associated with a shorter PFS, and this independent prognostic significance was preserved on multivariate analysis. Several studies have reported an independent adverse effect of TB on the survival of CRC patients [11-13,15,16]. However, in one study, an association of TB with poor outcome was shown only on univariate but not on multivariate analysis [27].
When evaluating OCT2 and TB in combination, patients with 2 favorable factors achieved the longest PFS whereas those with 0 favorable factors had the shortest PFS. On multivariate analysis, the combined OCT2/TB status was identified as a powerful independent prognostic factor, with a higher significance than that of individual OCT2 or TB status. However, this study is limited by its retrospective design and small number of patients. Thus, larger prospective studies are needed to evaluate the feasibility of our results.
In conclusion, a high OCT2 level may be an independent factor for longer PFS in mCRCs patients treated with first-line oxaliplatin-based chemotherapy, possibly because of its role in rendering invading cancer cells more susceptible to oxaliplatin. Combined analysis of OCT2 and TB status may provide important information for the selection of mCRC patients for oxaliplatin-based chemotherapy.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Misset JL, Bleiberg H, Sutherland W, Bekradda M, Cvitkovic E. Oxaliplatin clinical activity: a review. Crit Rev Oncol Hematol. 2000;35:75–93. doi: 10.1016/s1040-8428(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 3.Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1:227–235. [PubMed] [Google Scholar]
- 4.Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13:276–281. doi: 10.1097/PPO.0b013e3181570062. [DOI] [PubMed] [Google Scholar]
- 5.Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7:205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- 6.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3) J Pharmacol Exp Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 7.Cutler MJ, Choo EF. Overview of SLC22A and SLCO families of drug uptake transporters in the context of cancer treatments. Curr Drug Metab. 2011;12:793–807. doi: 10.2174/138920011798357060. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 10.Burger H, Zoumaro-Djayoon A, Boersma AW, Helleman J, Berns EM, Mathijssen RH, Loos WJ, Wiemer EA. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br J Pharmacol. 2010;159:898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–132. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 12.Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum. 2005;48:1597–1602. doi: 10.1007/s10350-005-0060-6. [DOI] [PubMed] [Google Scholar]
- 13.Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS. Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis. 2007;22:863–868. doi: 10.1007/s00384-006-0249-8. [DOI] [PubMed] [Google Scholar]
- 14.Prall F. Tumour budding in colorectal carcinoma. Histopathology. 2007;50:151–162. doi: 10.1111/j.1365-2559.2006.02551.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa H, Mitomi H, Nishiyama Y, Kishimoto I, Fukui N, Nakamura T, Watanabe M. Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis. 2008;10:41–47. doi: 10.1111/j.1463-1318.2007.01240.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, Nakagawa T, Uchimoto K, Nakamura S, Nonomura A, Nakajima Y. Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res. 2008;28:1831–1836. [PubMed] [Google Scholar]
- 17.Karamitopoulou E, Zlobec I, Kölzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, Lugli A. Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol. 2013;26:295–301. doi: 10.1038/modpathol.2012.155. [DOI] [PubMed] [Google Scholar]
- 18.International Union Against Cancer. TNM classification of malignant tumours. 7th ed. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 19.Japanese classification of colorectal carcinoma. 2nd English ed. Tokyo: Kanahara Shuppan; 2009. Japanese Scociety for Cancer of the Colon and Rectum. [Google Scholar]
- 20.Gao C, Wang AY, Han YJ. Microwave antigen retrieval blocks endogenous peroxidase activity in immunohistochemistry. Appl Immunohistochem Mol Morphol. 2008;16:393–399. doi: 10.1097/PAI.0b013e31815b074d. [DOI] [PubMed] [Google Scholar]
- 21.Bohanes P, Labonte MJ, Lenz HJ. A review of excision repair cross-complementation group 1 in colorectal cancer. Clin Colorectal Cancer. 2011;10:157–164. doi: 10.1016/j.clcc.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Fang YJ, Li F, Ou QJ, Chen G, Ma G. ERCC1, defective mismatch repair status as predictive biomarkers of survival for stage III colon cancer patients receiving oxaliplatin-based adjuvant chemotherapy. Br J Cancer. 2013;108:1238–1244. doi: 10.1038/bjc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14:22–34. doi: 10.1016/j.drup.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. 2010;1:651–661. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sis B, Sağol Ö, Küpelioğlu A, Sokmen S, Terzi C, Fuzun M, Ozer E, Bishop P. Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol Res Pract. 2004;200:379–387. doi: 10.1016/j.prp.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Baker EA, Bergin FG, Leaper DJ. Plasminogen activator system, vascular endothelial growth factor, and colorectal cancer progression. Mol Pathol. 2000;53:307–312. doi: 10.1136/mp.53.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sy J, Fung CL, Dent OF, Chapuis PH, Bokey L, Chan C. Tumor budding and survival after potentially curative resection of node-positive colon cancer. Dis Colon Rectum. 2010;53:301–307. doi: 10.1007/DCR.0b013e3181c3ed05. [DOI] [PubMed] [Google Scholar]