Abstract
Objective: Cardiac hypertrophy is a compensatory response of the heart to maintain its pumping capacity. Cardiac hypertrophy can be divided into pathological hypertrophy and physiological hypertrophy. The major forms of physiological hypertrophy include developing in response to developmental maturation, exercise, and pregnancy, which is adaptive and beneficial. Exercise has well-known beneficial cardiovascular effects and has recently been shown to be protective for myocardial ischemia-reperfusion injury. However, there are conflicting reports for the cardiac protective effects of pregnancy-induced hypertrophy. In the present study, we investigated the effects of pregnancy-induced physiological hypertrophy in cardiac ischemia-reperfusion injury and if cardiac progenitor cells were activated during pregnancy. Methods: Physiological hypertrophy was induced in pregnancy and the mRNA levels of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were determined by real-time polymerase chain reactions (RT-PCRs) analysis. Triphenyltetrazolium chloride staining was used to determine the cardiac ischemia-reperfusion injury. c-Kit and Nkx2.5 levels were determined by RT-PCRs, western blot and immunofluorescent staining. Results: Heart weight (HW) and the ratio of HW to tibia length were increased while mRNA levels of ANP and BNP remained unchanged. Pregnancy-induced physiological hypertrophy protected against cardiac ischemia-reperfusion injury. In pregnancy, c-Kit positive cardiac progenitor cells were activated. Conclusion: This study presents that pregnancy-induced physiological hypertrophy activates cardiac progenitor cells and thereafter protects against cardiac ischemia-reperfusion injury.
Keywords: Pregnancy, hypertrophy, physiological, ischemia/reperfusion, cardiac progenitor cells
Introduction
Cardiac hypertrophy, defined as the enlargement of the ventricle, develops as a result of the size increment of pre-existing cardiomyocytes [1]. Cardiac hypertrophy represents a compensatory response of the heart to maintain the pumping capacity, which can be either pathological or physiological changes [2]. Pathological hypertrophy, mainly caused by aortic stenosis, hypertension, ischemic heart disease, and infectious agents in the clinical setting, is maladaptive and detrimental, which can lead to cardiac fibrosis and heart failure if stimulus persists. Physiological hypertrophy, developing in response to developmental maturation, exercise, and pregnancy, is adaptive and beneficial, which does not induce sarcomere disarray, cardiac fibrosis, and dysfunction and is potential reversible without significant long-term detrimental effects on cardiac function [2-4].
Both being physiological hypertrophy, pregnancy is different from exercise training [1,5]. Exercise stimulates the cardiac growth intermittently while during pregnancy, the volume overload and increased heart rate is continuous by comparison [6]. The pregnant heart represents a better functioning heart in response to enhanced cardiac output and mechanical stress [6-8]. In addition, pregnancy is accompanied by significant changes in estrogens and progesterone levels and it has a distinct cardiac transcriptional profiles comparing to exercise [5,6,8]. Exercise has well-known beneficial cardiovascular effects and has recently been shown to be protective for myocardial ischemia-reperfusion injury [9-11]. Although pregnancy-induced hypertrophy is different from exercise-induced physiological hypertrophy to some extent, it is generally accepted as a physiological adaptation [1,8]. However, there are conflicting reports for the cardiac protective effects of pregnancy-induced hypertrophy [12-14]. Pregnant heart has been shown to be protected from cardiac fibrosis [12]. However, a recent study shows that cardiac vulnerability to ischemia-reperfusion injury drastically increases in late pregnancy in rat in vivo or in mice ex vivo [14]. Therefore, further studies are obviously needed to clarify whether pregnancy-induced hypertrophy has protective effects.
It is well-known that the heart has an attractive regenerative capacity owing to the endogenous stem cells that maintain the myocardium and replace the damaged cardiomyocytes [15-17]. The regenerative capacity can serve as a basis for regeneration of an damaged heart if it could be enhanced [18-20]. Therefore, in the present study, we investigated the effects of pregnancy-induced physiological hypertrophy in cardiac ischemia-reperfusion injury and if cardiac progenitor cells are activated during pregnancy.
Materials and methods
This study was approved by the local ethical committees and all animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996).
Animals
C57/BL6 female mice aged 10-12 weeks, purchased from Shanghai SLAC Laboratory Animal CO. LTD were used in nonpregnant diestrus stage (NP), and late pregnant (LP, day 19-20 of pregnancy) in the present study.
In vivo ischemia/reperfusion injury model in mice
Mice anesthetized with ketamine and sevoflulene underwent open-chest coronary artery ligation. The left coronary artery about 2 mm under the left auricle was ligated with 7-0 silk sutures. Following 30 minutes of left coronary artery occlusion, the ligature was released and reperfusion was visually confirmed. After that, the chests of the mice were closed and the mice were allowed to recover. After 24 hours of reperfusion, infarct size was determined with triphenyltetrazolium chloride (TTC) staining.
Total RNA isolation and real-time polymerase chain reactions (PCRs) analysis
Total RNA were isolated from cardiac tissues in NP and LP using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instruction. Real-time PCRs with SYBR Green I, which was validated with respect to reproducibility and linearity within the measuring range, was performed in quadruplicate with the Cycler System (Bio-Rad, Hercules, CA, USA), and SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA, USA) as reagent. To correct for potential variances between samples in reverse transcribed efficiency or in mRNA extraction, the mRNA expression of each gene was normalized to 18S, a reference gene. Sequences for all primers used in the present study were as follows: ANP, Forward, 5′-AGGCAGTCGATTCTGCTTGA-3′ and Reverse, 5′-CGTGATAGATGAAGGCAGGAAG-3′; BNP, Forward, 5′-TAGCCAGTCTC CAGAGCAATTC-3′ and Reverse, 5′-TTGGTCCTTCAAGAGCTGTCTC-3′; c-Kit, Forward, 5′-GAATCTCCGAAGAGGCC AGAA-3′ and Reverse, 5′-GCTGCAACAGGGGGTAACAT-3′; Nkx2.5, Forward, 5′-GACAAAGCCGA GACGGATGG-3′ and Reverse, 5′-CTGTCGCTTGCACTTGTAGC-3′; 18S, Forward, 5′-TCAAGAACGAAAGTCGGAGG-3′ and Reverse 5′-GGACATCT AAGGGCATCAC-3′. The cycling parameters were as follows: denaturation at 94°C for 1 min; annealing at 55-60°C for 1 min (depending on the primer); and elongation at 72°C for 1 min (40 cycles). All real time PCR reactions, including no-template controls, were performed in triplicate. The amplification products were assessed using melting curve analysis.
Western blot
Heart lysates were prepared in lysis buffer (20 mM Tris, 150 mM NaCl, 10% glycerol, 20 mM glycerophosphate, 1% NP40, 5 mM EDTA, 0.5 mM EGTA, 1 mM Na3VO4, 0.5 mM PMSF, 1 mM benzamidine, 1 mM DTT, 50 mM NaF, 4 μM leupeptin, pH=8.0). Samples were resolved by 10% SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with 5% non-fat milk in TBST (50 mM Tris, 150 mM NaCl, 0.5 mM Tween-20, pH=7.5) and then incubated with primary antibodies overnight. After washing three times with TBST and incubated with HRP-linked secondary antibodies for 1 h at room temperature. The signal was visualized using an ECL detection kit (Millipore, MA). The GAPDH was taken as the loading control. Antibodies used in this study were purchased from Abcam (anti-c-kit, ab5506) and Bioworld Technology (GAPDH, AP0063). Image J software (NIH) was used to perform densitometric analysis (http://rsb.info.nih.gov/ij/).
Immunofluorescent staining
Frozen sections from cardiac tissues in NP and LP were sliced into a thickness of 6 μM, and then were postfixed with 4% paraformaldehyde dissolved in 0.1M phosphate buffer (pH=7.4) for at least 15 minutes. After washed with phosphate buffer saline (PBS) for three times, sections were immersed in 10% goat serum for 1 hour. After that, sections were incubated overnight at 4°C with rabbit polyclonal anti-c-kit (Abcam, ab5506), which was diluted by 1:100 in 1×PBS with 0.25% Triton X-100. After that, sections were incubated to goat anti-rabbit labeled with rhodamine secondary antibodies (Santa Cruz, sc-362262) diluted by 1:200 in phosphate buffer for 1 hour. Finally, the sections were stained with DAPI (ProLong® Gold, Life technology). Semi-quantitative analyze was used to determine the percentage of c-Kit positive cells in a blinded manner. Slides (n=10) from each heart, 4 hearts from each group were counted at 40×magnification using confocal laser scanning microscope (LSM 710, Carl Zeiss MicroImaging GmbH, Germany).
Statistical analysis
Relative mRNA expression was calculated using the 2-ΔΔCt method. Data were presented as mean±SEM. An independent-samples t-test or chi-square test was conducted to evaluate the one-way layout data. All analyses were performed using SPSS 17.0, and all statistical tests were two-sided. P values less than 0.05 were considered to be statistically significant.
Results
Physiological hypertrophy is induced in pregnancy
As expected, pregnancy was associated with significant heart hypertrophy. Figure 1A showed that the heart weight (HW) in LP mice was significantly higher than that in NP mice. The larger body weight (BW) in LP mice made the ratio of HW/BW decrease in LP mice (Figure 1B and 1D), which is a feature of pregnancy-induced hypertrophy and is contrast to that of pathological hypertrophy where the ratio of HW/BW often increases. However, the ratio of HW to tibia length (TL) increased in LP mice (Figure 1C), indicating that hypertrophy is induced in pregnancy. To exclude the occurrence of pathological hypertrophy, mRNA levels of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were determined. Both genes were not changed in LP mice (Figure 1E and 1F), indicating that pregnancy-induced hypertrophy is physiological instead of pathological.
Figure 1.

Physiological hypertrophy is induced in pregnancy. A: Heart weight; B: Body weight; C: Heart weight/Tibia length; D: Heart weight/Body weight; E: ANP mRNA level; F: BNP mRNA level. *, compared to the non-pregnancy group, P less than 0.05.
Pregnancy-induced physiological hypertrophy protects against cardiac ischemia-reperfusion injury
To determine whether pregnancy-induced physiological hypertrophy attenuated myocardial injury after myocardial ischemia/reperfusion, LP or NP mice were subjected to 30 minutes of left coronary artery occlusion, followed by 24 hours of reperfusion, at which time point, infarct size was determined. Representative sections of NP and LP hearts were shown in Figure 2A. Interestingly, the infarct size was significant smaller in LP mice comparing to that in NP mice (Figure 2B), indicating that pregnancy-induced physiological hypertrophy protects against cardiac ischemia-reperfusion injury.
Figure 2.

Pregnancy-induced physiological hypertrophy protects against cardiac ischemia-reperfusion injury. A: Representative sections of NP and LP hearts in cardiac ischemia-reperfusion injury; B: Infarct size is reduced in LP mice. *, compared to the non-pregnancy group, P less than 0.05. NP (n=5), nonpregnant diestrus stage; LP (n=6), late pregnant.
Pregnancy upregulates the expression of c-Kit
In the present study, c-Kit was used as the markers for cardiomyocyte progenitor cells while Nkx2.5 was chosen as the markers for detecting early differentiation into cardiomyocytes [17,21-23]. As determined by real-time PCRs, c-kit and Nkx2.5 were not changed at the mRNA levels (Figure 3A and 3B). However, Western blot showed that pregnancy up-regulated c-Kit at the protein level (Figure 3C and 3D). These results indicate that the protective effects of pregnancy against ischemia/reperfusion injury might be related to the up-regulation of cardiac progenitor cells.
Figure 3.
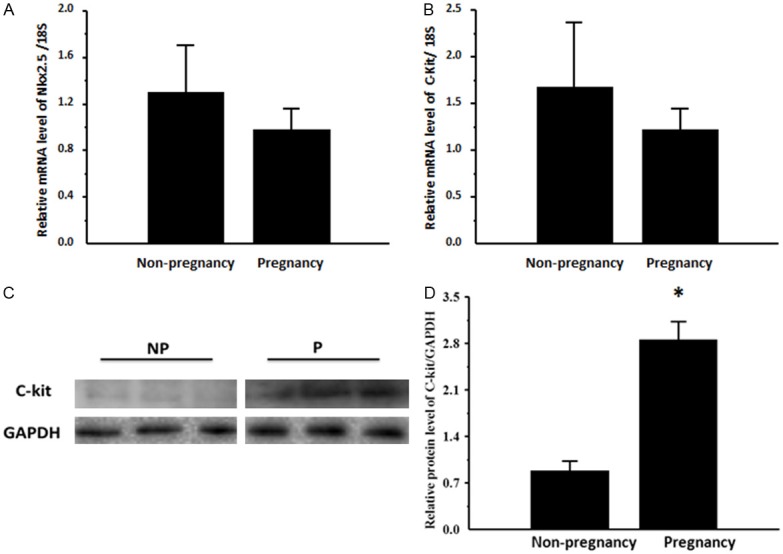
Pregnancy upregulates the expression of c-Kit. A: Nkx2.5 mRNA level; B: c-Kit mRNA level; C: Representative blots of c-Kit in non-pregnancy and pregnancy group; D: c-Kit protein level. *, compared to the non-pregnancy group, P less than 0.05. n=3 per group.
Pregnancy activates c-Kit positive cardiac progenitor cells
As c-Kit positive cells are the only one that has been proven to have all stem cell features such as clonogenicity, self-renewal and multipotency, blinded semi-quantitative immunofluorescent staining analyses of c-Kit positive cells were performed. As shown in Figure 4, pregnancy activates c-Kit positive cardiac progenitor cells, which might partly contributes to the cardiac protective effects during pregnancy.
Figure 4.

Pregnancy activates c-Kit positive cardiac progenitor cells. Representative pictures of c-Kit in non-pregnancy (A) and pregnancy group (B). Pregnancy activates c-Kit positive cardiac progenitor cells (C). *, compared to the non-pregnancy group, P less than 0.05. n=4 per group.
Discussion
Pregnancy-induced cardiac hypertrophy is a physiological adaptation similar to exercise-induced cardiac hypertrophy [7,8]. Although the ratio of HW/TW was decreased in LP, pregnancy-induced cardiac hypertrophy definitely occurred and the decreased ratio was only due to the dramatic increase of BW in LP. Supporting the concept that pregnancy induced a physiological adaptation, HW and the ratio of HW/TL in LP mice was increased while the fetal genes including ANP and BNP remained unchanged. These facts are consistent with the general concept of physiological hypertrophy, indicating that physiological hypertrophy is induced during pregnancy.
Exercise-induced physiological hypertrophy has been reported to be protective for pathological cardiac remodeling [24]. In addition, exercise consistently provides protection against myocardial infarction in animal models and most recently it has been shown to protect against myocardial ischemia-reperfusion injury [24,25]. Pregnancy has been proven to protect against pathological cardiac remodeling [13], which is consistent with the widely accepted protective role of physiological hypertrophy [12]. Here we provide direct evidence that pregnancy-induced physiological hypertrophy protects against cardiac ischemia-reperfusion injury. This is a rather surprising finding, given that a recent study has showed that cardiac vulnerability to ischemia-reperfusion injury drastically increased in late pregnancy in vivo rat model followed up by isolated mouse heart model [14], however this study is supportive of the evidence indicating the protective role of physiological hypertrophy. The main differences between the present study and that previous study are as follows. Firstly, this study was performed in vivo in mice while that study was in vivo in rat and ex vivo in mice. Secondly, the ischemia time in this study was 30 minutes while in that study was 45 minutes. Thus, the major reasons for the inconsistence of the present study with the previous one might be methodological or species differences.
The paradigm that the adult heart is a terminally differentiated organ has been challenged in recent years with accumulating evidences [16,26,27]. The adult mammalian heart retains the capacity for self-renewal though the source of newly formed cardiomyocytes is still on debate [19,20,28]. Cardiomyocytes replacement, especially after cardiac damage, is attributed to differentiation of a stem cell compartment or proliferation of adult cardiomyocytes [16,22,23,26,27]. Pregnancy has been reported to fail to promote proliferation of adult cardiomyocytes. Moreover, adult c-kit positive cardiac stem cells have been shown to be necessary and sufficient for functional cardiac regeneration and repair [28]. In the present study, we found that pregnancy initiated robust signs of up-regulation of cardiac progenitor cells, which might be the fundamental basis of the protective effects of LP in cardiac ischemia/reperfusion injury.
In conclusion, this study presents that pregnancy-induced physiological hypertrophy activates cardiac progenitor cells and thereafter protects against cardiac ischemia-reperfusion injury.
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (81200669 to J. Xiao; 81270314 to J. Xu), Innovation Program of Shanghai Municipal Education Commission (13YZ014 to J. Xiao), Foundation for University Young Teachers by Shanghai Municipal Education Commission (year 2012, to J. Xiao), Innovation Foundation of Shanghai university (sdcx2012038, to J. Xiao), and partially by Leading Academic Discipline Project of Shanghai Municipal Education Commission “Molecular Physiology”.
Disclosure of conflict of interest
None declared.
References
- 1.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 2.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- 4.Umar S, Nadadur R, Iorga A, Amjedi M, Matori H, Eghbali M. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol. 2012;113:1253–1259. doi: 10.1152/japplphysiol.00549.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol. 2012;112:1564–1575. doi: 10.1152/japplphysiol.00027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung E, Heimiller J, Leinwand LA. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One. 2012;7:e42297. doi: 10.1371/journal.pone.0042297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med. 2006;16:285–291. doi: 10.1016/j.tcm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Refitz-Zagrosek V, Eghbali M. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2:192–207. [PMC free article] [PubMed] [Google Scholar]
- 9.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakpva D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvert JW. Cardioprotective effects of nitrite during exercise. Cardiovasc Res. 2011;89:499–506. doi: 10.1093/cvr/cvq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aljabri MB, Songstad NT, Lund T, Serrano MC, Andreasen TV, Al-Saad S, Lindal S, Sitras V, Acharya G, Ytrehus K. Pregnancy protects against antiangiogenic and fibrogenic effects of angiotensin II in rat hearts. Acta Physiol (Oxf) 2011;201:445–456. doi: 10.1111/j.1748-1716.2010.02234.x. [DOI] [PubMed] [Google Scholar]
- 13.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300:H931–H942. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Umar S, Iorga A, Youn JY, Wang Y, Regitz-Zagrosek V, Cai H, Eghbali M. Cardiac vulnerability to ischemia/reperfusion injury drastically increases in late pregnancy. Basic Res Cardiol. 2012;107:271. doi: 10.1007/s00395-012-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3:S8–S13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 18.Allukian M 3rd, Xu J, Morris M, Caskey R, Dorsett-Martin W, Plappert T, Griswold M, Gorman JH 3rd, Gorman RC, Liechty KW. Mammalian cardiac regeneration after fetal myocardial infarction requires cardiac progenitor cell recruitment. Ann Thorac Surg. 2013;96:163–170. doi: 10.1016/j.athoracsur.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenzweig A. Medicine. Cardiac regeneration. Science. 2012;338:1549–1550. doi: 10.1126/science.1228951. [DOI] [PubMed] [Google Scholar]
- 20.Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs338. doi:10.1093/eurheartj/ehs338. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genead R, Fischer H, Hussain A, Jaksch M, Andersson AB, Ljung K, Bulatovic I, Franco-Cereceda A, Elsheikh E, Corbascio M, Smith CL, Sylven C, Grinnemo KH. Ischemia-reperfusion injury and pregnancy initiate time-dependent and robust signs of up-regulation of cardiac progenitor cells. PLoS One. 2012;7:e36804. doi: 10.1371/journal.pone.0036804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unno K, Jain M, Liao R. Cardiac side population cells: moving toward the center stage in cardiac regeneration. Circ Res. 2012;110:1355–1363. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang WE, Chen X, Houser SR, Zeng C. Potential of cardiac stem/progenitor cells and induced pluripotent stem cells for cardiac repair in ischaemic heart disease. Clin Sci (Lond) 2013;125:319–327. doi: 10.1042/CS20130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, Powers SK. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96:1299–1305. doi: 10.1152/japplphysiol.00920.2003. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi CM, Epstein JA. Heart-healthy hypertrophy. Cell Metab. 2011;13:3–4. doi: 10.1016/j.cmet.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koudstaal S, Jansen Of Lorkeers SJ, Gaetani R, Gho JM, van Slochteren FJ, Sluijter JP, Doevendans PA, Ellison GM, Chamuleau SA. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl Med. 2013;2:434–443. doi: 10.5966/sctm.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mollova M, Bersell K, Walsh K, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfò M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) Cardiac Stem Cells Are Necessary and Sufficient for Functional Cardiac Regeneration and Repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]


